Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.10158
Revised: February 11, 2014
Accepted: April 8, 2014
Published online: August 7, 2014
Processing time: 240 Days and 16.2 Hours
AIM: To investigate the potential role of oxidative stress and the possible therapeutic effects of N-acetyl cysteine (NAC), amifostine (AMF) and ascorbic acid (ASC) in methotrexate (MTX)-induced hepatotoxicity.
METHODS: An MTX-induced hepatotoxicity model was established in 44 male Sprague Dawley rats by administration of a single intraperitoneal injection of 20 mg/kg MTX. Eleven of the rats were left untreated (Model group; n = 11), and the remaining rats were treated with a 7-d course of 50 mg/kg per day NAC (MTX + NAC group; n = 11), 50 mg/kg per single dose AMF (MTX + AMF group; n = 11), or 10 mg/kg per day ASC (MTX + ASC group; n = 11). Eleven rats that received no MTX and no treatments served as the negative control group. Structural and functional changes related to MTX- and the various treatments were assessed by histopathological analysis of liver tissues and biochemical assays of malondialdehyde (MDA), superoxide dismutase (SOD), catalase, glutathione (GSH) and xanthine oxidase activities and of serum levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and total bilirubin.
RESULTS: Exposure to MTX caused structural and functional hepatotoxicity, as evidenced by significantly worse histopathological scores [median (range) injury score: control group: 1 (0-3) vs 7 (6-9), P = 0.001] and significantly higher MDA activity [409 (352-466) nmol/g vs 455.5 (419-516) nmol/g, P < 0.05]. The extent of MTX-induced perturbation of both parameters was reduced by all three cytoprotective agents, but only the reduction in hepatotoxicity scores reached statistical significance [4 (3-6) for NAC, 4.5 (3-5) for AMF and 6 (5-6) for ASC; P = 0.001, P = 0.001 and P < 0.005 vs model group respectively]. Exposure to MTX also caused a significant reduction in the activities of GSH and SOD antioxidants in liver tissues [control group: 3.02 (2.85-3.43) μmol/g and 71.78 (61.88-97.81) U/g vs model group: 2.52 (2.07-3.34) μmol/g and 61.46 (58.27-67.75) U/g, P < 0.05]; however, only the NAC treatment provided significant increases in these antioxidant enzyme activities [3.22 (2.54-3.62) μmol/g and 69.22 (61.13-100.88) U/g, P < 0.05 and P < 0.01 vs model group respectively].
CONCLUSION: MTX-induced structural and functional damage to hepatic tissues in rats may involve oxidative stress, and cytoprotective agents (NAC > AMF > ASC) may alleviate MTX hepatotoxicity.
Core tip: While the underlying mechanism of methotrexate (MTX)-induced hepatotoxicity remains to be fully elucidated, increases in oxidative stress have been linked to the effects of MTX; moreover, MTX-induced toxicity has been shown to be associated with increases in lipid peroxidation in various tissues, such as liver, using rat model systems. A few previous studies have shown that prophylactic delivery of antioxidant agents can prevent MTX-induced hepatotoxicity. Based on this information, we aimed to investigate the role of oxidative stress in MTX-induced hepatotoxicity and to evaluate the potential therapeutic effects of the cytoprotective antioxidants N-acetylcysteine, amifostine, and ascorbic acid.
- Citation: Akbulut S, Elbe H, Eris C, Dogan Z, Toprak G, Otan E, Erdemli E, Turkoz Y. Cytoprotective effects of amifostine, ascorbic acid and N-acetylcysteine against methotrexate-induced hepatotoxicity in rats. World J Gastroenterol 2014; 20(29): 10158-10165
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/10158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.10158
The folic acid antagonist methotrexate (MTX) disrupts cellular metabolism, effectively inhibiting cellular growth. As such, MTX is an effective cytotoxic agent and has been widely applied in chemotherapeutic-based treatments for malignancies as well as inflammatory diseases[1-3]. While MTX is generally well-tolerated by patients, its cytotoxic nature also lends a substantial risk of life-threatening side effects - the most severe of which are hematopoietic suppression, hepatotoxicity and pulmonary toxicity[1,2,4]. While the molecular mechanism of MTX-induced hepatotoxicity is not yet completely understood, considerable experimental and clinical evidence have implicated oxidative stress as a contributing factor[3,5]. Increased reactive oxygen species (ROS) generation, along with decreased antioxidant defense activities, have been shown to promote development and progression of MTX-induced hepatotoxicity in a rabbit model[6].
N-acetylcysteine (NAC) is a well established cytoprotective drug that has proven efficacy against drug (acetaminophen overdose)-induced hepatotoxicity[7]. As the acetylated precursor of glutathione (GSH), NAC exerts effective antioxidant and anti-inflammatory functions. In vitro studies of the molecular mechanisms of NAC have demonstrated that the drug can protect a cell from oxidative stress by inhibiting H2O2 formation[8].
Another cytoprotective drug with antioxidant properties is amifostine [AMF; S-2-(3-aminopropylamino) ethylphosphorothioic acid]. The efficacy of AMF as an anticancer therapy has prompted extensive research efforts to characterize its mode of action. Delivered as an inactive prodrug, interaction with membrane-bound alkaline phosphatase (ALP) is necessary to generate the activated metabolite form of AMF, known as WR-1065[9]. WR-1065 is capable of effective radical scavenging, especially for hydroxyl and oxygen radicals[9,10].
Ascorbic acid (ASC, vitamin C) is a naturally-occurring water-soluble antioxidant present in cells, body fluids, and plasma[11-13]. In addition to acting as a ROS scavenger[11,12], ASC plays a role as an essential coenzyme in the oxidative stress pathways; interactions have been demonstrated between ASC and proline hydroxylase, lysine hydroxylase, 4-hydroxyphenylpyruvate dioxygenase, dopamine-hydroxylase, tryptophan hydroxylase, and γ-butyrobetaine hydroxylase[13].
This study was designed to investigate the role of oxidative stress in MTX-induced hepatotoxicity (both structural and functional) and to evaluate the potential therapeutic effects of the cytoprotective antioxidants NAC, AMF and ASC. Specifically, a rat model of acute MTX-induced hepatotoxicity was established and the hepatoprotective mechanisms related to the antioxidant defense system were characterized by measuring changes in oxidative stress factors.
Fifty-five male Sprague Dawley rats (10-11 wk of age; weighing 230-320 g) were obtained from the Experimental Animal Research Center of Inonu University (Malatya, Turkey). The animals were housed under regular laboratory environment conditions (21 ± 2 °C, 60% ± 5% humidity, and 12:12 h light-dark cycle) with free access to standard rat chow and water. All procedures involving the animals were designed in accordance with the Guidelines for Animal Research from the National Institute of Health and were carried out with pre-approval from the Ethical Committee on Animal Research at Inonu University (No: 2011/A-109).
The 55 rats were randomly assigned to one of five groups, with an equal number of rats contained within each group. Simple randomization technique was used in this experimental study. Forty-four rats were administered a single intrapertioneal (ip) dose of 20 mg/kg methotrexate without (model group; n = 11) or with daily treatments of 50 mg/kg oral NAC (MTX + NAC group; n = 11), 50 mg/kg per day (single dose) ip AMF (MTX + AMF group; n = 11), or 10 mg/kg per day ip ASC (MTX + ASC group; n = 11). An additional 11 rats received ip injection of equivalent volumes of saline instead of MTX (n = 11), and represented the negative control group. The treatment course lasted 7 d for all groups. The body weights (BW) of the rats were measured at the last days of the study and recorded in grams (g). The dose and duration of MTX and antioxidant agents were selected according to results from previous studies[1,4,5,14-16].
All of the rats were sacrificed by anesthesia (ketamine) overdose at the end of the 7th day of treatment. The entire liver was excised and bisected; one-half was formalin-fixed and paraffin-embedded for subsequent use in histopathological analysis, and the other half was stored at -30 °C for subsequent biochemical analysis. Blood samples obtained from vena cava inferior and collected for biochemical analysis.
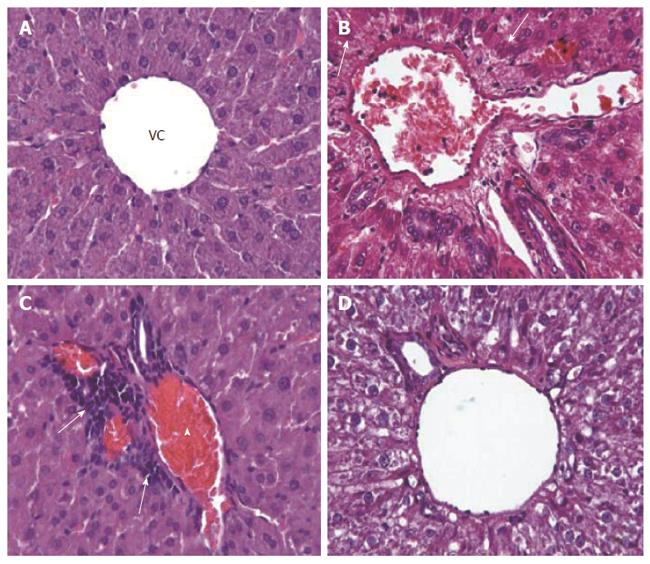
Histopathological analysis: Tissue sections (5 μm) were mounted on glass slides and stained with hematoxylin-eosin (H-E) to assess the general liver structure and with periodic acid-Schiff (PAS) to assess the presence of glycogen deposition in hepatocytes. Each stained section was semi-quantitatively evaluated under light microscope (DFC280 equipped with the QWin Image Processing System; Leica Micros Imaging Solutions Ltd., Cambridge, United Kingdom) by a histologist that was blinded to the treatment group. A scoring system was used to establish the severity of hepatic injury according to extent of (1) sinusoidal dilatation; (2) inflammatory cell infiltration; (3) congestion; and (4) hydropic degeneration (cytoplasmic vacuolization/swelling of hepatocyte), with features scored as 0 (normal), 1 (mild), 2 (moderate), or 3 (severe). The maximum score of 12 indicated the most severe hepatic injury.
Biochemical analyses: After thawing, the liver specimens were submerged in ice-cold 0.1 mol/L Tris-HCl buffer (pH 7.5; containing protease inhibitor and 1 mmol/L phenylmethylsulfonyl fluoride) and mechanically homogenized (Ultra Turrax T 25 basic; IKA, Wilmington, NC, United States) at 16000 g for 2 min at 4-8 °C.
The thiobarbituric acid substrate assay was used to measure malondialdehyde (MDA; nmol/g wet tissue) with a spectrophotometer (at 535 and 520 nm)[17]. Ellman’s method was used to measure reduced GSH (nmol/g wet tissue) with a spectrophotometer[18,19]. The xanthine/xanthine oxidase (XO) assay was used to estimate superoxide dismutase (SOD) activity (U/mg protein) by measuring the amount of reduced nitroblue tetrazolium (NBT), with one unit of SOD defined as the amount of protein that inhibits the rate of NBT reduction by 50%[20]. Aebi’s method was used to estimate catalase (CAT) activity (U/g protein) with a spectrophotometer (240 nm) to determine the rate constant k (dimension: s-1, k) for scavenging H2O2 (initial concentration 10 mmol/L)[21]. Finally, the Prajda and Weber method was used to estimate XO activity (U/g protein), which uses spectrophotometric (292 nm) measurement of uric acid formed from xanthine and defines one unit of activity as 1 μmol of uric acid formed per minute[1].
Plasma was separated from blood samples and directly applied to the Architect 16000c Autoanalyzer (Abbott Diagnostics, Abbott Park, IL, United States) to measure the concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin (TBil).
The automated colorimetric measurement methods developed by Erel[22,23] were used to determine the total antioxidant capacity (TAC) and total oxidant status (TOS) (a serum oxidant parameter) in serum samples.
All statistical analyses were carried out by the SPSS statistical software for Windows, version 13.0 (SPSS Inc., Chicago, IL, United States). All data are expressed as median (range of minimum to maximum values). The Shapiro-Wilk test was used to assess the distribution of continuous variables, with P < 0.05 indicating non-normal distribution. Intergroup differences were evaluated by the Kruskal-Wallis test or Mann-Whitney U test, as appropriate, with P < 0.05 indicating significance.
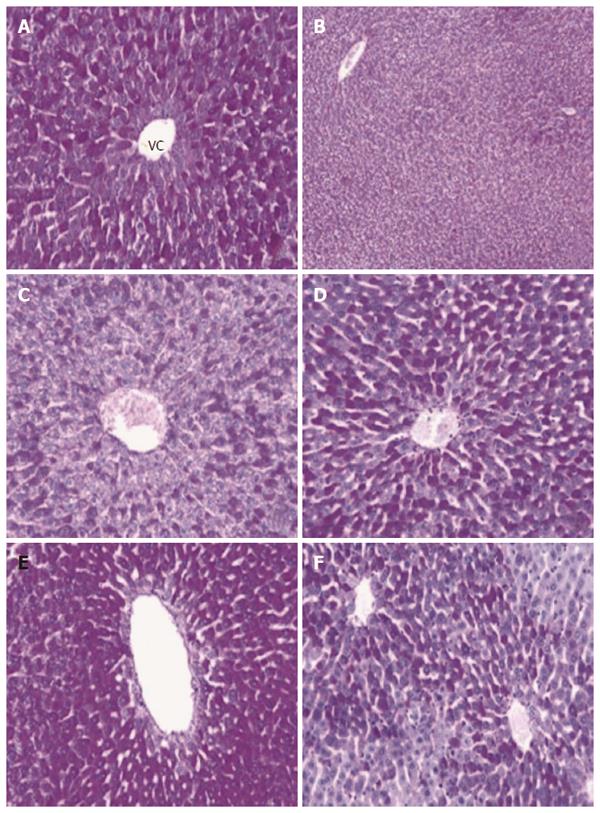
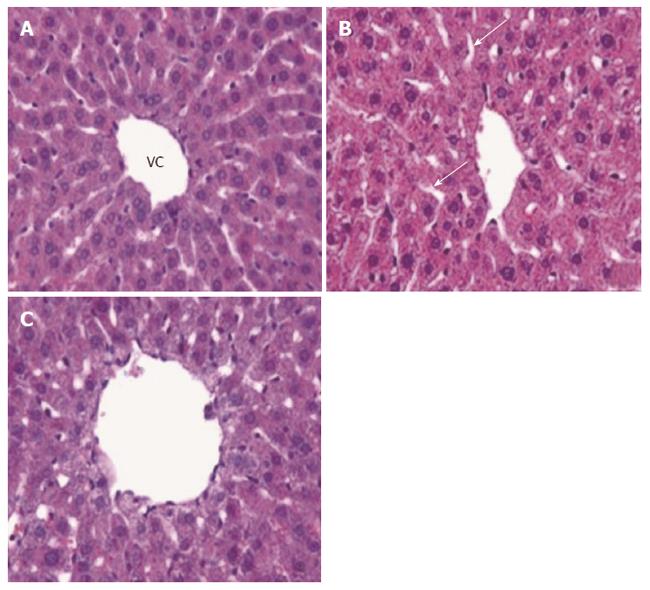
Compared to the normal histological appearance of liver sections from the control group (Figure 1A), liver sections from the model group had significantly worse histopathological scores (P = 0.001). In addition, the model group showed eosinophilic cytoplasm in hepatocytes surrounding the portal area (Figure 1B-D) and significantly reduced glycogen deposition in the hepatocytes (Figure 2A-C). The histological damage scores of all groups are presented in Table 1.
The MTX-induced structural aberrations were alleviated by all three antioxidant treatments, with the improved scores of the MTX + NAC group and the MTX + AMF group reaching statistical significance (Figure 3A-C). In addition, the improvements in histological scores produced by NAC and AMF were both significantly better than that produced by ASC (P < 0.05 and P < 0.005, respectively). The improvements in histological scores produced by NAC and AMF were not significantly different from one another (P > 0.05). The MTX-induced reduction in hepatocyte glycogen deposition was alleviated by all three antioxidant treatments (Figure 2D-F).
As shown in Table 2, the body weight of the model group was significantly decreased during the 7-d MTX exposure, as compared with that of the control group (P < 0.005). However, the changes in body weight (significant reduction from pre-treatment baseline) in the antioxidant treatment groups were not significantly different from that of the untreated model group (P > 0.05).
Changes in liver enzyme activities: As shown in Tables 3 and 4, compared to the control group, the model group showed a significantly higher level of MDA activity and significantly lower levels of GSH, SOD, and XO activities (all, P < 0.05); however, MTX exposure appeared to have no effect on CAT activity (P > 0.05). None of the antioxidant treatments produced a significant change in the MTX-stimulated MDA activity or in the MTX-reduced XO activity (all, P > 0.05). The NAC treatment produced a significant increase in both the MTX-reduced GSH activity (P < 0.05) and SOD activity (P < 0.01); neither the AMF nor ASC treatments produced a significant effect on either GSH or SOD activity (P > 0.05). However, the AMF treatment caused a significant decrease in normal CAT activity (P < 0.05 vs control group).
| Groups | MDA(nmol/g) | GSH(μmol/g) | SOD(U/g) | CAT(K/g) |
| Control | 409 (352-466) | 3.02 (2.85-3.43) | 71.78 (61.88-97.81) | 25.12 (21.08-29.28) |
| Model | 455.5 (419-516)a | 2.52 (2.07-3.34)a | 61.46 (58.27-67.75)a | 23.89 (20.89-30.75) |
| MTX + NAC | 436 (369-476) | 3.22 (2.54-3.62)b | 69.22 (61.13-100.88)c | 22.16 (19.45-22.48) |
| MTX + AMF | 442.5 (323-490) | 2.92 (2.21-3.53) | 63.78 (40.82-124.27) | 20.88 (17.27-21.78)d |
| MTX + ASC | 420.5 (355-463) | 2.84 (2.33-3.48) | 61.81 (46.76-85.71) | 21.51 (20.63-22.78) |
| Groups | TAC(mmol/L) | TOS(mmol/L) | XO(U/g) |
| Control | 1.3 (1.1-1.38) | 13.05 (9.3-27.3) | 2.41 (1.80-14.60) |
| Model | 1.2 (0.9-1.5) | 25.4 (16.1-49.7)a | 1.27 (0.56-17.35)a |
| MTX + NAC | 1.27 (1.15-1.4) | 26.7 (18.5-62.2)b | 1.41 (0.68-2.75)a |
| MTX + AMF | 1.22 (0.3-1.4) | 27.2 (10-61.2) | 1.60 (0.41-2.29)c |
| MTX + ASC | 1.31 (0.95-1.44) | 26.85 (12.2-97)a | 1.10 (0.24-1.48)cd |
Changes in serum levels of liver function markers: As shown in Table 5, compared to the control group, the model group showed significantly higher levels of serum ALT (P = 0.001) and significantly lower levels of AST (P = 0.001). None of the antioxidant treatments affected the MTX-induced ALT levels (all, P > 0.05), but the AMF treatment produced a significant decrease in the MTX-induced serum AST level (P < 0.05). MTX exposure appeared to have no effect on either ALP or TBil levels in serum (P > 0.05 vs control group).
| Groups | ALT(U/L) | AST(U/L) | ALP(U/L) | T. bilirubin(mg/dL) |
| Control | 36 (34-48) | 93.5 (74-135) | 197 (168-271) | 0.1 (0.1-0.2) |
| Model | 119.5 (62-141)a | 49 (33-73)a | 102 (76-437) | 0.1 (0.1-0.2) |
| MTX + NAC | 107.5 (66-161)b | 50 (35-80)b | 84.5 (72-123)a | 0.1 (0.1-0.2) |
| MTX + AMF | 74.5 (28-219) | 31 (11-49)ac | 57.5 (25-138)ae | 0.1 (0.1-0.8) |
| MTX + ASC | 91.5 (56-141)b | 45 (32-58)ad | 87 (64-108)a | 0.15 (0.1-0.2) |
Changes in serum TAC and TOS: As shown in Table 4, neither MTX exposure nor any of the antioxidant treatments appeared to affect TAC (all, P > 0.05). Compared to the control group, the MTX group showed significantly higher TOS (P < 0.05) but none of the antioxidant treatments produced a significant change in the MTX-induced TOS (all, P > 0.05).
While the underlying mechanism of MTX-induced hepatotoxicity remains to be fully elucidated[7,15,16,24], increases in oxidative stress (caused by ROS) have been linked to the effects of MTX; moreover, MTX-induced toxicity has been shown to be associated with increases in lipid peroxidation in various tissues, such as liver, kidney and ileum, using rat model systems[1,5,16,25,26].
In the present study, MTX was shown to significantly alter the oxidant/antioxidant balance in a rat model system. In addition, MTX was shown to increase the level of MDA activity as well as to decrease the levels of GSH and SOD activities in liver, but to have no observable affect on CAT activity. These results are similar to those from previous studies of MTX affects in liver tissue[4,5,7,14,24,27]. In addition, Hadi et al[6] reported that MTX treatment of rats led to increased serum MDA level and decreased serum GSH level.
Previous studies in rats have also shown that prophylactic delivery of multiple antioxidant agents can prevent MTX-induced hepatotoxicity. For example, Tunali-Akbay et al[14] and Dalaklioglu et al[3] demonstrated that resveratrol, a potent antioxidant in rats but with unknown and questionable efficacy in humans, protects against MTX-induced hepatotoxicity by decreasing hepatic MDA tissue level and increasing hepatic GSH and CAT activities. Ali et al[28] demonstrated the protective effect of chrysin against MTX-induced hepatotoxicity and showed that the drug was capable of enhancing the therapeutic index of MTX when the two were administered simultaneously. Demiryilmaz et al[29] demonstrated that administration of thiamine phosphate to rats led to significant reductions in oxidant parameters (i.e., MDA and MPO) and to increases in antioxidant parameters (i.e., GSH and SOD) in the liver tissue. Uraz et al[27] showed that ursodeoxycholic acid provided protection against MTX-induced hepatocyte, again by using the rat model system. Vardi et al[5] reported that therapeutic delivery of β-carotene, an important source of vitamin A in the human diet and having antioxidant properties, led to decreased hepatic MDA activity and increased hepatic SOD, CAT and GSH peroxidase (GPx) activities under conditions of MTX-induced liver injury in rats. Finally, Cetin et al[4] reported that therapeutic delivery of the bioactive resin propolis, another natural compound (produced by the honeybee Apis mellifera, L.) with antioxidant potential, led to the same effects on MDA, SOD, CAT and GPx activities in the MTX-induced liver injury rat model.
The antioxidant functions and mechanisms of NAC in humans are well-established. In addition to its targeted inhibition of H2O2 formation, NAC also protects cells from oxidative stress by directing cysteine into the GSH synthesis pathway and consequently increasing the intracellular content of GSH[8]. With regard to the condition of MTX-induced hepatotoxicity in rats, Cetinkaya et al[7] demonstrated that therapeutic delivery of NAC decreased MDA activity and increased SOD and CAT activities.
The MTX-induced changes in oxidative damage markers detected in the present study by biochemical analysis were in line with histological observations of liver specimens. MTX-induced structural damage to the liver, which includes sinusoidal dilatation, inflammatory cell infiltration, congestion and hydropic degeneration, was obvious in the livers of rats exposed to MTX for 7 d. Furthermore, perturbed glycogen deposition was observed in hepatocytes following the MTX exposure. Similar histopathologic results have been previously reported by a multitude of MTX studies using in vivo models[1,3,5,6,14,24]. In addition, Hadi et al[6] observed focal areas of necrosis in livers of rats exposed to MTX, and Dalaklioglu et al[3] observed increased numbers of activated Kupffer cells in liver tissues of MTX-administered rats.
The current study expanded the knowledge on MTX-induced damage to the hepatic structure by testing the ability of common clinical antioxidant therapeutic agents to alleviate the damage. Indeed, NAC, AMF and ASC were able to improve the MTX-related histopathological damage score, suggesting their potential benefit as adjunct protective agents in patients requiring MTX treatment; however, further studies are necessary to confirm this intriguing theory.
Another important finding of the present study is that MTX exposure led to a distinct increase in serum ALT levels and not in any of the other liver function serum markers. This finding is agreement with previous studies[14,24,27].
In conclusion, the mechanism of MTX-induced hepatic injury likely involves oxidative stress pathways; development or progression of this life-threatening condition may be prevented by prophylactic or therapeutic delivery of antioxidant agents, such as NAC, AMF or ASC.
While the underlying mechanism of methotrexate (MTX)-induced hepatotoxicity remains to be fully elucidated, the effects of this condition are known. Two of the most well studied effects are increases in oxidative stress and in lipid peroxidation involving various tissues, including liver. Previous investigations to characterize these deleterious conditions have relied on rat model systems, which are considered an adequate representation of the human system and may be further exploited to study the underlying molecular mechanisms as well.
Previous experimental studies using animal models and clinical studies of humans have indicated that prophylactic delivery of antioxidant agents can prevent MTX-induced hepatotoxicity. The present study, which relied on the well-established rat model system, detected MTX-induced changes in oxidative damage markers and showed that these biochemical profiles correlated with histologically detected features of liver damage in tissue specimens. These results are in agreement with results from previous studies, both experimental and clinical, and further indicate the important role played by oxidative stress signaling in MTX-induced liver damage.
The primary aim of this experimental study was to investigate the role of oxidative stress in MTX-induced hepatotoxicity, from both structural and functional perspectives, and to evaluate the potential therapeutic effects of cytoprotective antioxidants (e.g., N-acetyl cysteine, amifostine, and ascorbic acid). Specifically, a rat model of acute MTX-induced hepatotoxicity was established and the hepatoprotective mechanisms related to the antioxidant defense system were characterized by measuring changes in oxidative stress factors.
Methotrexate is an effective cytotoxic agent that has been widely applied in clinical practice as a chemotherapy-based treatment for malignancies and inflammatory diseases. N-acetylcysteine is a cytoprotective therapeutic agent with proven efficacy against drug-induced hepatotoxicity, such as that associated with acetaminophen overdose. Ascorbic acid is a naturally-occurring water-soluble antioxidant present in cells, body fluids, and plasma. Amifostine is an organic prodrug that exerts antioxidant and cytoprotective effects, via scavenging for and eliminating the DNA-damaging free oxygen radicals and reactive nucleophiles, upon hydrolysis of its thiophosphate by alkaline phosphatase in a biological system.
The results of the research are very interesting for both basic scientist and clinicians. Presentation and readability of the manuscript are good. The title accurately reflects the contents of the study. Readability of the abstract is good. The design of the study and statistical methods are appropriate. Standard materials and methods were used with a detailed description (the study could be easily reproduce or validate by other investigators). The sample size is big enough for experimental study. The manuscript is appropriate for publishing.
P- Reviewer: De Ponti F, Vujasinovic M S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Kose E, Sapmaz HI, Sarihan E, Vardi N, Turkoz Y, Ekinci N. Beneficial effects of montelukast against methotrexate-induced liver toxicity: a biochemical and histological study. ScientificWorldJournal. 2012;2012:987508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Soliman ME. Evaluation of the possible protective role of folic acid on the liver toxicity ınduced experimentally by methotrexate in adult male albino rats. Egypt J Histol. 2009;32:118-128. |
| 3. | Dalaklioglu S, Genc GE, Aksoy NH, Akcit F, Gumuslu S. Resveratrol ameliorates methotrexate-induced hepatotoxicity in rats via inhibition of lipid peroxidation. Hum Exp Toxicol. 2013;32:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Cetin A, Kaynar L, Eser B, Karada C, Saraymen B, Öztürk A. Beneficial effects of propolis on methotrexate-induced liver ınjury in rats. Acta Oncologica Turcica. 2011;44:18-23. |
| 5. | Vardi N, Parlakpinar H, Cetin A, Erdogan A, Cetin Ozturk I. Protective effect of beta-carotene on methotrexate-induced oxidative liver damage. Toxicol Pathol. 2010;38:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Hadi NR, Al-Amran FG, Swadi A. Metformin ameliorates methotrexate-induced hepatotoxicity. J Pharmacol Pharmacother. 2012;3:248-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken B. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006;12:BR274-BR278. [PubMed] |
| 8. | Kahraman H, Kurutas E, Tokur M, Bozkurt S, Cıralık H, Kabakcı B. Protective effectsof erythropoietin and n-acetylcysteine on methotrexate-induced lung injury in rats. Balkan Med J. 2013;30:99-104. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Stankiewicz A, Skrzydlewska E. Amifostine antioxidant effect on serum of rats treated with cyclophosphodamide. Polish J Environ Studies. 2005;14:341-346. |
| 10. | Maiguma T, Kaji H, Makino K, Teshima D. Protective effects of amifostine and cyclooxygenase-1 inhibitor against normal human epidermal keratinocyte toxicity induced by methotrexate and 5-fluorouracil. Basic Clin Pharmacol Toxicol. 2009;105:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Ray S, Roy K, Sengupta C. Exploring the protective effect of ascorbic acid and aqueous extract of spirulina platensis on methotrexate-induced lipid peroxidation. Iran J Pharm Sci. 2007;3:217-228. |
| 12. | Djuraševıć SF, Djordjevıć J, Drenca T, Jasnıć N, Cvıjıć G. Influence of vitamin c supplementation on the oxidative status of rat liver. Arch Biol Sci. 2008;60:169-173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Kojo S. Vitamin C: basic metabolism and its function as an index of oxidative stress. Curr Med Chem. 2004;11:1041-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Tunali-Akbay T, Sehirli O, Ercan F, Sener G. Resveratrol protects against methotrexate-induced hepatic injury in rats. J Pharm Pharm Sci. 2010;13:303-310. [PubMed] |
| 15. | Babiak RM, Campello AP, Carnieri EG, Oliveira MB. Methotrexate: pentose cycle and oxidative stress. Cell Biochem Funct. 1998;16:283-293. [PubMed] |
| 16. | Miyazono Y, Gao F, Horie T. Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand J Gastroenterol. 2004;39:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3306] [Cited by in RCA: 3468] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 18. | Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18594] [Cited by in RCA: 19226] [Article Influence: 769.0] [Reference Citation Analysis (0)] |
| 19. | Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158-169. [PubMed] |
| 20. | Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497-500. [PubMed] |
| 21. | Casado A, de La Torre R, Lopéz-Fernández E. Antioxidant enzyme levels in red blood cells from cataract patients. Gerontology. 2001;47:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 990] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 23. | Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1722] [Cited by in RCA: 2140] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 24. | Hemeida RA, Mohafez OM. Curcumin attenuates methotraxate-induced hepatic oxidative damage in rats. J Egypt Natl Canc Inst. 2008;20:141-148. [PubMed] |
| 25. | Jahovic N, Sener G, Cevik H, Ersoy Y, Arbak S, Yeğen BC. Amelioration of methotrexate-induced enteritis by melatonin in rats. Cell Biochem Funct. 2004;22:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Devrim E, Cetin R, Kiliçoğlu B, Ergüder BI, Avci A, Durak I. Methotrexate causes oxidative stress in rat kidney tissues. Ren Fail. 2005;27:771-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Uraz S, Tahan V, Aygun C, Eren F, Unluguzel G, Yuksel M, Senturk O, Avsar E, Haklar G, Celikel C. Role of ursodeoxycholic acid in prevention of methotrexate-induced liver toxicity. Dig Dis Sci. 2008;53:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Ali N, Rashid S, Nafees S, Hasan SK, Sultana S. Beneficial effects of Chrysin against Methotrexate-induced hepatotoxicity via attenuation of oxidative stress and apoptosis. Mol Cell Biochem. 2014;385:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Demiryilmaz I, Sener E, Cetin N, Altuner D, Suleyman B, Albayrak F, Akcay F, Suleyman H. Biochemically and histopathologically comparative review of thiamine’s and thiamine pyrophosphate’s oxidative stress effects generated with methotrexate in rat liver. Med Sci Monit. 2012;18:BR475-BR481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |