Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 28, 2012; 18(8): 754-766
Published online Feb 28, 2012. doi: 10.3748/wjg.v18.i8.754
Published online Feb 28, 2012. doi: 10.3748/wjg.v18.i8.754
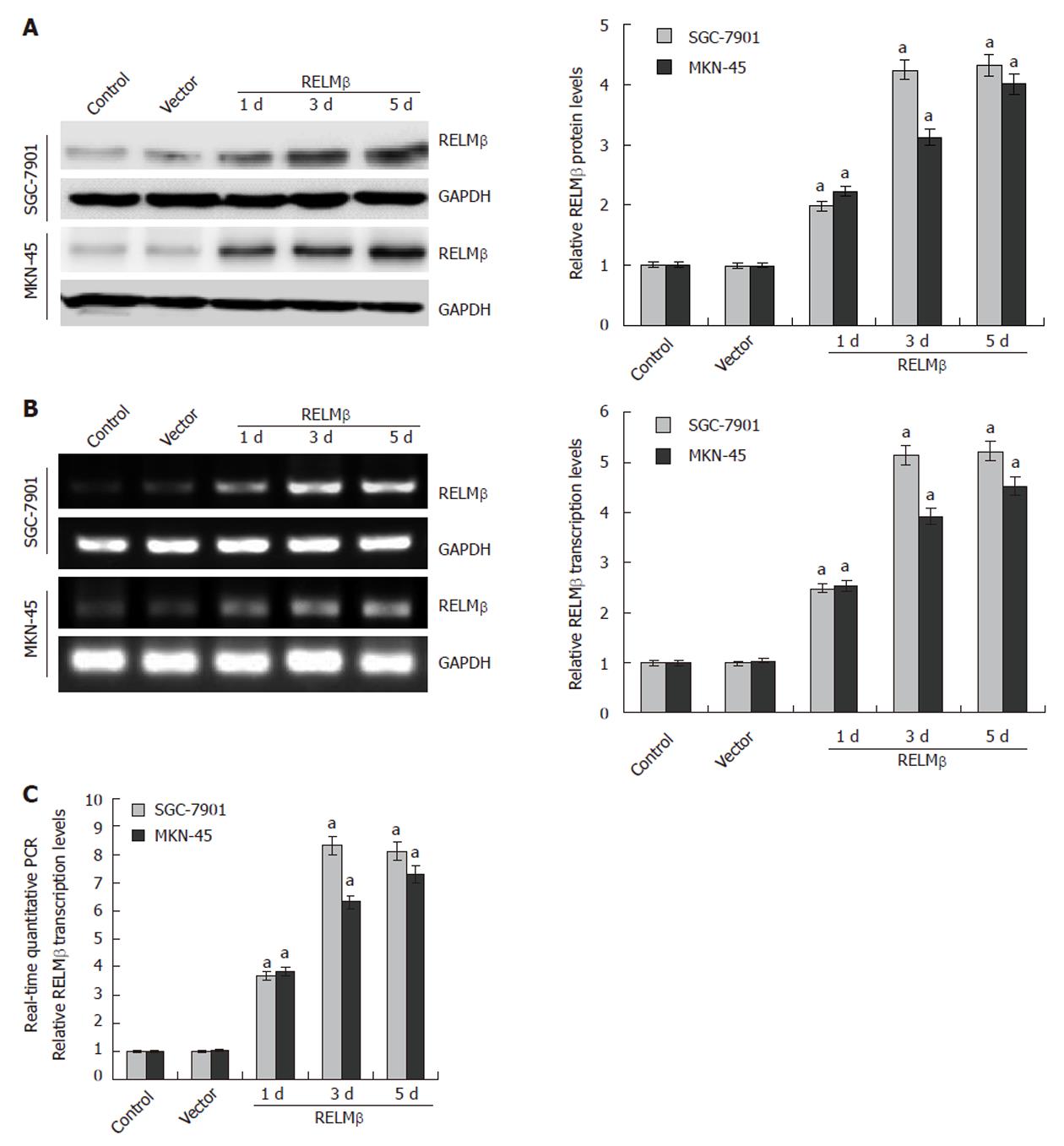
Figure 1 Transient transfection-mediated over-expression of resistin-like molecule β in gastric cancer cells.
A: Western blotting indicated that low resistin-like molecule β (RELMβ) protein was detected in the parental SGC-7901 and MKN-45 cells, and transient transfection of the empty vector (mock) did not affect the expression levels of RELMβ. However, transient transfection of pcDNA3.1-RELMβ for 24 h, 72 h and 120 h resulted in increased RELMβ expression; B: 24 h, 72 h and 120 h after transfection, reverse transcription polymerase chain reaction (RT-PCR) indicated the increased RELMβ transcription levels in pcDNA3.1-RELMβ transfected SGC-7901 and MKN-45 cells, but not in mock group; C: Real-time quantitative RT-PCR further demonstrated that transfection of pcDNA3.1-RELMβ for 24 h, 72 h and 120 h resulted in upregulation of RELMβ transcription levels in SGC-7901 and MKN-45 cells. The symbol (a) indicates a significant increase compared with parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results.
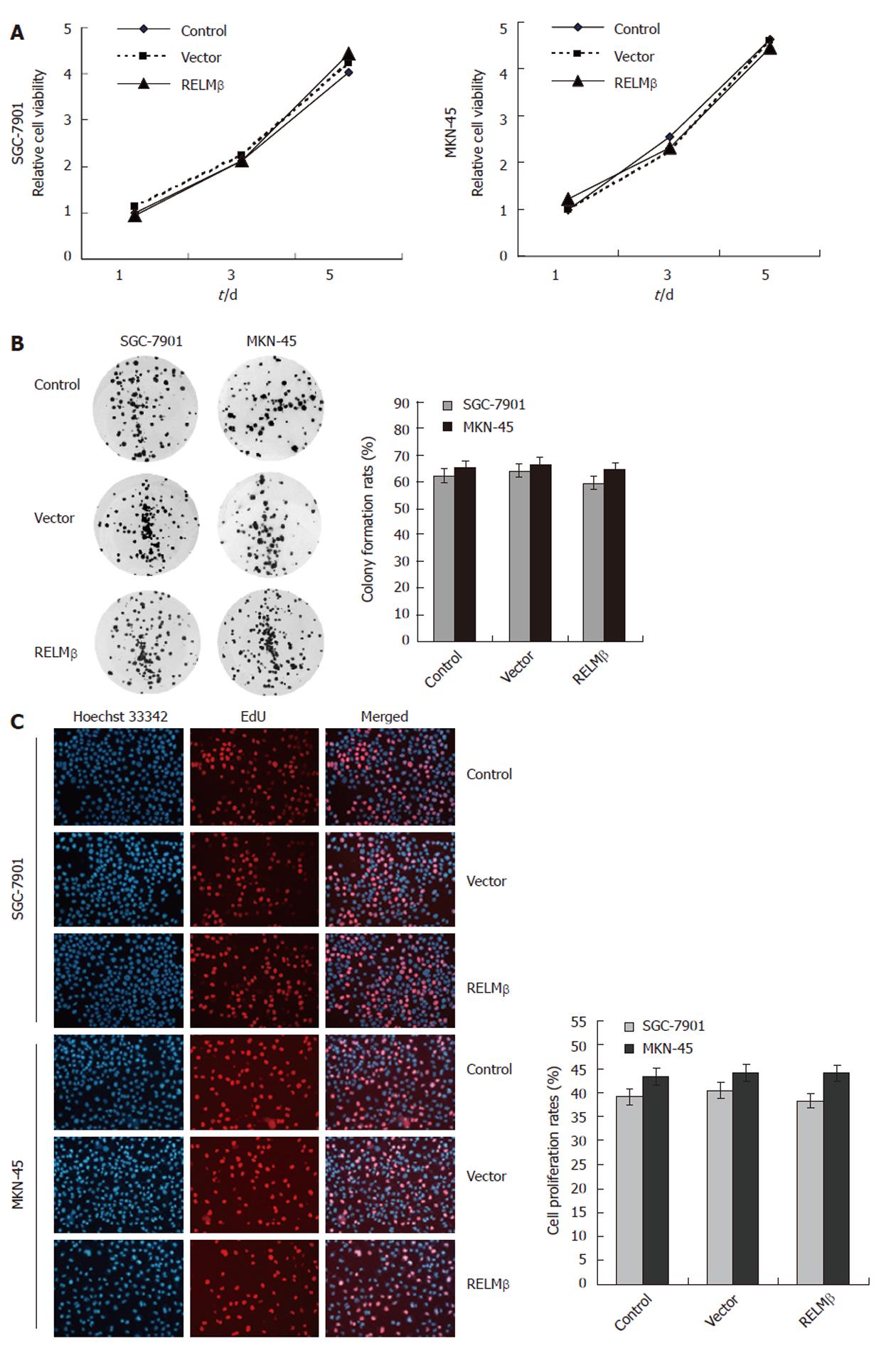
Figure 2 Upregulating resistin-like molecule β expression did not affect the in vitro proliferation of gastric cancer cells.
A: In 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric assay, transfection of pcDNA3.1-resistin-like molecule β (RELMβ) or empty vector (mock) for 24 h, 72 h and 120 h, did not affect the cell proliferation, when compared with the parental SGC-7901 and MKN-45 cells (P > 0.05); B: Colony formation assay indicated that 72 h after transfection, over-expression of RELMβ did not affect the in vitro proliferation of SGC-7901 and MKN-45 cells (P > 0.05); C: 5-ethynyl-20-deoxyuridine incorporation assay revealed that 72 h after transfection, over-expression of RELMβ did not influence the proliferation of cultured SGC-7901 and MKN-45 cells (P > 0.05). Triplicate experiments were performed with essentially identical results.
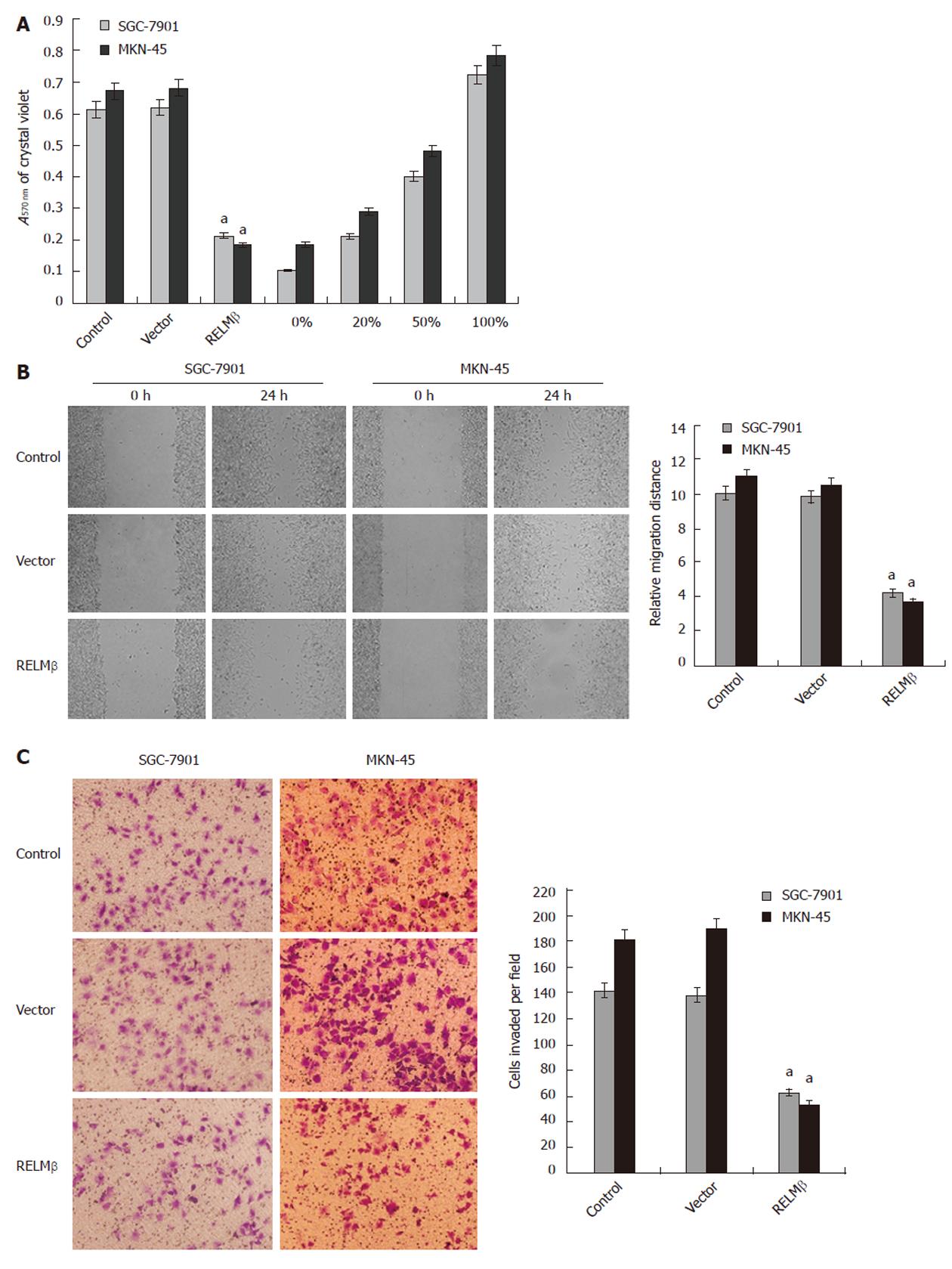
Figure 3 Over-expression of resistin-like molecule β attenuated the adhesion, migration and invasion of gastric cancer cells in vitro.
A: In the adhesion assay, SGC-7901 and MKN-45 cells transfected with pcDNA3.1-resistin-like molecule β (RELMβ) for 72 h exhibited markedly reduced ability in adhesion to the precoated matrigel, when compared with parental cells. However, the cells transfected with empty vector (mock) had a similar adhesive ability as parental cells; B: Scratch migration assay indicated that transfection of pcDNA3.1-RELMβ into SGC-7901 and MKN-45 cells for 72 h resulted in an impaired migration capacity, when compared with the parental cells and mock group; C: Transwell analysis indicated that transfection of pcDNA3.1-RELMβ for 72 h abolished the invasive capabilities of SGC-7901 and MKN-45 cells, when compared with the parental and mock cells. The symbol (a) indicates a significant decrease compared with parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results.
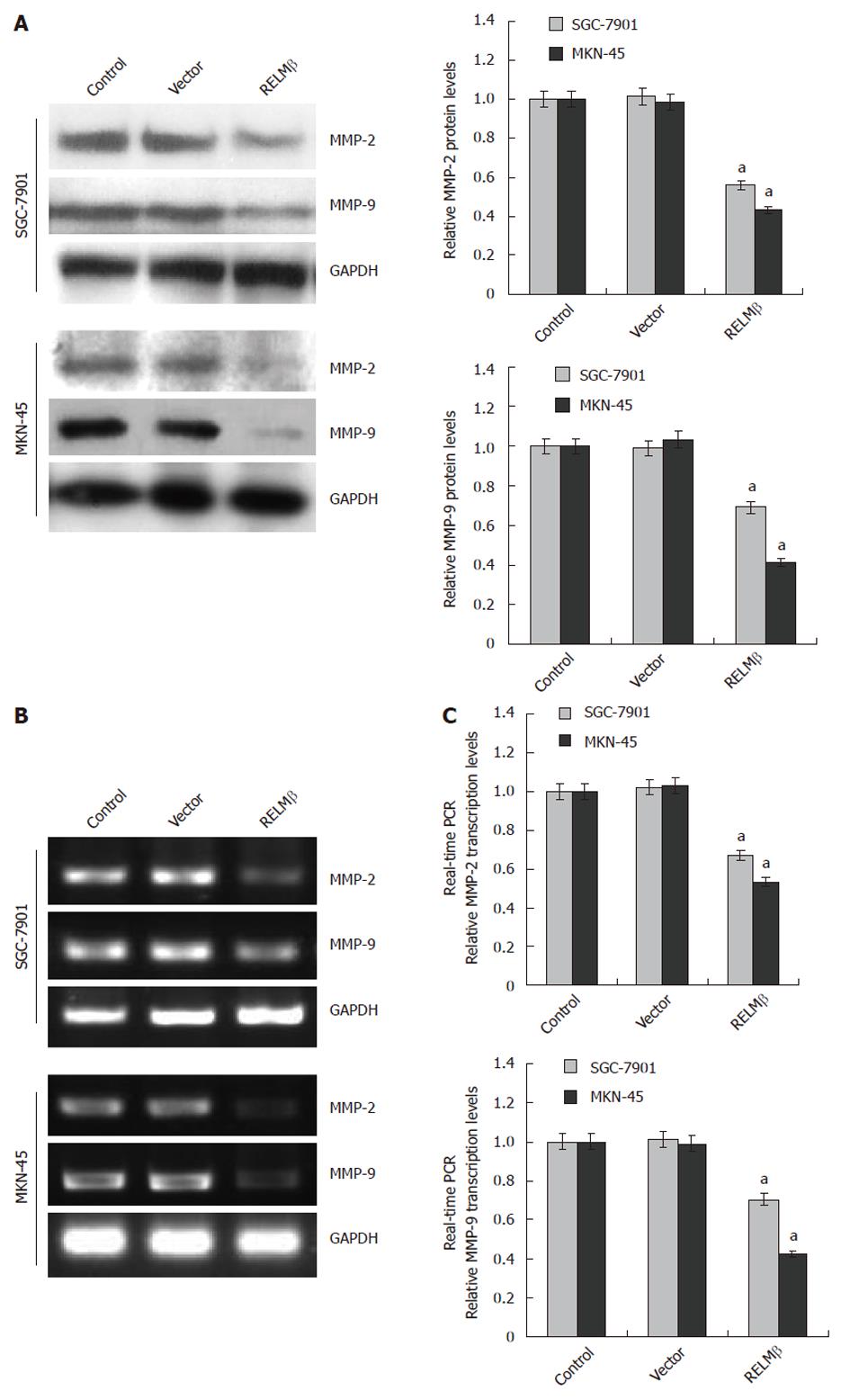
Figure 4 Over-expression of resistin-like molecule β decreased the expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 in gastric cancer cells.
A: Western blotting indicated that 72 h after transfection, over-expression of resistin-like molecule β (RELMβ) abolished the expression of matrix metalloproteinase (MMP)-2 and MMP-9 in SGC-7901 and MKN-45 cells. However, transfection of empty vector (mock) did not influence their expression; B: Reverse transcription polymerase chain reaction (RT-PCR) indicated the decreased MMP-2 and MMP-9 transcription levels in SGC-7901 and MKN-45 cells transfected with pcDNA3.1-RELMβ for 72 h, but not in mock group; C: Real-time quantitative RT-PCR further demonstrated that transfection of pcDNA3.1-RELMβ for 72 h resulted in decreased transcription levels of MMP-2 and MMP-9 in SGC-7901 and MKN-45 cells. The symbol (a) indicates a significant decrease compared with parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
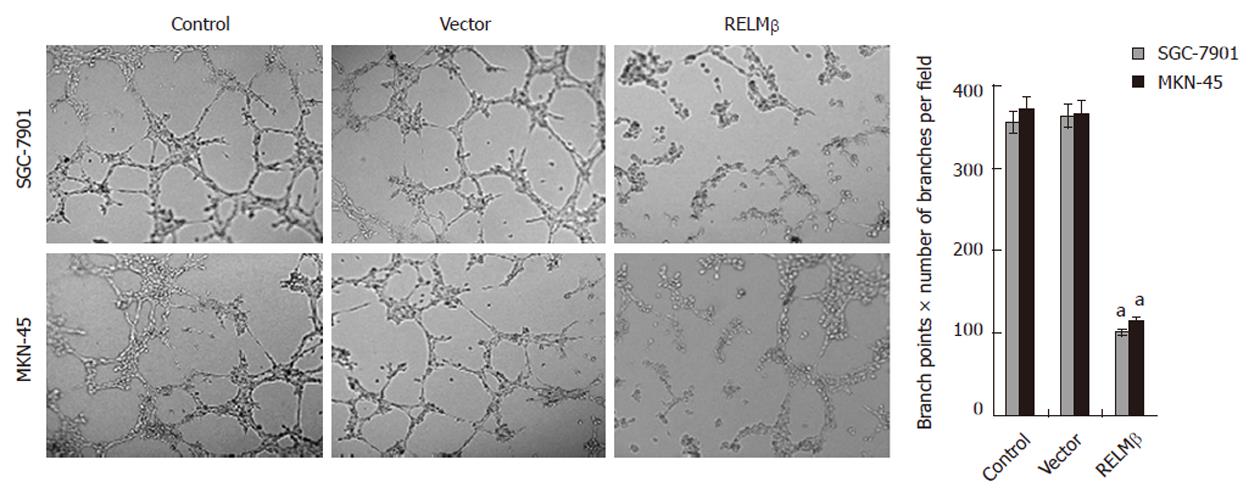
Figure 5 Over-expression of resistin-like molecule β inhibited the in vitro angiogenic capabilities of gastric cancer cells.
Extensive tube formation of endothelial cells was observed in parental and empty vector (mock) groups. However, when the endothelial cells were treated by the medium preconditioned with pcDNA3.1-resistin-like molecule β (RELMβ)-transfected SGC-7901 or MKN-45 cells, the tube formation was suppressed. The symbol (a) indicates a significant decrease compared with parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results.
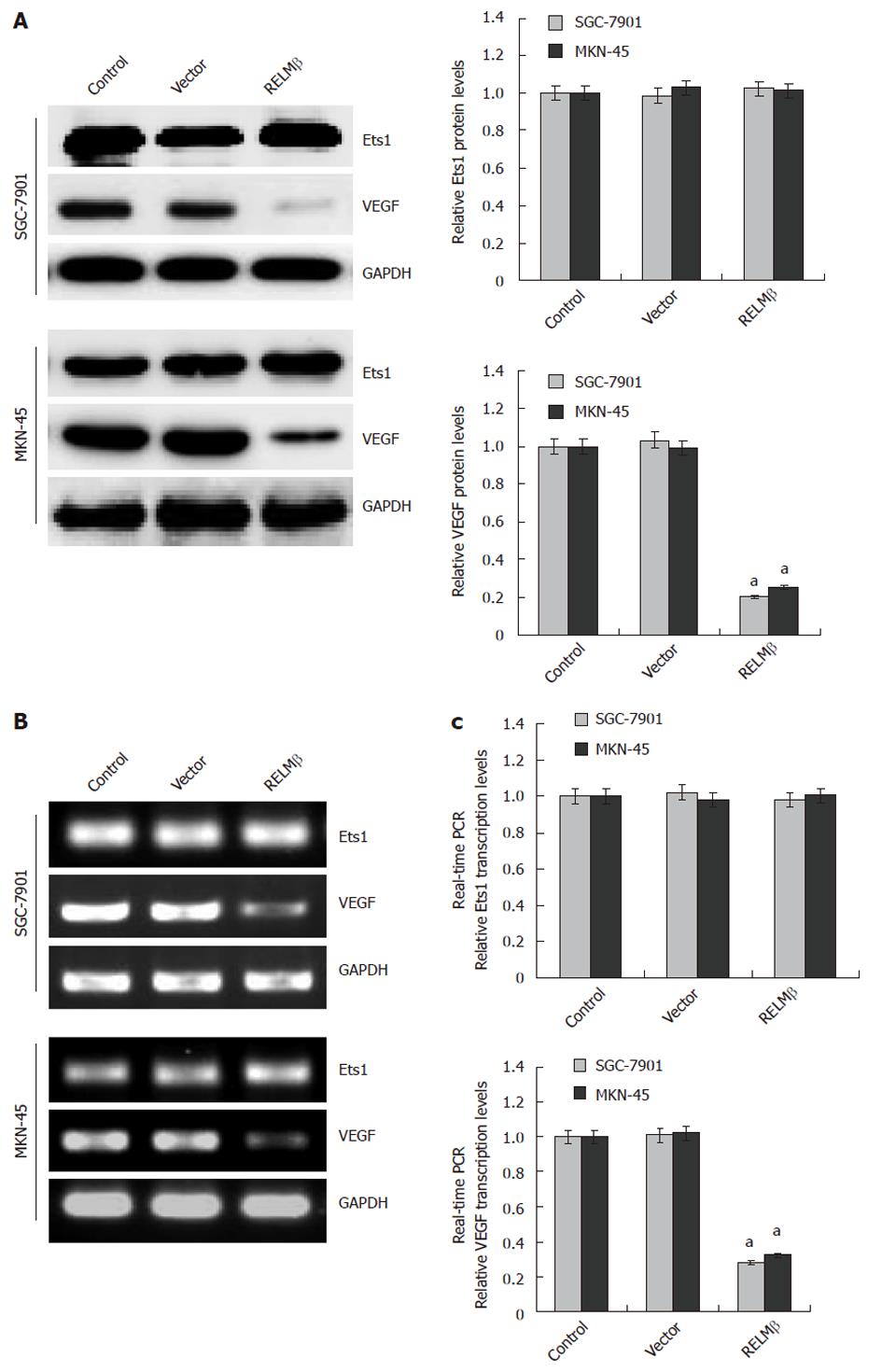
Figure 6 Over-expression of resistin-like molecule β decreased the expression of vascular endothelial growth factor, but not v-ets erythroblastosis virus E26 oncogene homolog 1, in gastric cancer cells.
A: Western blotting indicated that 72 h after transfection, over-expression of resistin-like molecule β (RELMβ) abolished the expression of vascular endothelial growth factor (VEGF), but not v-ets erythroblastosis virus E26 oncogene homolog 1 (Ets1), in SGC-7901 and MKN-45 cells. Moreover, transfection of empty vector (mock) did not influence the expression of VEGF and Ets1; B: Reverse transcription polymerase chain reaction (RT-PCR) indicated the decreased VEGF transcription levels in SGC-7901 and MKN-45 cells transfected with pcDNA3.1-RELMβ for 72 h, but not in mock group. Moreover, the Est1 transcription levels were not influenced by transfection of pcDNA3.1-RELMβ or empty vector (mock); C: Real-time quantitative RT-PCR further demonstrated that transfection of pcDNA3.1-RELMβ for 72 h resulted in decreased transcription levels of VEGF, but not of Ets1, in SGC-7901 and MKN-45 cells. The symbol (a) indicates a significant decrease from parental cells (P < 0.01). Triplicate experiments were performed with essentially identical results. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
-
Citation: Zheng LD, Yang CL, Qi T, Qi M, Tong L, Tong QS. Effects of resistin-like molecule β over-expression on gastric cancer cells
in vitro . World J Gastroenterol 2012; 18(8): 754-766 - URL: https://www.wjgnet.com/1007-9327/full/v18/i8/754.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i8.754