Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12007
Revised: May 6, 2014
Accepted: June 12, 2014
Published online: September 14, 2014
Gastric cancer is the fourth most common cancer in the world and the second leading cause of cancer-related death. More than 80% of diagnoses occur at the middle to late stage of the disease, highlighting an urgent need for novel biomarkers detectable at earlier stages. Recently, aberrantly expressed microRNAs (miRNAs) have received a great deal of attention as potential sensitive and accurate biomarkers for cancer diagnosis and prognosis. This review summarizes the current knowledge about potential miRNA biomarkers for gastric cancer that have been reported in the publicly available literature between 2008 and 2013. Available evidence indicates that aberrantly expressed miRNAs in gastric cancer correlate with tumorigenesis, tumor proliferation, distant metastasis and invasion. Furthermore, tissue and cancer types can be classified using miRNA expression profiles and next-generation sequencing. As miRNAs in plasma/serum are well protected from RNases, they remain stable under harsh conditions. Thus, potential functions of these circulating miRNAs can be deduced and may implicate their diagnostic value in cancer detection. Circulating miRNAs, as well as tissue miRNAs, may allow for the detection of gastric cancer at an early stage, prediction of prognosis, and monitoring of recurrence and/or lymph node metastasis. Taken together, the data suggest that the participation of miRNAs in biomarker development will enhance the sensitivity and specificity of diagnostic and prognostic tests for gastric cancer.
Core tip: Gastric cancer is the second leading cause of cancer-related death, and > 80% of cases are diagnosed at the middle to late disease stage. Novel biomarkers for the detection of early stage gastric cancer are therefore urgently needed. Recent recognition of a correlation between aberrantly expressed microRNAs (miRNAs) and cancer-related processes has highlighted the potential of miRNAs as diagnostic and prognostic markers. Detection of miRNAs, in tissue as well as in serum/plasma, may enhance the sensitivity and specificity of diagnostic and prognostic tests for early stage gastric cancer, and provide a means to monitor recurrence and/or lymph node metastasis.
- Citation: Liu HS, Xiao HS. MicroRNAs as potential biomarkers for gastric cancer. World J Gastroenterol 2014; 20(34): 12007-12017
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12007.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12007
Although the rate of gastric cancer (GC) has declined over the last 50 years, GC remains the fourth most common cancer worldwide, with a total of 989600 new cases and 738000 deaths estimated in 2008[1]. Over 70% of these new cases and deaths occurred in developing countries, with half of the world total occurring in Eastern Asia (mainly in China)[2]. Due to the absence of specific symptoms and early detection, gastric cancer is often diagnosed at an advanced stage when a cure is not possible, and in these cases, the prognosis remains unfavorable. Median survival is 7-9 mo and survival at 2 years is exceptionally > 10%. A combination of early diagnosis and the improvement in surgical techniques has extended survival in GC patients[3-5]. As the prognosis for these patients differs depending on the disease stage at the time of diagnosis, early detection of GC is crucial. While the combined use of tumor markers, such as α-fetoprotein, carcinoembryonic antigen and carbohydrate antigens 125 and 19-9, improves the sensitivity for the diagnosis of advanced gastric cancer, these markers yield inconsistent results when used for early detection of GC[6-10]. Therefore, identification of novel biomarkers for early GC diagnosis is a major focus of current investigation.
A class of small (about 22 nucleotides) noncoding RNA species, known as microRNAs (miRNAs), have been shown to regulate gene expression and play important roles in a wide range of physiological and pathological processes[11-18]. For example, the miR-15a-miR-16-1 cluster has been demonstrated to promote prostate cancer by targeting genes related to a multitude of oncogenic activities[19], and p53-mediated transactivation of miR-34a has been shown to influence the expression of genes related to apoptosis[20]; furthermore, miR-373, miR-520c and miR-10b have been shown to promote tumor invasion and metastasis[21-23]. Although the biological function of miRNA has yet to be fully understood, miRNA analyses indicate that a wide range of tumor types display significantly different expression profiles compared to normal tissues. Thus, miRNAs are now being utilized in development of various diagnostic tests as highly tissue specific biomarkers, with the potential for clinical determination of metastasis origin[24-26]. MiRNAs remain stable after incubation at room temperature for up to 24 h or after up to eight cycles of freeze-thawing. MiRNAs protected by various lipids and proteins in exosomes and other microparticles have been detected in plasma, serum, urine, saliva, and milk[27-30]. Their stability and easily testable length (about 22 bp) make miRNAs well suited for being utilized as biomarkers. Furthermore, circulating miRNAs meet the basic conditions for utility as a biomarker as tumor-derived miRNAs can be detected in plasma, with no significant difference in circulating levels in healthy male and female subjects[31-36]. Current knowledge concerning the diagnostic and prognostic applications of miRNAs in GC is reviewed and summarized below.
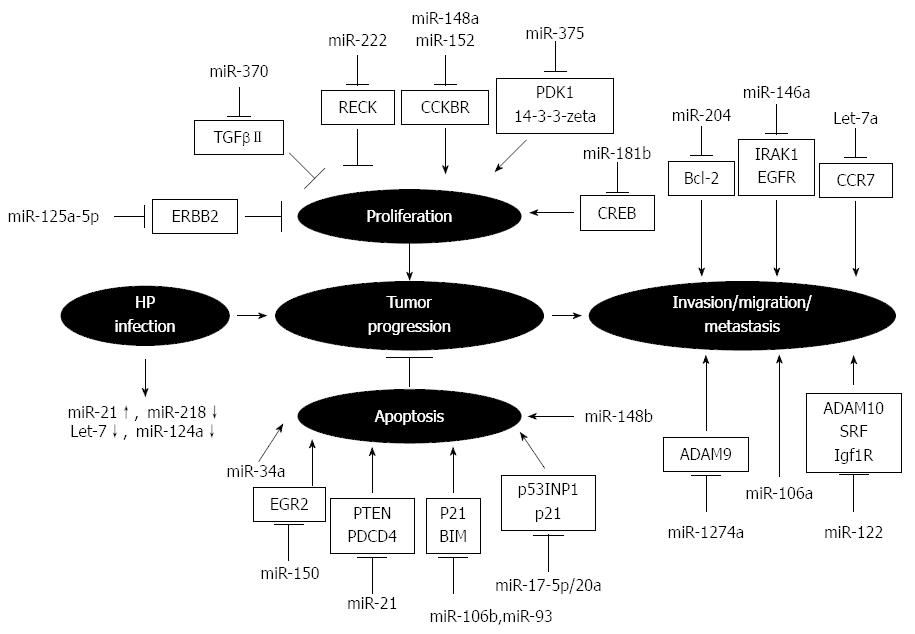
An increasing number of studies have shown dysregulation of miRNAs in Helicobacter pylori-infected gastric mucosa and GC, which correlates with cancer development and tumor progression[37-41] (Figure 1). For example, upregulation of miR-17-5p/20a promotes GC cell cycle progression and inhibits cell apoptosis via post-transcriptional modulation of tumor suppressor protein TP53INP1 and the cyclin-dependent kinase inhibitor p21[42]. MiR-106b and miR-93 impair TGFβ-induced apoptosis in GC cells through inhibition of BCL2L11[43]. In addition, miR-150 has been shown to promote GC growth by targeting EGR2[44], a tumor suppressive transcription factor that induces apoptosis by direct transactivation of BNIP3L and Bak[45]. Other miRNAs, such as miR-204, are downregulated in gastric tumors, and their ectopic expression inhibits the colony formation, migration and tumor engraftment of GC cells by targeting Bcl-2 mRNA[46]. Additionally, miR-375 is one of the most downregulated miRNAs in GC and its overexpression substantially reduces cell viability by targeting PDK1 and 14-3-3zeta[47]. Furthermore, ectopic expression of miR-146a, whose levels are significantly lower in cancerous tissues, inhibits migration and invasion of GC cells and downregulates the expression of the epidermal growth factor receptor and interleukin receptor-associated kinase IRAK1[48].
MiRNAs have also been shown to correlate with tumor proliferation and pathology. Low expression levels of miR-125a-5p are associated with an enhanced malignant potential, such as tumor size and depth, and a poor clinical prognosis. In vitro assays have shown that the proto-oncogene ERBB2 is a direct target of miR-125a-5p, which potently suppresses the proliferation of GC cells[49]. Song et al[50] showed that miR-148b is significantly downregulated in GC tissues and cell lines compared with their non-tumor counterparts, and is associated with tumor size. Reduced expression of miR-148a, as well as miR-152, was also shown in human GC by Chen et al[51], which correlated with an increased tumor size and advanced tumor stage.
Invasiveness and metastasis are essential aspects of cancer cells that have also been associated with miRNA gene targeting. Recent studies have demonstrated that the expression of the novel tumor suppressor protein programmed cell death 4 (PDCD4) is suppressed by miR-21 and downregulated in GC. Tumor size and depth, lymph node metastasis, venous invasion, advanced stage, and poor clinical prognosis are all significantly correlated with reduced PDCD4 and elevated miR-21 expression[52], as well as higher expression of miR-106a[53]. In contrast, the levels of let-7a miRNA are significantly lower in the serum and tumor tissues of gastric adenocarcinoma patients compared to the peritumoral tissues and serum from healthy controls. In vitro transfection of MNK-45 cells with let-7a significantly inhibits the protein expression of the chemokine receptor CCR7, and impedes their migratory and invasive capabilities[54-56].
Furthermore, in vivo studies have also shown the significant impactions of miRNAs in gastric carcinogenesis[57-102]. For example, tumor suppressor gene miR-338 can decrease migratory, invasive, proliferative behaviors as well as EMT by targeting NRP1 in GC[103]. By using gain or loss-of-function in in vitro and in vivo experiments, overexpressed miR-19a/b in GC tissues have been observed to have a pro-metastatic function by attenuating the expression of MXD1[104]. The in vivo roles of miR-133b and miR-202-3p have been shown to inhibit GC metastasis by directly targeting Gli1 and suppressing Gli1 target genes, OPN and Zeb2[105,106]. In addition, miR-1207-5p and miR-1266 are significantly decreased in GC tissues, and their ectopic expression inhibits gastric tumor growth in vitro and in vivo by suppressing hTERT[107]. Other miRNAs, such as miR-26a and miR-212, can inhibit proliferation of GC by directly repressing the expression of their targets FGF9 and RBP2[108,109].
Due to the absence of specific symptoms and early detection[55], GC is often diagnosed at an advanced stage with a median survival of about 7-9 mo[56]. Thus, a longstanding goal of GC research has been to identify methods for the early diagnosis and management of cancer. Over the past five years, scientists have begun to explore the feasibility of utilizing miRNAs as biomarkers, as many are involved in GC tumorigenesis, proliferation, invasion and metastasis (Table 1, Table 2, Table 3 and Table 4).
| Ref. | Sample | Method (normalization) | MiRNA | Clinical application |
| Jiang et al[60] (CN) | 60 GC (46A, 6M, 8S)/18 C | qRT-PCR (U6) | miR-421 | Diagnostic biomarker |
| Zhang et al[59] (CN) | 63 GC/10 C | real-time qRT-PCR (RNU6B) | miR-31 | Diagnostic biomarker |
| Xiao et al[53] (CN) | 55 GC/17 C | real-time qRT-PCR (U6) | miR-106a | Diagnostic biomarker |
| Chan et al[58] (CN) | 37 GC/37 C | qPCR (U6) | miR-21 | Diagnostic marker |
| Tsai et al[67] (CN) | 72 GC/72 C | qRT-PCR | miR-196a | Detecting GC and monitoring recurrence |
| Xiao et al[98] (CN) | 20 GC/20 C | qRT-PCR (U6) | miR-146a | Diagnostic biomarker and therapeutic target |
| Song et al[50] (CN) | 4 GC cell lines | real-time qRT-PCR (U6) | miR-148b | Diagnostic biomarker |
| 106 GC/106 C tissues | ||||
| Chen et al[51] (CN) | 101 GC(34I, 67B)/101CC/101 C | real-time qRT-PCR (U6) | miR-148a and miR-152 | Detection of gastrointestinal cancer |
| Su et al[101] (CN) | 20 GC/20 C | miRNA microarray and qRT-PCR (U6) | miR-574-3p | Early detection of GC |
| Ref. | Sample | Method (normalization) | MiRNA | Clinical application |
| Wang et al[42] (CN) | 110 GC/110 C | qRT-PCR (U6) | miR-17-5p/20a | Therapeutic marker |
| Yan et al[66] (CN) | 31 with/43 without recurrent GC | miRNA microarray and qPCR (U6) | miR-335 | Recognition of recurrence risk |
| Liu et al[62] (CN) | 92 GC/92 C | real-time qRT-PCR (U6) | miR-221 | Prognostic factor for overall survival |
| Inoue et al[96] (JP) | 161 GC/161 C | miRNA qPCR array, Taqman miRNA assay and real-time qRT-PCR (U6) | miR-107 | Prediction of prognosis |
| Brenner et al[97] (IL) | 45 GC (39A, 6S) | miRNA microarray and qRT-PCR (six miRs) | miR-451, miR-199a-3p and miR-195 | Prediction of recurrence |
| Zhang et al[68] (CN) | 29 with/36 without recurrent GC (A) | miRNA microarray and qPCR (U6) | miR-375 and miR-142-5p | Prediction of recurrence risk for GC |
| Kim et al[99] (KR) | 15 GC cell lines | miRNA methylome profiling, bisulfite pyrosequencing and qRT-PCR (U6) | miR-10b | Assessing the risk of GC |
| 100 GC(44I, 56D)/100 C tissues | ||||
| Nishida et al[49] (JP) | 87 GC | qRT-PCR (RNU6B) | miR-125a-5p | Prognostic marker |
| Li et al[65] (CN) | 100 GC | real-time RT-PCR | miR-10b, miR-21, miR-223, miR-338, let-7a, miR-30a-5p, miR-126 | Survival prediction |
| Xu et al[64] (CN) | 86 GC | RT-PCR (Let-7a) | miR-21 | Prediction of LN metastasis |
| Wu et al[63] (CN) | 52 GC/52 C | real-time RT-PCR (U6, Let7a, miR-191, 103) | miR-212 and miR-195 | Prediction of LN metastasis |
| Liu et al[102] (CN) | 84 GC/84 C | qRT-PCR (U6) | let-7i | Prediction of chemotherapeutic sensitivity and prognosis |
| Chen et al[61] (CN) | 3 GC/1 gastric mucosal lines | miRNA array and qPCR (U6) | miR-10a | Prediction of LN metastasis |
| 38 GC (28A, 8T, 2S)/10 C tissues | ||||
| Valladares-Ayerbes et al[100] (ES) | 28 CC/7 GC/3 pancreas | qRT-PCR (5S and U6) | miR-17-92 | Prognostic markers |
| Kogo et al[48] (JP) | 90 GC/90 C | qRT-PCR (RNU6B) | miR-146a | Prognostic factor |
| Sacconi et al[46] (IT) | 123 GC (75I, 24D, 24U)/123 C | miRNA microarray and qPCR (RNU6B) | miR-204 | Prognostic value |
| Ref. | Sample | Method (normalization) | MiRNA | Clinical application |
| Li et al[74] (CN) | 80 EGC/70 C plasma | real-time qRT-PCR(U6, U44) | miR-199-3p | Early detection of GC |
| Rotkrua et al[72] (JP) | 6 DCKO mice with DGC/6 C tissue | miRNA microarray and | miR-103, miR-107, | Early detection of DGC |
| 21 DCKO mice with DGC/23 C serum | TaqMan qRT-PCR (cel-miR-39) | miR-194 and miR-210 | ||
| Li et al[75] (CN) | 230 GC/130 C and | miRNA microarray and | miR-199-3p | GC detection |
| 20 gastric precancerous plasma | real-time qRT-PCR (U6, U44) | |||
| Cai et al[76] (CN) | 90 GC(70A, 16M, 3S, 1SC)/90 C plasma | qRT-PCR (cel-miR-39) | miR-106b, miR-20a, | Early detection of GC |
| and miR-221 | ||||
| Li et al[91] (CN) | 8 GC/8 C tissue | qRT-PCR (U6, cel-miR-39) | miR-223, miR-21 | GC detection |
| 70 GC(56A, 13M, 1S)/70 C plasma | and miR-218 | |||
| Wang et al[92] (CN) | 174 solid cancers/39 C serum | qRT-PCR (miR-16) | miR-21 | Detection of some solid cancers |
| Song et al[93] (CN) | 82 GC(71A, 3S, 1SC)/46 DYS/128 C serum | TaqMan low-density array | miR-221, miR-376c | Early detection of GC |
| and TaqMan qRT-PCR (cel-miR-39) | and miR-744 | |||
| Konishi et al[94] (JP) | 56 GC/30 C plasma | miRNA microarray | miR-451 and miR-486 | Screening GC |
| and qRT-PCR (U6) | ||||
| Liu et al[77] (CN) | 61 GC(A, M)/61 C serum | miRNA microarray | miR-378 | Early detection of GC |
| and real-time qRT-PCR (U6) | ||||
| Liu et al[82] (CN) | 164 GC/127 C serum | Solexa sequencing | miR-1, miR-20a, miR-27a, | GC detection |
| and qRT-PCR (the serum volume) | miR-34 and miR-423-5p | |||
| Tsujiura et al[73] (JP) | 8 GC/8 C tissue | qRT-PCR (RNU6B) | miR-17-5p, miR-21, miR-106a, | GC detection |
| 79 GC/30 C plasma | miR-106b and let-7a | |||
| Gorur et al[95] (TR) | 20 GC/190 C plasma | real-time qPCR (global means) | miR-195-5p | Early detection of GC |
| Wang et al[54] (CN) | 80 gastric adenocarcinomas/40 peritumoral tissues | real-time qRT-PCR (U6) | let-7a | GC detection |
| 80 gastric adenocarcinomas/45 C serum |
| Ref. | Sample | Method (normalization) | MiRNA | Clinical application |
| Valladares-Ayerbes et al[81] (ES) | 52 GC/31 C peripheral blood and 2 GC cell lines | qRT-PCR (U6, 5S) | miR-200c | Predictor of progression and survival |
| Komatsu et al[78] (JP) | 69 GC plasma | qRT-PCR (U6) | miR-21 | Prognostic marker |
| Wang et al[79] (CN) | 79 pre-operative GC/30 post-/6 relapse plasma | qRT-PCR (RNU6B) | miR-17-5p/20a | Prediction of prognosis and monitoring of chemotherapeutic effects |
| Kim et al[80] (KR) | 16 LN-positive GC/15 LN-negative GC/10 C serum | qRT-PCR | miR-21, miR-146a, and miR-148a | Predicting LN metastasis |
MiRNA expression profiles and next-generation sequencing have revealed that miRNA aberrant expression could be used for tissue specificity and to classify cancer types, highlighting the potential of miRNAs for cancer diagnosis. For example, miR-375 is significantly downregulated in distal gastric adenocarcinoma tissues as well as in the circulating serum. At a normalized cutoff of 0.218, miR-375 yields a receiver operating characteristic (ROC) area under the curve (AUC) of 0.835 with a specificity of 80% and a sensitivity of 85%, in the discrimination of distal gastric adenocarcinoma from control tissues[57]. The levels of miR-106a and miR-21 are significantly higher in GC tissues[53,58], while the level of miR-31 is significantly lower[59]. MiR-421 is overexpressed in GC tissues, and while it is not associated with clinicopathological features, it may be involved in the early stage of gastric carcinogenesis[60]. As the positive detection rate of miR-421 is higher than that of serum carcinoembryonic antigen, it thus may serve as an efficient early diagnostic biomarker. These new data lend further support to the notion that miRNAs may represent efficient diagnostic biomarkers.
The connection between miRNA expression and GC progression and metastasis suggests that miRNAs can be used as prognosis monitoring tools. The expression levels of miR-10a, miR-221, miR-212 and miR-195 are associated with lymph node metastasis[61-63], and in addition, miR-21 expression is also significantly correlated with histologic type, tumor stage, and pathologic tumor-node-metastasis (pTNM) stage[64]. Meanwhile, a seven-miRNA signature (miR-10b, miR-21, miR-223, miR-338, let-7a, miR-30a-5p, miR-126) has been identified for overall survival and relapse-free survival in GC patients[65]. A high frequency of recurrence and poor survival are observed in GC cases with high levels of miR-335, miR-196a or miR-375, while low expression levels of miR-146a, miR-142-5p or miR-204 are correlated with increased tumor size, pTNM stage and worse overall survival[66-68,46,48]. Furthermore, low expression of miR-125a-5p is associated with a poor prognosis and enhanced malignancy potential, measured by tumor size, tumor invasion and liver metastasis.
Several studies to date have investigated the molecular mechanisms underlying miRNA targeting as an anticancer therapy. Overexpression of miR-34a has been shown to induce apoptosis and accumulation of cells in the G1 phase, ultimately inhibiting tumorsphere formation[69]. Both miR-15b and miR-16 were shown to promote chemotherapy (mitotic inhibitor: vincristine)-induced apoptosis in a human GC cell line (SGC7901/VCR), suggesting the potential of these miRNAs to modulate the sensitivity of GC cells to certain anticancer drugs[70]. The potential anticancer property of miR-508-5p has been shown to involve its targeting of the 3’-untranslated regions of ABCB1 and zinc ribbon domain-containing 1 (ZNRD1), which sensitizes cancer cells to an array of known chemotherapeutic agents[71]. Furthermore, increased expression of miR-150 and miR-146a, and reduced expression of miR-142-3p and miR-199b-5p have been observed in blood samples from chronic myeloid leukemia patients after two weeks of imatinib therapy, suggesting that miRNAs may serve as a novel clinically useful biomarker in this disease[110]. In this regard, miR-451 has also been considered as a potential predictor marker of imatinib therapy[111]. In addition, miR-1274a is shown to be involved in sorafenib therapy of hepatocellular carcinomas (HCC) by targeting ADAM9[112]. Other miRNAs, such as miR-122, can directly inhibit angiogenesis in vitro with concomitant suppression of its target genes, namely ADAM10, SRF, and Igf1R[113]. Ectopic expression of miR-122 potentiates growth inhibitory function of sorafenib in HCC cells. Additionally, Kaplan-Meier survival analysis reveals that low expression levels of miR-21 and miR-181b are closely associated with better GC patient’s overall survival for both S-1 and doxifluridine based therapies[114]. Collectively, these data suggest the potential application of miRNAs not only as targets of anticancer therapeutic approaches but also as predictive markers of drug resistance and treatment response.
The search for non-invasive tools for the diagnosis and management of cancer has led to the investigation of circulating nucleic acids, including miRNAs, in plasma and serum. Finding miRNAs in plasma/serum has suggested the potential of miRNA signatures in cancer diagnosis. Endogenous circulating miRNAs, which are protected from RNases and remain stable in harsh conditions, exhibit specific tissue and cancer type expression patterns[31,32], demonstrating their diagnostic potential. Circulating levels of miR-103, miR-107, miR-194 and miR-210 were upregulated in sera from double conditional knockout mice with early or advanced-stage diffuse-type GC[72]. Tsujiura et al[73] have shown that plasma miR-17-5p, miR-21, miR-106a, and miR-106b are significantly higher, whereas let-7a is lower, in GC patients, with AUCs of 0.721 and 0.879 for the miR-106b and miR-106a/let-7a ratio assays, respectively. Furthermore, the expression of miRNA-199a-3p, miR-106b, miR-20a, and miR-221 are significantly elevated in plasma of GC patients with AUCs of 0.818, 0.7733, 0.8593, and 0.7960, respectively[74-76]. However, there were no significant differences in the plasma levels of these miRNAs among the four TNM stages. Our group has found that serum miR-378 is significantly elevated in early GC patients, in an assay yielding an ROC curve area of 0.861 with a 87.5% sensitivity and a 70.73% specificity[77]. These findings indicate that elevated circulating miRNAs can be detected in early stages of tumor growth, suggesting their potential as noninvasive biomarkers for early GC detection.
There is also evidence to suggest that circulating miRNA levels are associated with the progression and prognosis of GC. MiR-21, miR-17-5p/20a, miR-146a, and miR-148a have been reported as non-invasive biomarker candidates to predict prognosis, monitor chemotherapeutic effects and predict the presence of lymph node metastasis[48,50,78-80]. In addition, Valladares-Ayerbes et al[81] have found that expression levels of miR-200c correlate with the number of lymph node metastases, and are significantly associated with poor overall and disease-free survival rates, suggesting that miR-200c has the potential to be a predictor of cancer progression and survival. Moreover, Liu et al[82] used Solexa sequencing and qRT-PCR to identify a profile of five serum miRNAs (miR-1, miR-20a, miR-27a, miR-34 and miR-423-5p) correlating with tumor stage that can serve as biomarkers for detecting GC. In their study, the AUCs for this five-serum miRNA signature were 0.879 and 0.831 in two sets of serum samples, which are markedly higher than those of the currently used biomarkers carcinoembryonic antigen (0.503) and carbohydrate antigen 19-9 (0.600). Furthermore, their data demonstrated that higher sensitivity and specificity of monitoring prognosis can be achieved by circulating miRNAs as compared to the other commonly used non-miRNA testing methods.
Recently, the detection of circulating tumor cells in peripheral blood has received a great deal of attention for the prediction of postoperative cancer recurrence and the evaluation of novel adjuvant therapies. A significant correlation has been identified between the number of circulating cancer cells and the levels of miR-106a, miR-17, miR-421 and miR-21[83-85]. In this regard, miRNAs are being evaluated as a new molecular diagnostic marker for the detection of these cells. These data highlight a novel potential use for miRNAs in monitoring circulating tumor cells. In addition to serum, the levels of miRNAs in gastric juice are under investigation. Levels of miR-21 are higher in specimens of intestinal type GC compared to diffuse or mixed GC type, whereas the levels of miR-129-1-3p and miR-129-2-3p in gastric juice are significantly lower, with AUCs of up to 0.969 for miR-21 and 0.656 for a combination test of miR-129-1-3p and miR-129-2-3p[86,87]. Furthermore, the addition of gastric juice miR-421 for the detection of early GC shows a remarkable improvement over serum carcinoembryonic antigen alone[88]. These results indicate that gastric juice miRNAs provide additional novel non-invasive biomarkers for screening GC.
Although miRNAs show promise as detection and prognosis biomarkers, there are methodological and technical limitations regarding the analyses. The variety of methodologies, types of carcinomas included, analysis software and normalization strategies used in the various studies in the published literature have led to a considerable amount of variability and inconsistency among the findings reported. Therefore, detection methods should be standardized and include normalization controls, such as the housekeeping miRNAs, miR-16[89] and RUN6B[77]. MiR-93 is also recommended as a suitable reference gene for serum miRNA analysis between GC patients and healthy controls[89]. Other protocols call for samples to be processed from identical input volumes, then corrected for technical variability using spiked-in synthetic non-human (Caenorhabditis elegans) miRNA as a normalizing control[31,90]. The use of “invariant” miRNAs as endogenous controls has been proposed by some investigators, however, biological variability may preclude this approach. As no consensus concerning the ideal normalization control has been reached, additional studies are needed for sufficient sensitivity and precision in the quantification of miRNAs.
Though thousands of miRNAs have been demonstrated to be related to GC, the variability among different patients, even with the same type of cancer, makes it impossible to use just one marker as a reliable method for determining cancer status. For this reason only the combination of several miRNAs could be effective for diagnostic purpose. For example, low expression of miR-106b and high expression of miR-181b are observed in patients with liver cirrhosis. The AUC for miR-106b and -181b are 0.715 and 0.833, respectively. The ROC curve of the combined miRNAs has an AUC of 0.882. These data demonstrate that the combined detection of miR-106b and miR-181b has more considerable clinical value to diagnose patients with liver cirrhosis[115]. The combination of four serum miRNAs (miR-22, miR-572, miR-638 and miR-1234) signature and TNM stage had better prognostic value in personalized therapy for nasopharyngeal carcinoma than the TNM stage or miRNA signature alone[116]. In addition, high expressed miR-223, miR-21 and low expressed miR-218 in GC patients yield the AUC values of 0.9089, 0.7944, and 0.7432, respectively. While the combined ROC analysis reveals the highest AUC value of 0.9531 in discriminating GC patients from healthy controls[92]. Therefore, a cluster of biomarkers for one disease would be a better diagnostic tool with much higher sensitivity, specificity, and accuracy.
In conclusion, GC-specific miRNAs have been associated with tumorigenesis, tumor proliferation and metastasis. While further investigation with large-scale validation is needed before miRNAs can serve as a non-invasive screening tool in routine clinical trials, their inclusion in biomarker development will enhance the sensitivity and specificity of diagnostic and prognostic tests for GC.
P- Reviewer: Ierardi E, Link A, Nagahara A, Sugimura H, Wang YH S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 3. | Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v50-v54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1140] [Cited by in F6Publishing: 1280] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 5. | Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: current status of diagnosis and treatment. Cancers (Basel). 2013;5:48-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | He CZ, Zhang KH, Li Q, Liu XH, Hong Y, Lv NH. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol. 2013;13:87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Sisik A, Kaya M, Bas G, Basak F, Alimoglu O. CEA and CA 19-9 are still valuable markers for the prognosis of colorectal and gastric cancer patients. Asian Pac J Cancer Prev. 2013;14:4289-4294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Cervantes A, Roselló S, Roda D, Rodríguez-Braun E. The treatment of advanced gastric cancer: current strategies and future perspectives. Ann Oncol. 2008;19 Suppl 5:v103-v107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Nakane Y, Okamura S, Akehira K, Boku T, Okusa T, Tanaka K, Hioki K. Correlation of preoperative carcinoembryonic antigen levels and prognosis of gastric cancer patients. Cancer. 1994;73:2703-2708. [PubMed] [Cited in This Article: ] |
| 10. | Marrelli D, Pinto E, De Stefano A, Farnetani M, Garosi L, Roviello F. Clinical utility of CEA, CA 19-9, and CA 72-4 in the follow-up of patients with resectable gastric cancer. Am J Surg. 2001;181:16-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 3280] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 12. | Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1568] [Cited by in F6Publishing: 1543] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 13. | Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301:336-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1353] [Cited by in F6Publishing: 1241] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 14. | He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2729] [Cited by in F6Publishing: 2776] [Article Influence: 146.1] [Reference Citation Analysis (0)] |
| 15. | Pasquinelli AE, Ruvkun G. Control of developmental timing by micrornas and their targets. Annu Rev Cell Dev Biol. 2002;18:495-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 264] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5705] [Cited by in F6Publishing: 5849] [Article Influence: 324.9] [Reference Citation Analysis (0)] |
| 17. | Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA, Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E. The colorectal microRNAome. Proc Natl Acad Sci USA. 2006;103:3687-3692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 710] [Cited by in F6Publishing: 725] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 18. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25833] [Cited by in F6Publishing: 26776] [Article Influence: 1338.8] [Reference Citation Analysis (0)] |
| 19. | Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, D’Urso L, Pagliuca A, Biffoni M, Labbaye C. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271-1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 725] [Cited by in F6Publishing: 735] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 20. | Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1481] [Cited by in F6Publishing: 1531] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 21. | Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 740] [Cited by in F6Publishing: 733] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 22. | Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1936] [Cited by in F6Publishing: 1950] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 23. | Tavazoie SF, Alarcón C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massagué J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1441] [Cited by in F6Publishing: 1456] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 24. | Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7124] [Cited by in F6Publishing: 7177] [Article Influence: 377.7] [Reference Citation Analysis (0)] |
| 25. | Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257-2261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4162] [Cited by in F6Publishing: 4422] [Article Influence: 245.7] [Reference Citation Analysis (0)] |
| 26. | Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S, Levy A. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26:462-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 728] [Cited by in F6Publishing: 698] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 27. | Sun Y, Zhang K, Fan G, Li J. Identification of circulating microRNAs as biomarkers in cancers: what have we got. Clin Chem Lab Med. 2012;50:2121-2126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Zandberga E, Kozirovskis V, Ābols A, Andrējeva D, Purkalne G, Linē A. Cell-free microRNAs as diagnostic, prognostic, and predictive biomarkers for lung cancer. Genes Chromosomes Cancer. 2013;52:356-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1730] [Cited by in F6Publishing: 1817] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 30. | Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003-5008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2345] [Cited by in F6Publishing: 2488] [Article Influence: 191.4] [Reference Citation Analysis (0)] |
| 31. | Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513-10518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5636] [Cited by in F6Publishing: 6046] [Article Influence: 377.9] [Reference Citation Analysis (0)] |
| 32. | Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3:e3148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 996] [Cited by in F6Publishing: 1042] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 33. | Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3218] [Cited by in F6Publishing: 3383] [Article Influence: 211.4] [Reference Citation Analysis (0)] |
| 34. | Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 855] [Cited by in F6Publishing: 938] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 35. | Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009;112:55-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 491] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 36. | Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1258] [Cited by in F6Publishing: 1278] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 37. | Carrasco G, Corvalan AH. Helicobacter pylori-Induced Chronic Gastritis and Assessing Risks for Gastric Cancer. Gastroenterol Res Pract. 2013;2013:393015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Conteduca V, Sansonno D, Lauletta G, Russi S, Ingravallo G, Dammacco F. H. pylori infection and gastric cancer: state of the art (review). Int J Oncol. 2013;42:5-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 39. | Song J, Bai Z, Zhang Z. MicroRNAs are implicated in the initiation and progression of gastric cancer. Chin Med J (Engl). 2014;127:554-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 40. | Song S, Ajani JA. The role of microRNAs in cancers of the upper gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2013;10:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Yasui W, Sentani K, Sakamoto N, Anami K, Naito Y, Oue N. Molecular pathology of gastric cancer: research and practice. Pathol Res Pract. 2011;207:608-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 42. | Wang M, Gu H, Qian H, Zhu W, Zhao C, Zhang X, Tao Y, Zhang L, Xu W. miR-17-5p/20a are important markers for gastric cancer and murine double minute 2 participates in their functional regulation. Eur J Cancer. 2013;49:2010-2021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 654] [Cited by in F6Publishing: 682] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 44. | Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K, Ren G, Su T, Pan Y, Feng B. MiR-150 promotes gastric cancer proliferation by negatively regulating the pro-apoptotic gene EGR2. Biochem Biophys Res Commun. 2010;392:340-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 45. | Unoki M, Nakamura Y. EGR2 induces apoptosis in various cancer cell lines by direct transactivation of BNIP3L and BAK. Oncogene. 2003;22:2172-2185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Sacconi A, Biagioni F, Canu V, Mori F, Di Benedetto A, Lorenzon L, Ercolani C, Di Agostino S, Cambria AM, Germoni S. miR-204 targets Bcl-2 expression and enhances responsiveness of gastric cancer. Cell Death Dis. 2012;3:e423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 47. | Tsukamoto Y, Nakada C, Noguchi T, Tanigawa M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M. MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res. 2010;70:2339-2349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 341] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 48. | Kogo R, Mimori K, Tanaka F, Komune S, Mori M. Clinical significance of miR-146a in gastric cancer cases. Clin Cancer Res. 2011;17:4277-4284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 49. | Nishida N, Mimori K, Fabbri M, Yokobori T, Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y, Mori M. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17:2725-2733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 50. | Song YX, Yue ZY, Wang ZN, Xu YY, Luo Y, Xu HM, Zhang X, Jiang L, Xing CZ, Zhang Y. MicroRNA-148b is frequently down-regulated in gastric cancer and acts as a tumor suppressor by inhibiting cell proliferation. Mol Cancer. 2011;10:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 51. | Chen Y, Song Y, Wang Z, Yue Z, Xu H, Xing C, Liu Z. Altered expression of MiR-148a and MiR-152 in gastrointestinal cancers and its clinical significance. J Gastrointest Surg. 2010;14:1170-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 52. | Motoyama K, Inoue H, Mimori K, Tanaka F, Kojima K, Uetake H, Sugihara K, Mori M. Clinicopathological and prognostic significance of PDCD4 and microRNA-21 in human gastric cancer. Int J Oncol. 2010;36:1089-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Xiao B, Guo J, Miao Y, Jiang Z, Huan R, Zhang Y, Li D, Zhong J. Detection of miR-106a in gastric carcinoma and its clinical significance. Clin Chim Acta. 2009;400:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 54. | Wang WN, Chen Y, Zhang YD, Hu TH. The regulatory mechanism of CCR7 gene expression and its involvement in the metastasis and progression of gastric cancer. Tumour Biol. 2013;34:1865-1871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | di Mario F, Cavallaro LG. Non-invasive tests in gastric diseases. Dig Liver Dis. 2008;40:523-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-2909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 833] [Cited by in F6Publishing: 872] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 57. | Zhang WH, Gui JH, Wang CZ, Chang Q, Xu SP, Cai CH, Li YN, Tian YP, Yan L, Wu B. The identification of miR-375 as a potential biomarker in distal gastric adenocarcinoma. Oncol Res. 2012;20:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Chan SH, Wu CW, Li AF, Chi CW, Lin WC. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res. 2008;28:907-911. [PubMed] [Cited in This Article: ] |
| 59. | Zhang Y, Guo J, Li D, Xiao B, Miao Y, Jiang Z, Zhuo H. Down-regulation of miR-31 expression in gastric cancer tissues and its clinical significance. Med Oncol. 2010;27:685-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Jiang Z, Guo J, Xiao B, Miao Y, Huang R, Li D, Zhang Y. Increased expression of miR-421 in human gastric carcinoma and its clinical association. J Gastroenterol. 2010;45:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Chen W, Tang Z, Sun Y, Zhang Y, Wang X, Shen Z, Liu F, Qin X. miRNA expression profile in primary gastric cancers and paired lymph node metastases indicates that miR-10a plays a role in metastasis from primary gastric cancer to lymph nodes. Exp Ther Med. 2012;3:351-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Liu K, Li G, Fan C, Diao Y, Wu B, Li J. Increased Expression of MicroRNA-221 in gastric cancer and its clinical significance. J Int Med Res. 2012;40:467-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 63. | Wu WY, Xue XY, Chen ZJ, Han SL, Huang YP, Zhang LF, Zhu GB, Shen X. Potentially predictive microRNAs of gastric cancer with metastasis to lymph node. World J Gastroenterol. 2011;17:3645-3651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Xu Y, Sun J, Xu J, Li Q, Guo Y, Zhang Q. miR-21 Is a Promising Novel Biomarker for Lymph Node Metastasis in Patients with Gastric Cancer. Gastroenterol Res Pract. 2012;2012:640168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Li X, Zhang Y, Zhang Y, Ding J, Wu K, Fan D. Survival prediction of gastric cancer by a seven-microRNA signature. Gut. 2010;59:579-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 66. | Yan Z, Xiong Y, Xu W, Gao J, Cheng Y, Wang Z, Chen F, Zheng G. Identification of hsa-miR-335 as a prognostic signature in gastric cancer. PLoS One. 2012;7:e40037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 67. | Tsai KW, Liao YL, Wu CW, Hu LY, Li SC, Chan WC, Ho MR, Lai CH, Kao HW, Fang WL. Aberrant expression of miR-196a in gastric cancers and correlation with recurrence. Genes Chromosomes Cancer. 2012;51:394-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 68. | Zhang X, Yan Z, Zhang J, Gong L, Li W, Cui J, Liu Y, Gao Z, Li J, Shen L. Combination of hsa-miR-375 and hsa-miR-142-5p as a predictor for recurrence risk in gastric cancer patients following surgical resection. Ann Oncol. 2011;22:2257-2266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 69. | Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 722] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 70. | Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 546] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 71. | Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li K, Zhou L, Sun Y, Li M, Zhou J. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267-3276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 72. | Rotkrua P, Shimada S, Mogushi K, Akiyama Y, Tanaka H, Yuasa Y. Circulating microRNAs as biomarkers for early detection of diffuse-type gastric cancer using a mouse model. Br J Cancer. 2013;108:932-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 487] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 74. | Li C, Li JF, Cai Q, Qiu QQ, Yan M, Liu BY, Zhu ZG. MiRNA-199a-3p: A potential circulating diagnostic biomarker for early gastric cancer. J Surg Oncol. 2013;108:89-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Li C, Li JF, Cai Q, Qiu QQ, Yan M, Liu BY, Zhu ZG. miRNA-199a-3p in plasma as a potential diagnostic biomarker for gastric cancer. Ann Surg Oncol. 2013;20 Suppl 3:S397-S405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Cai H, Yuan Y, Hao YF, Guo TK, Wei X, Zhang YM. Plasma microRNAs serve as novel potential biomarkers for early detection of gastric cancer. Med Oncol. 2013;30:452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 77. | Liu H, Zhu L, Liu B, Yang L, Meng X, Zhang W, Ma Y, Xiao H. Genome-wide microRNA profiles identify miR-378 as a serum biomarker for early detection of gastric cancer. Cancer Lett. 2012;316:196-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 78. | Komatsu S, Ichikawa D, Tsujiura M, Konishi H, Takeshita H, Nagata H, Kawaguchi T, Hirajima S, Arita T, Shiozaki A. Prognostic impact of circulating miR-21 in the plasma of patients with gastric carcinoma. Anticancer Res. 2013;33:271-276. [PubMed] [Cited in This Article: ] |
| 79. | Wang M, Gu H, Wang S, Qian H, Zhu W, Zhang L, Zhao C, Tao Y, Xu W. Circulating miR-17-5p and miR-20a: molecular markers for gastric cancer. Mol Med Rep. 2012;5:1514-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 80. | Kim SY, Jeon TY, Choi CI, Kim DH, Kim DH, Kim GH, Ryu DY, Lee BE, Kim HH. Validation of circulating miRNA biomarkers for predicting lymph node metastasis in gastric cancer. J Mol Diagn. 2013;15:661-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 81. | Valladares-Ayerbes M, Reboredo M, Medina-Villaamil V, Iglesias-Díaz P, Lorenzo-Patiño MJ, Haz M, Santamarina I, Blanco M, Fernández-Tajes J, Quindós M. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J Transl Med. 2012;10:186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 82. | Liu R, Zhang C, Hu Z, Li G, Wang C, Yang C, Huang D, Chen X, Zhang H, Zhuang R. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47:784-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 342] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 83. | Zhou H, Xiao B, Zhou F, Deng H, Zhang X, Lou Y, Gong Z, Du C, Guo J. MiR-421 is a functional marker of circulating tumor cells in gastric cancer patients. Biomarkers. 2012;17:104-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Zheng Y, Cui L, Sun W, Zhou H, Yuan X, Huo M, Chen J, Lou Y, Guo J. MicroRNA-21 is a new marker of circulating tumor cells in gastric cancer patients. Cancer Biomark 2011-. 2012;10:71-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Zhou H, Guo JM, Lou YR, Zhang XJ, Zhong FD, Jiang Z, Cheng J, Xiao BX. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using microRNA as a marker. J Mol Med (Berl). 2010;88:709-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 86. | Cui L, Zhang X, Ye G, Zheng T, Song H, Deng H, Xiao B, Xia T, Yu X, Le Y. Gastric juice MicroRNAs as potential biomarkers for the screening of gastric cancer. Cancer. 2013;119:1618-1626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 87. | Yu X, Luo L, Wu Y, Yu X, Liu Y, Yu X, Zhao X, Zhang X, Cui L, Ye G. Gastric juice miR-129 as a potential biomarker for screening gastric cancer. Med Oncol. 2013;30:365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Zhang X, Cui L, Ye G, Zheng T, Song H, Xia T, Yu X, Xiao B, Le Y, Guo J. Gastric juice microRNA-421 is a new biomarker for screening gastric cancer. Tumour Biol. 2012;33:2349-2355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Song J, Bai Z, Han W, Zhang J, Meng H, Bi J, Ma X, Han S, Zhang Z. Identification of suitable reference genes for qPCR analysis of serum microRNA in gastric cancer patients. Dig Dis Sci. 2012;57:897-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 90. | Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods. 2010;50:298-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 867] [Cited by in F6Publishing: 901] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 91. | Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo X, Mao XH, Zou QM, Yu PW, Zuo QF. Plasma microRNAs, miR-223, miR-21 and miR-218, as novel potential biomarkers for gastric cancer detection. PLoS One. 2012;7:e41629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 92. | Wang B, Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol. 2012;138:1659-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 93. | Song MY, Pan KF, Su HJ, Zhang L, Ma JL, Li JY, Yuasa Y, Kang D, Kim YS, You WC. Identification of serum microRNAs as novel non-invasive biomarkers for early detection of gastric cancer. PLoS One. 2012;7:e33608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 94. | Konishi H, Ichikawa D, Komatsu S, Shiozaki A, Tsujiura M, Takeshita H, Morimura R, Nagata H, Arita T, Kawaguchi T. Detection of gastric cancer-associated microRNAs on microRNA microarray comparing pre- and post-operative plasma. Br J Cancer. 2012;106:740-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 95. | Gorur A, Balci Fidanci S, Dogruer Unal N, Ayaz L, Akbayir S, Yildirim Yaroglu H, Dirlik M, Serin MS, Tamer L. Determination of plasma microRNA for early detection of gastric cancer. Mol Biol Rep. 2013;40:2091-2096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 96. | Inoue T, Iinuma H, Ogawa E, Inaba T, Fukushima R. Clinicopathological and prognostic significance of microRNA-107 and its relationship to DICER1 mRNA expression in gastric cancer. Oncol Rep. 2012;27:1759-1764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 97. | Brenner B, Hoshen MB, Purim O, David MB, Ashkenazi K, Marshak G, Kundel Y, Brenner R, Morgenstern S, Halpern M. MicroRNAs as a potential prognostic factor in gastric cancer. World J Gastroenterol. 2011;17:3976-3985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 70] [Cited by in F6Publishing: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 98. | Xiao B, Zhu ED, Li N, Lu DS, Li W, Li BS, Zhao YL, Mao XH, Guo G, Yu PW. Increased miR-146a in gastric cancer directly targets SMAD4 and is involved in modulating cell proliferation and apoptosis. Oncol Rep. 2012;27:559-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 99. | Kim K, Lee HC, Park JL, Kim M, Kim SY, Noh SM, Song KS, Kim JC, Kim YS. Epigenetic regulation of microRNA-10b and targeting of oncogenic MAPRE1 in gastric cancer. Epigenetics. 2011;6:740-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 100. | Valladares-Ayerbes M, Blanco M, Haz M, Medina V, Iglesias-Díaz P, Lorenzo-Patiño MJ, Reboredo M, Santamarina I, Figueroa A, Antón-Aparicio LM. Prognostic impact of disseminated tumor cells and microRNA-17-92 cluster deregulation in gastrointestinal cancer. Int J Oncol. 2011;39:1253-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Su Y, Ni Z, Wang G, Cui J, Wei C, Wang J, Yang Q, Xu Y, Li F. Aberrant expression of microRNAs in gastric cancer and biological significance of miR-574-3p. Int Immunopharmacol. 2012;13:468-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Liu K, Qian T, Tang L, Wang J, Yang H, Ren J. Decreased expression of microRNA let-7i and its association with chemotherapeutic response in human gastric cancer. World J Surg Oncol. 2012;10:225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 103. | Peng Y, Liu YM, Li LC, Wang LL, Wu XL. MicroRNA-338 inhibits growth, invasion and metastasis of gastric cancer by targeting NRP1 expression. PLoS One. 2014;9:e94422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 104. | Wu Q, Yang Z, An Y, Hu H, Yin J, Zhang P, Nie Y, Wu K, Shi Y, Fan D. MiR-19a/b modulate the metastasis of gastric cancer cells by targeting the tumour suppressor MXD1. Cell Death Dis. 2014;5:e1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 105. | Zhao Y, Huang J, Zhang L, Qu Y, Li J, Yu B, Yan M, Yu Y, Liu B, Zhu Z. MiR-133b is frequently decreased in gastric cancer and its overexpression reduces the metastatic potential of gastric cancer cells. BMC Cancer. 2014;14:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 106. | Zhao Y, Li C, Wang M, Su L, Qu Y, Li J, Yu B, Yan M, Yu Y, Liu B. Decrease of miR-202-3p expression, a novel tumor suppressor, in gastric cancer. PLoS One. 2013;8:e69756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 107. | Chen L, Lü MH, Zhang D, Hao NB, Fan YH, Wu YY, Wang SM, Xie R, Fang DC, Zhang H. miR-1207-5p and miR-1266 suppress gastric cancer growth and invasion by targeting telomerase reverse transcriptase. Cell Death Dis. 2014;5:e1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 108. | Deng M, Tang HL, Lu XH, Liu MY, Lu XM, Gu YX, Liu JF, He ZM. miR-26a suppresses tumor growth and metastasis by targeting FGF9 in gastric cancer. PLoS One. 2013;8:e72662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 109. | Jiping Z, Ming F, Lixiang W, Xiuming L, Yuqun S, Han Y, Zhifang L, Yundong S, Shili L, Chunyan C. MicroRNA-212 inhibits proliferation of gastric cancer by directly repressing retinoblastoma binding protein 2. J Cell Biochem. 2013;114:2666-2672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Flamant S, Ritchie W, Guilhot J, Holst J, Bonnet ML, Chomel JC, Guilhot F, Turhan AG, Rasko JE. Micro-RNA response to imatinib mesylate in patients with chronic myeloid leukemia. Haematologica. 2010;95:1325-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 111. | Scholl V, Hassan R, Zalcberg IR. miRNA-451: A putative predictor marker of Imatinib therapy response in chronic myeloid leukemia. Leuk Res. 2012;36:119-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Zhou C, Liu J, Li Y, Liu L, Zhang X, Ma CY, Hua SC, Yang M, Yuan Q. microRNA-1274a, a modulator of sorafenib induced a disintegrin and metalloproteinase 9 (ADAM9) down-regulation in hepatocellular carcinoma. FEBS Lett. 2011;585:1828-1834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 113. | Bai S, Nasser MW, Wang B, Hsu SH, Datta J, Kutay H, Yadav A, Nuovo G, Kumar P, Ghoshal K. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284:32015-32027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 114. | Jiang J, Zheng X, Xu X, Zhou Q, Yan H, Zhang X, Lu B, Wu C, Ju J. Prognostic significance of miR-181b and miR-21 in gastric cancer patients treated with S-1/Oxaliplatin or Doxifluridine/Oxaliplatin. PLoS One. 2011;6:e23271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 115. | Chen YJ, Zhu JM, Wu H, Fan J, Zhou J, Hu J, Yu Q, Liu TT, Yang L, Wu CL. Circulating microRNAs as a Fingerprint for Liver Cirrhosis. PLoS One. 2013;8:e66577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 116. | Liu N, Cui RX, Sun Y, Guo R, Mao YP, Tang LL, Jiang W, Liu X, Cheng YK, He QM. A four-miRNA signature identified from genome-wide serum miRNA profiling predicts survival in patients with nasopharyngeal carcinoma. Int J Cancer. 2014;134:1359-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |