Published online Jun 18, 2025. doi: 10.13105/wjma.v13.i2.107997
Revised: April 10, 2025
Accepted: May 8, 2025
Published online: June 18, 2025
Processing time: 75 Days and 12.6 Hours
Hepatobiliary and pancreatic cancers are among the most lethal malignancies due to late-stage diagnosis and limited treatment options. Liquid biopsy has emerged as a minimally invasive tool for early cancer detection, prognosis, and therapeutic monitoring.
To concise the available data on liquid biopsy and establish its role in hepatobiliary surgeries.
This systematic review was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 guidelines. A comprehensive lite
Liquid biopsy demonstrated significant potential for early cancer detection, perioperative risk stratification, intraoperative surgical decision-making, and postoperative monitoring of minimal residual disease. However, challenges remain regarding standardization, sensitivity, and clinical validation.
Liquid biopsy represents a paradigm shift in hepatobiliary and pancreatic cancer management. Advancements in next-generation sequencing and artificial intelligence may enhance its clinical utility. Further large-scale studies are needed to establish standardized protocols for routine implementation.
Core Tip: Liquid biopsy is a minimally invasive technique that detects circulating tumor DNA, circulating tumor cells, and other biomarkers in bodily fluids. It offers real-time monitoring of tumor dynamics, treatment response, and minimal residual disease. Unlike traditional tissue biopsies, liquid biopsy allows for repeated sampling, providing insights into tumor heterogeneity and evolution. It has applications in early cancer detection, prognosis, and guiding targeted therapies. Despite its advantages, challenges such as standardization, sensitivity, and specificity remain. Further research and technological advancements are needed to enhance its clinical utility.
- Citation: Agrawal H, Goswami B, Gupta N, Singh N. Liquid biopsy in hepatobiliary and pancreatic cancers: A paradigm shift in early detection, prognostic stratification, and perioperative monitoring. World J Meta-Anal 2025; 13(2): 107997
- URL: https://www.wjgnet.com/2308-3840/full/v13/i2/107997.htm
- DOI: https://dx.doi.org/10.13105/wjma.v13.i2.107997
Hepatobiliary and pancreatic tumours are one of the most fatal malignancies. They are the second and third most common cause of global cancer related mortalities respectively. They are mostly associated with poor survival outcome primarily due to late presentation and subsequent late diagnosis. Once diagnosed, a very limited effective therapeutic options are available for their management. Therefore, these represent a vast subset of tumours with a critical therapeutic challenge[1].
Typical perioperative management for these malignancies involves surgical resection along with chemotherapy. While for hepatocellular carcinoma (HCC) it could be liver transplantation or resection, of pancreatic ductal adenocarcinoma (PDAC) generally requires a Whipple procedure. It has been estimated that only 20% PDAC are eligible for R0-surgical resection at the time of presentation. Even after potentially curative resection, many pancreatic cancer patients experience early relapse, particularly hepatic metastases, which significantly worsen the prognosis[2].
The current standard for diagnosis and monitoring of hepatobiliary and pancreatic cancer relies heavily on protein biomarkers such as carbohydrate Antigen (CA) 19-9, alpha feto-protein (AFP), but their utility remains limited due to suboptimal sensitivity and specificity. Imaging modalities like triple phase CECT and magnetic resonance imaging has an important role in staging and treatment planning, but they are not always sensitive enough to detect small tumours or micro metastasis. Tissue biopsy, which is the gold standard for sampling tumours, is firstly an invasive procedure that has a limited role when the anatomical positioning of such cancers makes them challenging to sample, and secondly, they are often unable to adequately scope the tumour heterogeneity. Moreover, tissue biopsies may not capture the dynamic molecular evolution of the tumour, resulting in suboptimal treatment decisions[3].
These obstacles underscore the need for alternative approaches that can provide comprehensive molecular information while minimizing invasiveness and patient discomfort. Recent advances in molecular diagnostics have introduced liquid biopsy as a promising, minimally invasive approach that may transform the perioperative management of these aggressive cancers. The integration of liquid biopsy techniques into clinical practice represents a paradigm shift in the management of these challenging malignancies, offering new opportunities to detect disease earlier, monitor treatment response more accurately, and potentially improve survival rates through personalized treatment approaches.
This systematic review synthesizes current evidence regarding the application of liquid biopsy in the perioperative setting for hepatobiliary and pancreatic cancers, examining its potential to improve patient outcomes through enhanced diagnosis, risk stratification, treatment selection, and surveillance.
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies were selected based on predefined inclusion and exclusion criteria. Inclusion criteria encompassed studies evaluating the application of liquid biopsy in hepatobiliary and pancreatic cancers, those assessing the diagnostic, prognostic, and therapeutic implications of liquid biopsy markers such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), exosomes, and other circulating biomarkers, as well as clinical trials, cohort studies, case-control studies, and systematic reviews published in peer-reviewed journals. Studies were excluded if they were non-English, lacked sufficient data, were in vitro or preclinical without human data, or were case reports/case series involving fewer than two patients.
A comprehensive literature search was performed using PubMed, Scopus, Web of Science, and EMBASE to identify relevant studies published up to March 2025. The search utilized keywords such as "liquid biopsy", "circulating tumor DNA", "circulating tumor cells", "exosomes", "cell-free DNA", "hepatobiliary cancer", "pancreatic cancer", "biliary tract cancer", "diagnosis", "prognosis", and "therapeutic monitoring", with Boolean operators (AND/OR) applied to refine the results. References of relevant articles were manually screened to identify additional studies.
Two independent authors conducted the study selection process by screening titles and abstracts, followed by a full-text review of eligible articles. Any discrepancies between authors were resolved through discussion with a third author. Data extraction was performed using a standardized form that captured information on study design, sample size, type of liquid biopsy, biomarkers analysed, methodologies used, key findings, and clinical implications.
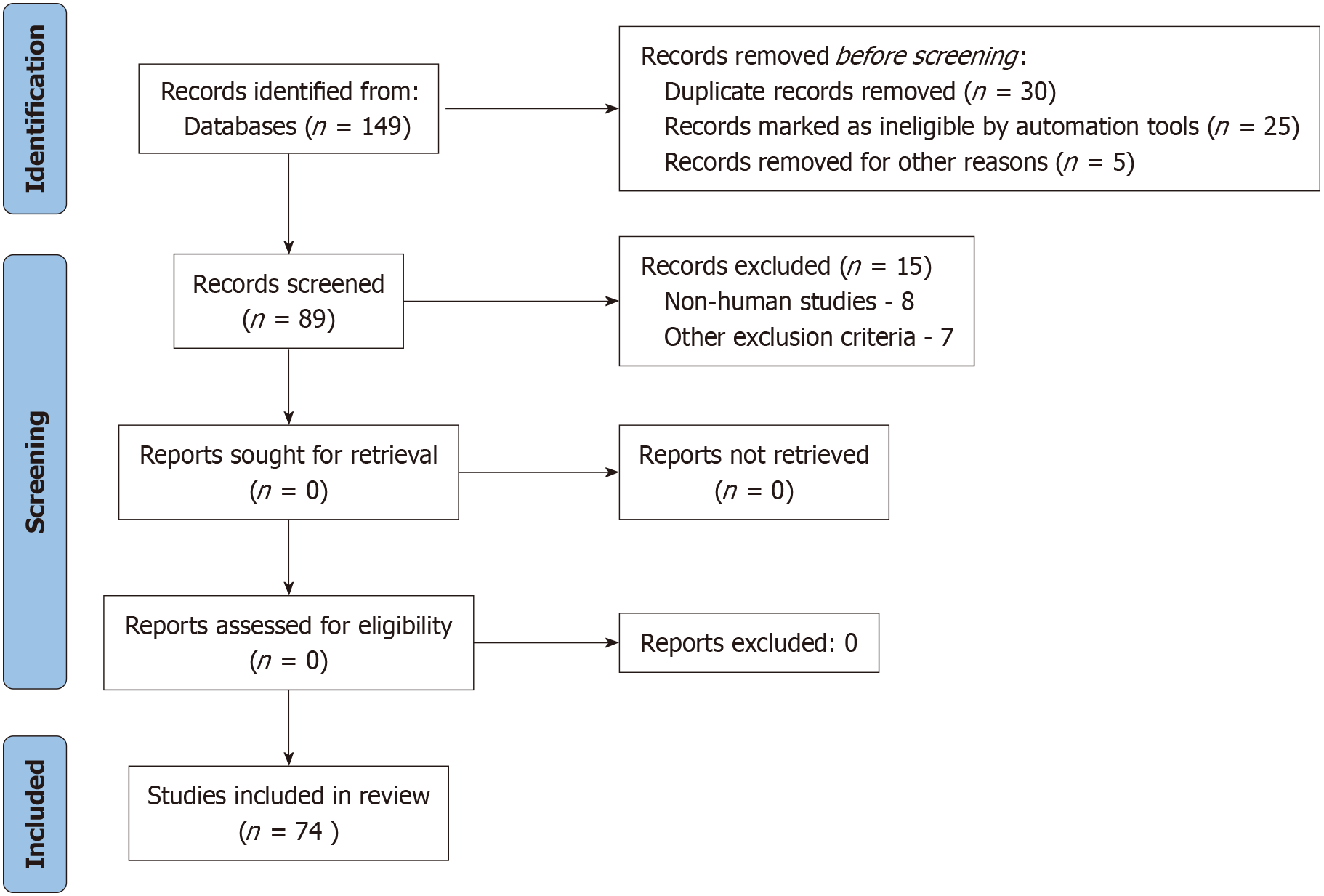
The risk of bias was assessed using the Newcastle-Ottawa Scale for observational studies and the Cochrane Risk of Bias Tool for clinical trials. Studies were categorized as having low, moderate, or high risk of bias based on these assessments. Given the heterogeneity in study designs, patient populations, and analytical methodologies, a qualitative synthesis of the findings was conducted. Where possible, summary statistics such as sensitivity, specificity, and hazard ratios were reported. Total of 149 articles were sorted out during the search. However, after exclusion of non-relevant articles, a total of 74 articles were included in this review. The PRISMA flow chart is depicted in Figure 1.
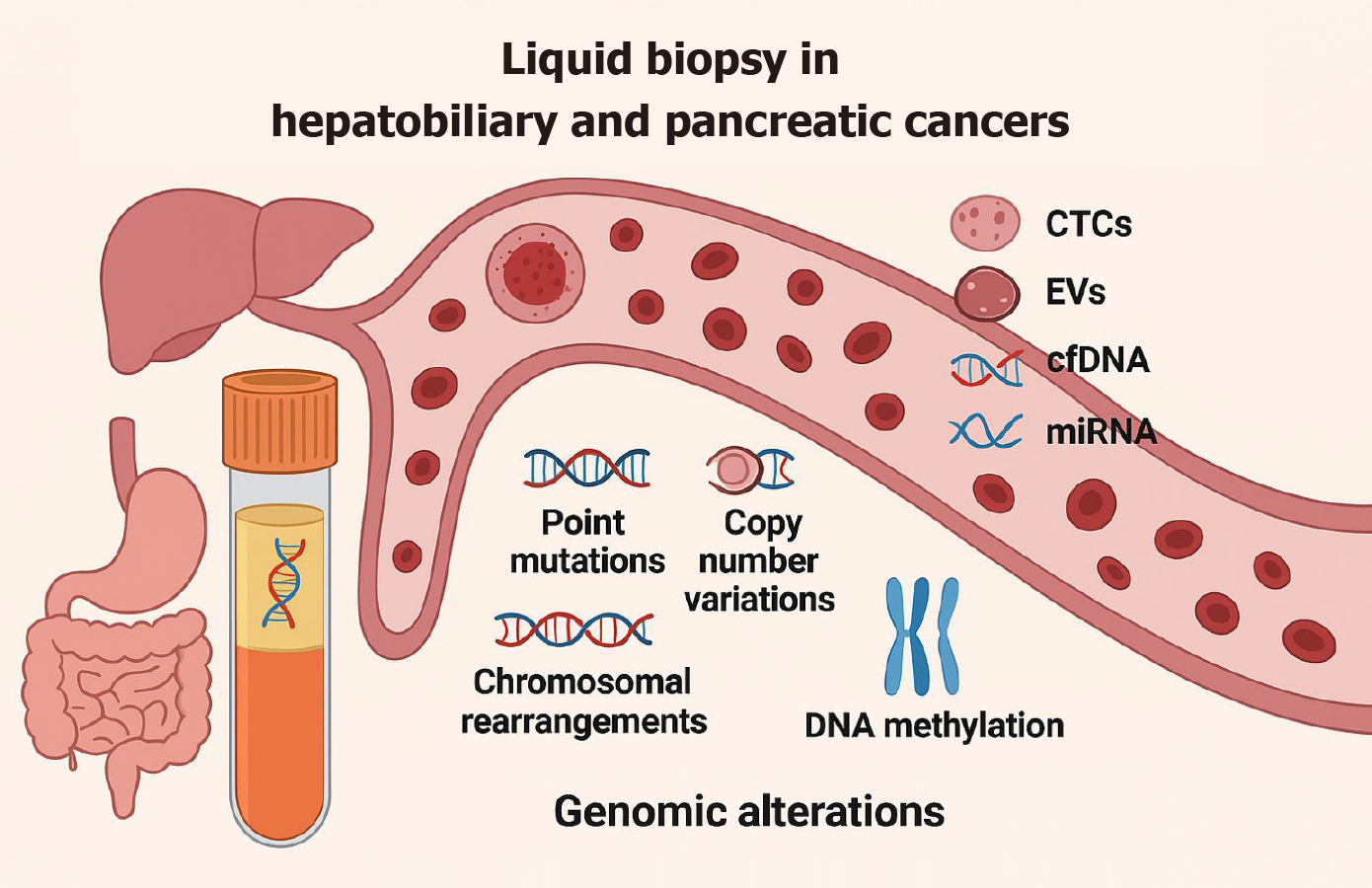
CTC represent intact cancer cells that have detached from the primary tumour or metastatic sites and entered the bloodstream, potentially serving as seeds for distant metastasis. These cells can be isolated and analysed to provide information about tumour characteristics, including genetic mutations, protein expression, and potential drug targets. Liquid biopsy refers to the analysis of these tumour-derived components in body fluids as a less invasive alternative to traditional tissue biopsy for obtaining genetic and epigenetic information relevant to oncogenesis and cancer progression. Tumour-derived components, such as exosomes, microRNAs, ctDNA, and circulating free DNA (cfDNA), as well as extracellular vesicles (EVs) and protein markers found in blood or other biological fluids are analysed in liquid biopsy[4] (Figure 2). Each of these markers provides a unique and complementary information about the underlying malignancy. ctDNA and carries tumour-specific genetic and epigenetic alterations. EVs can carry proteins, lipids, and nucleic acids reflecting the molecular characteristics of their cells of origin. EVs have mainly been explored as a liquid biopsy tool specifically for biliary cancer screening and hepatobiliary cancer differentiation. These approaches have gained significant attention in recent years due to its potential to overcome the limitations of conventional tissue biopsy, including invasiveness, sampling bias, and inability to capture spatial and temporal tumour heterogeneity[5].
Liquid biopsy offers a real time assessment of tumour’s molecular profile. This can prove to be critical for both early cancer diagnosis and assessing tumour response to various treatment modalities. It has stood out to be an effective tool in assessing the tumour’s susceptibility to genetic alterations, tumour burden and its ability to identify tumour heterogeneity and early signs of relapse[6].
cfDNA are analyzed through spectrophotometry by using specific primers such as ALU247 and ALU115 to determine the ratio of long to short DNA fragments[7]. These help in concentration measurement and assessment of DNA integrity. Detection of specific genetic alterations in ctDNA requires more sensitive approaches which includes high-resolution melting following real-time polymerase chain reaction (PCR) magnification combined with parallel internal controls. This approach has been successfully employed to identify mutations such as PIK3CA in various cancer types[8]. For circulating tumor cell detection, specialized techniques have been developed, including the identification of folate receptor-positive CTCs using platforms such as the CytoploRare® Detection Kit (GenoSaber Biotech Co., Ltd., Shanghai, China), which has shown promising results specifically in hepatobiliary- pancreatic cancers. Protein biomarker analysis typically relies on conventional serum immunoassays, but newer multiplex approaches allow for the simultaneous evaluation of multiple protein markers. Highly sensitive biomarker analyses using platforms like Olink® and LEGENDplexTM using Proximity Extension Assay have identified significant differences in proteins involved in chemotaxis, migration of immune cells, and cell growth in the serum of patients with early vs late onset of liver metastasis in pancreatic cancer[2].
The selection of appropriate methodological approaches depends on the specific clinical question being addressed, with consideration of factors such as sensitivity, specificity, cost, turnaround time, and availability. Standardization of these methodologies represents an ongoing challenge, with efforts focused on establishing reproducible protocols that can be implemented across different clinical settings. The technical complexity of liquid biopsy analysis necessitates specialized expertise and infrastructure, which may currently limit its widespread adoption outside of academic or specialized centers. Despite these challenges, continuing technological advancements are progressively improving the sensitivity and specificity of liquid biopsy approaches, enhancing their potential for clinical application in hepatobiliary and pancreatic cancers.
Early cancer detection: Liquid biopsies are pivotal in this respect, especially in hepatobiliary and pancreatic malignancies, where early-stage diagnosis remains extremely challenging. Indeed, ctDNA, as well as other biomarkers, can be detected in the blood long before clinical symptoms are present, allowing for earlier intervention and improved survival. The ability of liquid biopsy to detect minimum residual disease (MRD), or the rare residual cancer cells that manage to survive therapy and may might cause recurrence, is among its most promising features. Liquid biopsy has shown high sensitivity for the detection of MRD, even in patients classified as being in remission based on conventional imaging and clinical assessment[9]. Additionally, liquid biopsy allows the dynamic monitoring of tumour heterogeneity and genetic mutations, which are important for monitoring disease progression and response to ongoing therapies[10].
In a multicentre observational study by He et al[2] exploring the clinical significance of folate receptor-positive CTCs in hepatobiliary-pancreatic cancers, researchers determined that with 8.65 FU/3 mL as the cut-off value, CTC analysis demonstrated impressive sensitivity and specificity of 98.1% and 79.1%, respectively, for differential diagnosis. This performance exceeded the detection capabilities of conventional serum biomarkers, with folate receptor CTC detection rates superior to CA19-9, CA125, and CEA. These findings suggest that liquid biopsy approaches may significantly enhance the diagnostic accuracy for hepatobiliary and pancreatic cancers, potentially enabling earlier intervention. He et al[2] also investigated the clinical significance of FR+CTC in hepatobiliary-pancreatic cancers (HBPs). The study found that FR+CTC analysis had high sensitivity and specificity in differentiating HBPs from healthy individuals, outperforming conventional serum biomarkers.
Adamo et al[11] explored the potential of cfDNA in profiling tumor heterogeneity in pancreatic cancer patients. The study demonstrated that sequencing cfDNA could identify key mutations, including KRAS, offering insights into PDAC's genomic profile. While KRAS mutations were associated with poor survival, cfDNA's role in early detection remains inconclusive, necessitating further research.
Baba et al[12] developed a non-invasive urinary miRNA-based assay for detecting pancreatic cancer at various stages. This method demonstrated high sensitivity and specificity, especially in early-stage detection, outperforming CA19-9. The assay reflects miRNA patterns from both the tumor and its microenvironment, offering potential for broader use in early detection and screening, particularly in underserved areas.
Chen et al[13] developed the PreCar Score, a cell-free DNA-based tool for early HCC detection in liver cirrhosis patients. The PreCar Score demonstrated higher sensitivity for detecting early-stage HCC compared to ultrasound alone, and its combination with ultrasound improved screening performance, offering a promising strategy for routine HCC surveillance.
Chen et al[14] explored the diagnostic potential of plasma cell-free RNAs (cfRNAs), derived from both humans and microbes, for cancer classification. By profiling cfRNAs in various cancers, the study found that both human and microbe-derived cfRNAs could distinguish cancer patients from healthy donors, with microbial features improving the classification accuracy. This research supports the clinical relevance of cfRNAs in cancer detection and tumor site identification.
Cohen et al[15] developed a noninvasive blood test combining ctDNA and protein biomarkers for early detection of pancreatic cancer. The study found that this combination improved sensitivity while maintaining high specificity, detecting nearly two-thirds of resectable pancreatic cancers without distant metastasis. This approach may offer broader applications for early cancer detection.
Espejo-Cruz et al[1] critically reviewed the role of CTCs in HCC, emphasizing their potential as biomarkers for diagnosis and therapeutic monitoring. While CTCs showed promise, technical challenges remained, including optimal surface marker identification and efficient ex-vivo expansion.
Gongye et al[16] explored the potential of EVs in the early diagnosis of hepatobiliary and pancreatic tumors through multi-omics analysis. Thay concluded that EVs, due to their role as carriers of active substances, are increasingly recognized for their contribution to tumor progression and highlighted the diagnostic potential of EVs.
Guo et al[17] developed HepaAiQ, a non-invasive blood-based assay that detects HCC using differentially methylated regions in circulating tumor DNA. The assay demonstrated high sensitivity and specificity for diagnosing HCC, with strong prognostic value, correlating higher postoperative scores with increased recurrence risk. This model offered a promising tool for early diagnosis and prognosis evaluation of HCC in at-risk populations.
Han et al[18] investigated the potential of bile as a liquid biopsy fluid for detecting ctDNA in biliary tract cancer (BTC) patients. The study found that KRAS mutations in bile ctDNA were significantly associated with poorer survival outcomes. Bile ctDNA showed high concordance with tissue samples, suggesting bile as an effective alternative for ctDNA analysis in BTC diagnosis and prognosis.
Hao et al[19] reviewed the clinical application of liquid biopsy for early screening and recurrence prediction in HCC. Despite challenges in current screening methods and recurrence prediction, liquid biopsy offered a promising, non-invasive alternative for early detection and monitoring. The article highlighted the potential of liquid biopsy to complement traditional approaches, improving diagnosis and treatment outcomes for HCC patients.
He et al[3] developed BileScreen, a sensitive detection method for pancreatobiliary tract cancer based on mutations and methylation changes in bile samples. The assay demonstrated high sensitivity and specificity in multiple cohorts, outperforming serum CA19-9, particularly in terms of specificity. BileScreen offered a promising, less-invasive alternative for diagnosing pancreatobiliary tract cancer, improving early detection and reducing unnecessary treatments.
Hong et al[20] reviewed the potential of EV proteins as biomarkers for pancreatic ductal adenocarcinoma. EVs, due to their minimal invasiveness and easy sampling, were highlighted as promising tools for early PDAC detection and prognostication. The review emphasized the role of EV proteins in cancer progression and diagnostic applications.
Hu et al[21] reviewed emerging biomolecules for the diagnosis of hepatocellular carcinoma. The study emphasized the potential of secretory proteins, DNA methylation, miRNAs, and genome sequencing for early-stage detection. It also highlighted the promise of combining molecular biomarkers with advanced imaging and clinical indices, supported by deep machine learning and multiplex immunohistochemistry, to improve HCC diagnosis and treatment.
Hu et al[22] reviewed the potential of liquid biopsy using cfDNA and ctDNA for the early diagnosis of HCC. Despite the use of traditional screening methods like ultrasound, computed tomography, and AFP, the low sensitivity of these approaches makes early detection challenging. The review highlights recent advancements in cfDNA-based liquid biopsy as a promising method for improving HCC early screening and diagnosis.
Kim et al[23] explored the potential of combining tumor-specific miRNA signatures with CA19-9 for liquid biopsy-based detection of PDAC. The study identified five miRNAs in extracellular vesicles from PDAC patients, which, when combined with CA19-9, showed high sensitivity and specificity for early PDAC diagnosis, offering a promising non-invasive biomarker approach.
Koo et al[24] introduced a biocomposite-based rapid sampling platform for isolating cell-free nucleic acids from liquid biopsy samples. By combining amine-modified diatomaceous earth and cucurbituril, the platform achieved high capture efficiency and yielded high-purity cfDNAs from cancer patients, offering a simple and efficient method for cancer diagnosis and treatment monitoring.
Lapitz et al[25] identified protein biomarkers in EVs for the early diagnosis, risk prediction, and prognostication of cholangiocarcinoma (CCA). High-throughput proteomics and machine learning algorithms revealed biomarkers for differentiating CCA subtypes and predicting CCA development in high-risk patients. These biomarkers, detectable in total serum, offer a promising liquid biopsy tool for personalized medicine in CCA management.
Li et al[26] reviewed the clinical applications of liquid biopsy, specifically CTCs and ctDNA, in HCC. Given the challenges in early diagnosis and treatment resistance, liquid biopsy offers a promising non-invasive method for early detection, monitoring progression, and guiding individualized treatment strategies for HCC patients.
Li et al[27] discovered a novel piRNA, piR-162725, associated with PIWIL3, which significantly enhances the sensitivity of CA19-9 for identifying pancreatic cancer in liquid biopsies. This piRNA, identified through next-generation sequencing in pancreatic cancer samples, holds promise as a non-invasive biomarker for early detection, potentially improving prognosis and management of pancreatic cancer.
Liu et al[28] introduced a novel cfDNA analysis method, SLHC-seq, which enhances detection of pancreatic cancer by targeting short mutant cfDNA fragments. This approach improved the sensitivity of KRAS mutation detection, identifying mutations in 70% of patients. The study demonstrated that small mutant fragments are prevalent in early-stage pancreatic cancer, offering new insights for liquid biopsy applications in early cancer detection.
Luo et al[29] reviewed the role of CTCs in pancreatic cancer prognosis and treatment. CTCs, as a non-invasive liquid biopsy technique, have shown potential for early detection, real-time monitoring, and assessing treatment response. The review emphasized the need for personalized assays specific to tumor types, as well as the promising future of CTCs in clinical applications, despite challenges in standardization and validation.
Miura et al[30] evaluated the efficacy of bile liquid biopsy in diagnosing biliary tract cancer, finding that genomic analysis significantly improved the sensitivity of diagnosis compared to bile cytology alone. The study also identified therapeutic mutations in a subset of malignant patients, suggesting that bile liquid biopsy could aid in both diagnosis and treatment decision-making.
Nakamura et al[31] developed an exosome-based transcriptomic signature for the early, non-invasive detection of PDAC. The study identified a panel of 13 miRNAs that successfully discriminated PDAC patients from controls, including those with early-stage disease. When combined with CA19-9, the signature significantly improved diagnostic accuracy, offering a promising tool for early PDAC detection.
Ning et al[32] conducted a comprehensive evaluation of cfRNAs for HCC detection, identifying cfRNA fragments as key biomarkers for early-stage detection. The study developed the HCCMDP panel, which combined six cfRNA markers and AFP, achieving high diagnostic accuracy across multiple cohorts, including early-stage HCC patients.
Qu et al[33] developed the HCCscreen liquid biopsy assay to detect early-stage HCC in asymptomatic HBsAg-positive individuals. The assay demonstrated high sensitivity and specificity in a training cohort and identified early-stage HCC in a validation cohort with normal liver ultrasonography and serum AFP levels. This study supports the feasibility of using combined cfDNA alterations and protein markers for early detection of HCC in asymptomatic populations.
Sok et al[34] reviewed the role of molecular pathology and protein biomarkers in PDAC diagnosis, treatment, and surveillance. While CA19-9 remained the most widely used biomarker, its limited sensitivity and specificity highlighted the need for new biomarkers. The review discusses emerging protein markers that could enhance early-stage detection, predict therapy response, and monitor recurrence.
Urban et al[35] explored the use of EV phenotyping as a liquid biopsy tool for differentiating hepatobiliary cancers, including biliary cancer and HCC. The study identified tumor-related antigen combinations in serum EVs that showed strong diagnostic performance. Combining EV phenotyping with serum AFP provided perfect differentiation between biliary cancer and HCC, offering a promising, minimally invasive method for cancer screening and diagnosis.
Xing et al[36] developed a proteomics-driven approach for the early diagnosis of HCC using a serum protein panel. The P4 panel (HABP2, CD163, AFP, and PIVKA-II) demonstrated high sensitivity and specificity, outperforming existing clinical methods. The panel also successfully predicted liver cirrhosis to HCC conversion and detected HCC months before imaging, highlighting its potential as a non-invasive liquid biopsy tool for early HCC diagnosis.
Xiong et al[37] identified a novel panel of somatic mutations in plasma cfDNA for diagnosing HCC. The study found that these mutations, detected in the plasma cfDNA of HCC patients, demonstrated high diagnostic performance with 100% specificity and an area under the curve (AUC) of 0.92. Combining these mutations with AFP improved diagnostic accuracy, especially in AFP-negative patients. The study suggests that this cfDNA mutation panel could serve as a promising non-invasive diagnostic tool for HCC.
Xu et al[38] developed a circulating circular RNA (circRNA) biomarker panel for the non-invasive and early detection of PDAC. The panel, consisting of five circRNA candidates, was effective in identifying PDAC patients, distinguishing early-stage from late-stage disease, and improving diagnostic performance when combined with CA19-9 levels. The panel showed high potential in identifying clinically CA19-9-negative patients, offering a promising approach for early PDAC diagnosis.
Yadav et al[39] reviewed the potential of liquid biopsy in pancreatic cancer diagnosis and treatment, highlighting its role in monitoring genetic mutations and epigenetic alterations. Liquid biopsy, coupled with genomic tests, offers a minimally invasive method to detect early-stage pancreatic cancer and track treatment progress. The article discusses the potential of liquid biopsy in creating circulating tumor cell-derived xenografts, 3D organoid systems, and real-time mutation monitoring using circulating tumor DNA and exosomes.
Yan et al[40] reviewed advances in the use of liquid biopsy for the early detection of pancreatic cancer, a malignancy often diagnosed at advanced stages due to the lack of symptoms. The review highlights the potential of liquid biopsy as a unique diagnostic tool that could enable earlier tumor detection, which is crucial for improving survival rates in pancreatic cancer patients.
Ye et al[41] reviewed the clinical applications of CTCs and ctDNA in HCC, emphasizing their role in non-invasive early detection and monitoring of recurrence. Liquid biopsy, as a less invasive alternative to tissue biopsy, enables repeated analyses to track tumor progression, metastasis, and treatment responses in real-time. The review highlights the promising results of CTC and ctDNA detection techniques, offering potential for improved diagnosis and personalized treatment strategies for HCC patients.
Yi et al[42] developed a novel liquid biopsy approach using a SiO2-chip to enhance exosome enrichment for the early detection and monitoring of HCC. The study identified two HCC-related long non-coding RNAs, LUCAT-1 and EGFR-AS-1, which, when combined with traditional markers like AFP and DCP, significantly improved diagnostic accuracy and monitored disease progression. This method offers a non-invasive and reliable alternative for HCC detection and prognosis monitoring.
Yi et al[43] developed a tidal microfluidic chip-based method for the isolation and profiling of EVs in plasma to monitor high-risk patients for HCC and its precursors. The study identified HMMR and B4GALT2 as potential HCC biomarkers, demonstrating that bi-mRNAs in EVs outperform AFP, especially for early-stage and AFP-negative cases. This approach enhances early HCC detection and complements existing imaging techniques, offering a promising non-invasive tool for improved surveillance and diagnosis of high-risk individuals, such as those with chronic hepatitis B and liver cirrhosis.
Yokose et al[44] identified O-glycan-altered EVs as specific serum markers elevated in pancreatic cancer (PC). Using lectin microarray and ExoCounter technology, the study found that EVs recognized by O-glycan-binding lectins ABA and ACA were significantly increased in the serum of PC patients, including those negative for CA19-9. These elevated levels of O-glycan-altered EVs returned to normal after pancreatectomy, and histological examination confirmed their presence in tumor tissues. This research suggests that O-glycan-altered EVs could serve as potential biomarkers for early-stage PC detection through liquid biopsy.
Zhao et al[45] developed a cfDNA-based liquid biopsy assay for early diagnosis of PDAC. The study identified cancer-specific cfDNA methylation alterations and created a diagnostic model using six methylation markers, which showed high sensitivity (88.7%) and specificity (96.8%), outperforming mutation-based models. Combining this methylation-based model with CA19-9 further improved diagnostic accuracy (sensitivity: 95.7%; specificity: 93.3%), indicating that cfDNA methylation profiling and integrated assays are promising non-invasive tools for early PDAC detection.
Beyond initial diagnosis, liquid biopsy may assist in more accurate staging by detecting occult metastatic disease not visible on conventional imaging. This application is particularly relevant for pancreatic cancer, where early liver metastases significantly impact prognosis and treatment decisions. Research has identified significant differences in serum protein profiles between patients with early vs late onset of liver metastasis in pancreatic cancer, with differentially expressed proteins involved in processes such as chemotaxis, immune cell migration, and cell growth[46]. The findings of this study suggest potential for developing biomarker panels that could identify patients at higher risk for early metastatic spread, potentially influencing surgical decision- making and perioperative treatment planning.
Boons et al[47] reported the detection of tumor-specific mutations and copy number variations (CNVs) in cfDNA from metastatic pancreatic neuroendocrine tumor (PNET) patients. The study highlighted that cfDNA analysis can track tumor progression, with changes in variant allele fractions and CNV profiles correlating with increasing tumor burden, suggesting cfDNA's potential as a liquid biopsy for metastatic PNET.
Hata et al[48] demonstrated that ctDNA is an effective predictor of occult metastases in pancreatic cancer patients with radiographically non-metastatic disease. The study found a higher prevalence of ctDNA in patients with occult metastases compared to those without, suggesting its potential as a diagnostic and prognostic marker for early detection and recurrence prediction in pancreatic cancer.
Hu et al[49] reviewed the potential of tumor-educated platelet RNA and cfRNA as liquid biopsy markers for various cancers. These biomarkers, due to their non-invasive nature, offer significant advantages in early diagnosis, monitoring tumor progression, and assessing drug resistance.
Mehdorn et al[46] investigated serum biomarkers for predicting early liver metastases in PDAC patients. The study found that Olink® analysis identified significant differences in inflammatory mediators between patients with early and late hepatic metastasis, providing a potential biomarker panel for predicting liver metastasis.
Umemoto et al[50] examined the clinical significance of ctDNA analysis in PDAC, focusing on KRAS mutations across different metastatic sites. The study found that KRAS mutations were more prevalent and had higher variant allele frequencies in patients with liver metastasis compared to those with metastasis to other organs. The findings highlight the potential utility of ctDNA analysis for assessing PDAC with liver metastases, offering a non-invasive method for monitoring disease progression (Table 1).
| No. | Title of study | Ref. | Conclusion of study |
| 1 | A preliminary study of the clinical significance of folate receptor-positive CTC in the management of hepatobiliary-pancreatic cancers | He et al[2], 2020 | Suggests that folate receptor-positive CTCs could serve as a potential biomarker for diagnosing and monitoring hepatobiliary-pancreatic cancers |
| 2 | Profiling tumor heterogeneity through circulating tumor DNA in patients with pancreatic cancer | Adamo et al[11], 2017 | Suggests that circulating tumor DNA (ctDNA) analysis can reveal tumor heterogeneity in pancreatic cancer, aiding in personalized treatment strategies |
| 3 | A noninvasive urinary microRNA-based assay for the detection of pancreatic cancer from early to late stages: A case control study | Baba et al[12], 2024 | Demonstrates that a urinary microRNA-based assay can detect pancreatic cancer across different stages, offering a noninvasive diagnostic tool |
| 4 | Cell-free DNA testing for early HCC surveillance | Chen et al[13], 2024 | Highlights the potential of cell-free DNA testing for early HCC detection, suggesting its role in surveillance programs |
| 5 | Cancer type classification using plasma cell-free RNAs derived from human and microbes | Chen et al[14], 2022 | Shows that plasma-derived cell-free RNAs from both human and microbial sources can aid in cancer type classification |
| 6 | Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers | Cohen et al[15], 2017 | Suggests that integrating ctDNA and protein biomarkers improves early detection of pancreatic cancer |
| 7 | CTCs in HCC: A comprehensive review and critical appraisal | Espejo-Cruz et al[1], 2021 | Discusses the role of CTCs in HCC diagnosis, prognosis, and therapy. Highlights the need for further research on CTC detection techniques |
| 8 | Multi-omics analysis revealed the role of extracellular vesicles in hepatobiliary & pancreatic tumor | Gongye et al[16], 2022 | Identifies extracellular vesicles as key players in hepatobiliary and pancreatic cancer progression using multi-omics analysis |
| 9 | Early detection and prognosis evaluation for hepatocellular carcinoma by circulating tumor DNA methylation: A multicenter cohort study | Guo et al[17], 2024 | Supports ctDNA methylation analysis as a promising tool for early HCC detection and prognosis evaluation |
| 10 | Liquid biopsy from bile-circulating tumor DNA in patients with biliary tract cancer | Han et al[18], 2021 | Suggests that bile-based liquid biopsy can help in diagnosing and monitoring biliary tract cancer |
| 11 | Application of liquid biopsy in early screening and recurrence prediction of HCC | Hao et al[19], 2022 | Discusses the role of liquid biopsy in early detection and recurrence prediction in HCC patients |
| 12 | Molecular diagnosis of pancreatobiliary tract cancer by detecting mutations and methylation changes in bile samples | He et al[3], 2023 | Demonstrates the utility of bile-based molecular diagnosis for pancreatobiliary cancers, suggesting it could improve early detection and treatment |
| 13 | Deciphering extracellular vesicles protein cargo in pancreatic cancer | Hong et al[20], 2024 | Highlights the role of extracellular vesicle proteins in pancreatic cancer progression and their potential as biomarkers |
| 14 | Emerging biomolecules for practical theranostics of liver HCC | Hu et al[21], 2023 | Reviews novel biomolecules for theranostics in HCC, emphasizing their potential for diagnosis and targeted therapy |
| 15 | Liquid biopsy using cell-free DNA in the early diagnosis of HCC | Hu et al[22], 2023 | Supports cfDNA as a noninvasive approach for early HCC detection, highlighting its diagnostic accuracy |
| 16 | Tumor-Specific miRNA signatures in combination with CA19-9 for liquid biopsy-based detection of PDAC | Kim et al[23], 2021 | Suggests that miRNA signatures combined with CA19-9 improve PDAC detection using liquid biopsy |
| 17 | A biocomposite-based rapid sampling assay for circulating cell-free DNA in liquid biopsy samples from human cancers | Koo et al[24], 2020 | Introduces a novel biocomposite assay for efficient cfDNA isolation, enhancing liquid biopsy applications |
| 18 | Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma | Lapitz et al[25], 2023 | Identifies protein biomarkers from liquid biopsy for early detection and prognosis of cholangiocarcinoma |
| 19 | Clinical applications of liquid biopsy as prognostic and predictive biomarkers in HCC: CTC and circulating tumor DNA | Li et al[26], 2018 | Reviews the prognostic value of CTCs and ctDNA in HCC, highlighting their clinical applications |
| 20 | A novel PiRNA enhances CA19-9 sensitivity for pancreatic cancer identification by liquid biopsy | Li et al[27], 2022 | Suggests a novel piRNA that enhances CA19-9 sensitivity, improving pancreatic cancer detection via liquid biopsy |
| 21 | Enrichment of short mutant cell-free DNA fragments enhanced detection of pancreatic cancer | Liu et al[28], 2019 | Demonstrates that short mutant cfDNA fragments improve pancreatic cancer detection accuracy |
| 22 | The value of CTC in the prognosis and treatment of pancreatic cancer | Luo et al[29], 2022 | Highlights CTCs as potential prognostic and treatment monitoring biomarkers in pancreatic cancer |
| 23 | The efficacy of bile liquid biopsy in the diagnosis and treatment of biliary tract cancer | Miura et al[30], 2024 | Supports bile liquid biopsy as a promising tool for diagnosing and managing biliary tract cancer |
| 24 | An exosome-based transcriptomic signature for noninvasive, early detection of patients with pancreatic ductal adenocarcinoma: A multicenter cohort study | Nakamura et al[31], 2022 | Identifies an exosome-derived transcriptomic signature for noninvasive early detection of PDAC |
| 25 | A comprehensive evaluation of full-spectrum cell-free RNAs highlights cell-free RNA fragments for early-stage hepatocellular carcinoma detection | Ning et al[32], 2023 | Demonstrates that cfRNA fragments can improve early HCC detection, expanding the utility of liquid biopsy |
| 26 | Detection of early-stage HCC in asymptomatic HBsAg-seropositive individuals by liquid biopsy | Qu et al[33], 2019 | Supports liquid biopsy for early HCC detection in high-risk asymptomatic HBV-infected individuals |
| 27 | Molecular pathology and protein markers for pancreatic cancer: Relevance in staging, in adjuvant therapy, in determination of minimal residual disease, and follow-up | Sok et al[34], 2024 | Discusses molecular pathology and protein markers as key tools for pancreatic cancer staging and monitoring |
| 28 | Synergistic effects of extracellular vesicle phenotyping and AFP in hepatobiliary cancer differentiation | Urban et al[35], 2020 | Suggests that extracellular vesicle profiling combined with AFP improves hepatobiliary cancer differentiation |
| 29 | Proteomics-driven noninvasive screening of circulating serum protein panels for the early diagnosis of HCC | Xing et al[36], 2023 | Identifies serum protein panels as potential noninvasive biomarkers for early HCC diagnosis |
| 30 | Detection of a novel panel of somatic mutations in plasma cell-free DNA and its diagnostic value in HCC | Xiong et al[37], 2019 | Proposes a novel somatic mutation panel in cfDNA for improving HCC diagnosis |
| 31 | A Circulating panel of circRNA biomarkers for the noninvasive and early detection of pancreatic ductal adenocarcinoma | Xu et al[38], 2024 | Suggests circRNA panels as potential noninvasive biomarkers for early detection of PDAC |
| 32 | Liquid biopsy in pancreatic cancer: The beginning of a new era | Yadav et al[39], 2018 | Reviews the role of liquid biopsy in pancreatic cancer management, emphasizing its growing clinical significance |
| 33 | Advances in the detection of pancreatic cancer through liquid biopsy | Yan et al[40], 2021 | Summarizes advances in pancreatic cancer detection using liquid biopsy, highlighting key biomarkers and techniques |
| 34 | Liquid biopsy in HCC: CTC and circulating tumor DNA | Ye et al[41], 2019 | Reviews the roles of CTCs and ctDNA in HCC, emphasizing their diagnostic and prognostic potential |
| 35 | Transcriptomic signature of 3D hierarchical porous chip enriched exosomes for early detection and progression monitoring of HCC | Yi et al[42], 2024 | Suggests that exosome-based transcriptomic signatures can enable early detection and monitoring of HCC |
| 36 | Tidal microfluidic chip-based isolation and transcriptomic profiling of plasma extracellular vesicles for clinical monitoring of high-risk patients with hepatocellular carcinoma-associated precursors | Yi et al[43], 2025 | Introduces a novel microfluidic chip-based method for isolating extracellular vesicles, improving HCC monitoring |
| 37 | O-Glycan-Altered extracellular vesicles: A specific serum marker elevated in pancreatic cancer | Yokose et al[44], 2020 | Identifies O-glycan-altered extracellular vesicles as a specific serum biomarker for pancreatic cancer detection |
| 38 | Circulating cell-free DNA methylation-based multi-omics analysis allows early diagnosis of pancreatic ductal adenocarcinoma | Zhao et al[45], 2024 | Supports cfDNA methylation analysis as an effective early diagnostic tool for PDAC |
| 39 | Biomarkers in liquid biopsies for prediction of early liver metastases in pancreatic cancer | Mehdorn et al[46], 2022 | Suggests liquid biopsy biomarkers as predictors of early liver metastases in pancreatic cancer patients |
| 40 | Cell-free DNA from metastatic pancreatic neuroendocrine tumor patients contains tumor-specific mutations and copy number variations | Boons et al[47], 2018 | Finds tumor-specific mutations and CNVs in cfDNA of metastatic pancreatic neuroendocrine tumor patients, supporting its use in diagnosis and monitoring |
| 41 | Circulating tumor DNA as a predictive marker for occult metastases in pancreatic cancer patients with radiographically non-metastatic disease | Hata et al[48], 2021 | Suggests that ctDNA can serve as a predictive marker for occult metastases in pancreatic cancer |
| 42 | Tumor-educated platelet RNA and circulating free RNA: Emerging liquid biopsy markers for different tumor types | Hu et al[49], 2024 | Reviews tumor-educated platelet RNA and cfRNA as emerging biomarkers for various cancer types |
| 43 | Clinical significance of circulating-tumor DNA analysis by metastatic sites in pancreatic cancer | Umemoto et al[50], 2023 | Demonstrates that ctDNA analysis varies by metastatic site, offering insights into pancreatic cancer progression |
Liquid biopsy has many advantages in the preoperative setting in the assessment of molecular signatures and mutations in tumours. ctDNA testing in blood can identify individual genetic mutations that can be both unique to the tumour and can be associated with the aggressiveness and metastatic potential of the tumour[9].
Per-operative molecular profiling allows for a more holistic understanding of tumour biology and genetic predisposition, leading to key insights for individualized therapeutic approaches. For example, mutations in such genes as KRAS, TP53, or epidermal growth factor receptor (EGFR) may guide which targeted therapies or immunotherapies could provide a more suitable treatment plan[51].
Furthermore, liquid biopsy enables the evaluation of tumour heterogeneity, which is essential for studying the different populations of cells within a tumour and their potential for response or resistance to treatment[52].
Specifically for biliary tract cancers, several targetable alterations have been identified, including isocitrate dehydrogenase-1, fibroblast growth factor receptor-2 B-Raf proto-oncogene, and human epidermal growth factor receptor-2. Similarly, in pancreatic cancer, potential therapeutic targets include KRAS G12C mutations, neuregulin, and BRCA1/2 gene alterations[53].
Liu et al[54] reviewed the potential of bile liquid biopsy for biliary tract cancer, highlighting its role in understanding tumor biology and monitoring disease progression. Bile, due to its unique anatomical location, is a promising fluid for detecting biomarkers such as cells, extracellular vesicles, nucleic acids, proteins, and metabolites.
Martins et al[10] reviewed the applications of liquid biopsies in cancer diagnosis and monitoring, highlighting their potential to detect genomic and transcriptomic alterations in biofluids. Liquid biopsies offer advantages over tissue biopsies by providing a more comprehensive tumor profile, aiding in diagnosis, prognosis, and treatment selection. They also facilitate real-time monitoring of disease progression and treatment response, though challenges remain for their widespread clinical implementation.
Shen et al[55] investigated the potential of bile cfDNA as a liquid biopsy for detecting somatic variants in BTC. Using targeted deep sequencing, the study found high sensitivity and specificity for detecting SNVs and insertions/deletions (Indels), and moderate sensitivity for CNVs. The study highlights the potential of bile cfDNA, offering a non-invasive alternative to tissue biopsy for molecular characterization of BTC.
This information also can assist in devising the most effective and suitable preoperative treatment approaches, like neoadjuvant chemotherapy, which could downsize the tumour and increase the chances of a successful surgical resection (Table 2).
| No. | Title of study | Ref. | Conclusion of study |
| 1 | The growing role of precision and personalized medicine for cancer treatment | Krzyszczyk et al[51], 2018 | Highlights advancements in precision medicine and its role in tailoring cancer treatments based on genetic and molecular markers |
| 2 | CTC as liquid biomarker for high HCC recurrence risk after curative liver resection | von Felden et al[52], 2017 | Suggests that CTCs can serve as a prognostic biomarker for high recurrence risk in HCC patients after liver resection |
| 3 | Precision medicine of hepatobiliary and pancreatic cancers: Focusing on clinical trial outcomes | Tsumura et al[53], 2022 | Reviews the role of precision medicine in hepatobiliary and pancreatic cancers, emphasizing clinical trial findings |
| 4 | Bile liquid biopsy in biliary tract cancer | Liu et al[54], 2023 | Supports the use of bile liquid biopsy for diagnosing and monitoring biliary tract cancer |
| 5 | Bile cellfree DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer | Shen et al[55], 2019 | Demonstrates that bile cfDNA can detect somatic mutations, aiding in the diagnosis of biliary tract cancer |
The ability of liquid biopsy to provide prognostic information represents a significant advancement in perioperative risk stratification for hepatobiliary and pancreatic cancer patients. Studies examining the perioperative dynamics of cell-free DNA and circulating tumour DNA have demonstrated their potential to predict outcomes and stratify patients according to recurrence risk. Research in breast cancer has shown that both preoperative and postoperative cfDNA concentrations and DNA integrity were significantly higher in patients who developed recurrence compared to those who remained disease-free. While these findings come from breast cancer studies, they suggest a methodology that may be applicable to hepatobiliary and pancreatic cancers, where similar perioperative monitoring of cfDNA dynamics could help identify high-risk patients who might benefit from more intensive surveillance or adjuvant therapy[7].
Li et al[56] investigated the use of exosomal DNA (exoDNA) for liquid biopsy in HCC patients, specifically detecting TP53 mutations via droplet digital PCR. The study found that high-frequency mutations in exoDNA were associated with shorter recurrence-free survival (RFS) and poor prognosis, suggesting that exoDNA mutations can serve as prognostic biomarkers for HCC.
Pelizzaro et al[57] reviewed the potential of liquid biopsy in HCC, highlighting its promise for early diagnosis, prognostic stratification, and therapeutic monitoring. Unlike traditional biomarkers like AFP, which have limited accuracy, liquid biopsy offers a non-invasive alternative by analyzing cancer-related molecular by-products in the bloodstream. The review discusses various biomarkers, including extracellular vesicles, circulating tumor cells, cell-free DNA, and non-coding RNA, and suggests that these could significantly improve the clinical management of HCC patients in the near future.
Qi et al[58] investigated the significance of CTCs undergoing epithelial-mesenchymal transition (EMT) in HCC. The study found that high CTC count and mesenchymal-CTC (M-CTC) percentage before resection were associated with early recurrence and metastasis.
Qiao et al[59] conducted a meta-analysis to evaluate the diagnostic and prognostic value of circulating exosomal glypican-1 (GPC-1) in pancreatic cancer. The analysis showed high sensitivity and specificity for GPC-1 in diagnosing pancreatic cancer (AUC = 0.93) and found that elevated GPC-1 Levels were associated with poorer overall survival (hazard ratio = 4.59). The study suggests that GPC-1 could be a valuable biomarker for both diagnosis and prognosis.
Wang et al[60] reviewed the clinical significance of small extracellular vesicles (sEVs) in cholangiocarcinoma, an aggressive cancer with poor prognosis. The review highlighted the role of sEVs in tumor proliferation, invasion, and metastasis. Due to their unique properties, sEVs have emerged as promising biomarkers for diagnosing and predicting outcomes in cholangiocarcinoma. Additionally, their potential as drug carriers and in targeted therapies for this cancer type was discussed, suggesting a new approach for both diagnosis and treatment.
Wang et al[61] reviewed the role of liquid biopsy in PC, focusing on its applications in diagnosis, monitoring, and prognosis evaluation. Liquid biopsy techniques, including the analysis of CTCs, ctDNA, noncoding RNAs, and EVs, aids in early diagnosis, treatment monitoring, and assessing chemotherapy resistance, thereby supporting more personalized and precise treatment decisions for PC patients.
Zhang et al[62] reviewed biomarkers and prognostic factors for PD-1/PD-L1 inhibitor-based therapy in advanced HCC. Key factors include PD-L1 expression, tumor-infiltrating lymphocytes, and preclinical markers like dendritic cells and macrophages. Genomic characteristics, tumor mutational burden, and signaling pathways (e.g., WNT/β-catenin) were found to correlate with prognosis.
Zhao et al[63] demonstrated that CTCs, particularly proliferative CTCs (PCP) and CTC clusters, can predict aggressiveness and early recurrence in HCC patients. Their study showed that CTC number, PCP, and CTC clusters correlated with tumor characteristics like microvascular invasion and tumor size. These markers were more effective than AFP in predicting recurrence, with high sensitivity and specificity in both training and validation sets. A nomogram model incorporating these factors had strong predictive ability (AUC = 0.947), highlighting their potential to enhance HCC prognosis monitoring (Table 3).
| No. | Title of study | Ref. | Conclusion of study |
| 1 | TP53 mutation detected in circulating exosomal DNA is associated with prognosis of patients with hepatocellular carcinoma | Li et al[56], 2022 | Identifies TP53 mutation in exosomal DNA as a prognostic marker for HCC |
| 2 | Liquid Biopsy in Hepatocellular Carcinoma: Where Are We Now? | Pelizzaro et al[57], 2021 | Reviews current advancements and challenges in liquid biopsy for HCC |
| 3 | Circulating Tumor Cells Undergoing EMT Provide a Metric for Diagnosis and Prognosis of Patients with Hepatocellular Carcinoma | Qi et al[58], 2018 | Shows that EMT markers in CTCs can aid in HCC diagnosis and prognosis |
| 4 | Diagnostic and prognostic value of circulating exosomal glypican-1 in pancreatic cancer: A meta-analysis | Qiao et al[59], 2024 | Supports exosomal glypican-1 as a valuable biomarker for pancreatic cancer detection and prognosis |
| 5 | Clinical significance of small extracellular vesicles in cholangiocarcinoma | Wang et al[60], 2024 | Highlights the role of small extracellular vesicles as potential biomarkers for cholangiocarcinoma |
| 6 | Liquid biopsy techniques and pancreatic cancer: Diagnosis, monitoring, and evaluation | Wang et al[61], 2023 | Reviews liquid biopsy techniques for pancreatic cancer diagnosis, treatment monitoring, and prognosis evaluation |
| 7 | Biomarkers and prognostic factors of PD-1/PD-L1 inhibitor-based therapy in patients with advanced hepatocellular carcinoma | Zhang et al[62], 2024 | Identifies biomarkers and prognostic factors influencing response to PD-1/PD-L1 inhibitors in advanced HCC |
| 8 | Tumor-derived proliferative CTCs and CTC clusters predict aggressiveness and early recurrence in hepatocellular carcinoma patients | Zhao et al[63], 2023 | Suggests that proliferative CTCs and CTC clusters are predictive markers for aggressive HCC and early recurrence |
Liquid biopsy can identify genetic alterations associated with a poor prognosis in hepato-bio-pancreatic tumours, such as KRAS, P53 and EGFR mutations through the detection of ctDNA. These mutations largely dictate the appropriateness of certain targeted medicines. For example, based on the commonality of KRAS mutations seen in pancreatic cancer, KRAS inhibitors and other targeted medicines that utilize this mutation have been developed and can be used to tailor treatment plans. These mutations can be tracked over time so that therapies can be adjusted based on the evolving genetic landscape of the tumour[9].
Matsudera et al[8] conducted a pilot study (PROFILE) to evaluate the clinical utility of comprehensive genomic profiling using plasma cell-free DNA in Japanese solid tumor patients. The study found that liquid biopsy identified actionable mutations in the majority of patients, with biomarker-matched therapies leading to positive responses in some. Patients with higher matching scores showed a significantly better disease control rate, supporting the potential of liquid biopsy for precision cancer medicine.
Qi et al[64] reviewed the role of EVs in liver cancer, focusing on HCC and cholangiocarcinoma. EVs, by carrying proteins, RNA, DNA, lipids, and metabolites, play key roles in promoting tumor progression by supporting cancer cell proliferation, invasion, and metastasis. The review highlighted the potential of EVs as biomarkers for liver cancer diagnosis and prognosis, as well as their emerging role in EV-based therapeutics and antigen delivery in preclinical liver cancer studies.
Shu et al[65] explored the role of bile exosomal miR-182/183-5p in cholangiocarcinoma progression. The study found that high levels of miR-182/183-5p in cholangiocarcinoma tissues and bile were associated with poor prognosis. Bile exosomal miR-182/183-5p promoted cholangiocarcinoma stemness and progression by targeting hydroxyprostaglandin dehydrogenase, increasing prostaglandin E2 production, and activating PTGER1. Additionally, miR-182/183-5p induced mast cell-derived VEGF-A release, facilitating angiogenesis. These findings highlight a self-driven progression of cholangiocarcinoma influenced by bile exosomal miR-182/183-5p and its interaction with mast cells.
Wang et al[5] explored the correlation between genomic alterations in CTC DNA and mesenchymal CTCs or CTC-associated white blood cell (CTC-WBC) clusters in HCC. Using the CanPatrol™ system and next-generation sequencing (NGS), the study identified somatic mutations in PTEN, MET, EGFR, RET, and FGFR3. Mesenchymal CTC counts were associated with PTEN and MET mutations, while CTC-WBC clusters were linked to RET mutations. The findings highlight the potential of this method for monitoring tumor heterogeneity and guiding clinical applications in HCC.
Wang et al[66] developed a technology called mutation capsule plus (MCP) that enables simultaneous analysis of genetic mutations and methylation alterations in cfDNA for HCC detection. The study demonstrated that MCP could detect HCC with high sensitivity and specificity, both in HCC patients and in asymptomatic hepatitis B virus carriers at risk of developing HCC. The technology shows promise for non-invasive cancer detection through the identification of multiomics biomarkers, offering a comprehensive approach to monitoring HCC progression and risk.
Yin et al[67] conducted a study on bile cfDNA to explore the whole-genome mutation landscape and structural variations in cholangiocarcinoma. The analysis revealed TP53 and KRAS as the most frequently mutated genes, with significant mutations found in the RTK/RAS, homologous recombination (HR), and HIPPO pathways. Structural variants such as chromothripsis and kataegis were identified, and mutations in the HR pathway were associated with poorer overall survival, highlighting the potential of bile cfDNA as a prognostic tool for cholangiocarcinoma (Table 4).
| No. | Title of study | Ref. | Conclusion of study |
| 1 | The role and potential application of extracellular vesicles in liver cancer | Qi et al[64], 2021 | Highlights extracellular vesicles as potential biomarkers and therapeutic targets in liver cancer |
| 2 | Bile exosomal miR-182/183-5p increases cholangiocarcinoma stemness and progression by targeting HPGD and increasing PGE2 generation | Shu et al[65], 2024 | Demonstrates that bile exosomal miR-182/183-5p contributes to cholangiocarcinoma progression and stemness, making it a potential therapeutic target |
| 3 | Simultaneous analysis of mutations and methylations in circulating cell-free DNA for hepatocellular carcinoma detection | Wang et al[66], 2022 | Shows that simultaneous mutation and methylation analysis of cfDNA improves HCC detection |
| 4 | Identification of whole-genome mutations and structural variations of bile cell-free DNA in cholangiocarcinoma | Yin et al[67], 2024 | Suggests that whole-genome analysis of bile cfDNA can identify mutations and structural variations in cholangiocarcinoma |
Intraoperatively, liquid biopsy provides a solid platform for real-time molecular profiling to impact surgical decision making. Detection of circulating tumour-derived biomarkers in the blood during surgery means that surgeons have access to the real-time information, potentially impacting resection of the tumour and assessment of residual disease[66]. An example of this would be the use of liquid biopsy for the assessment of the molecular status of surgical margins, ensuring that the tumour is completely removed. Intraoperative liquid biopsy could also assist in determining resection extent, particularly in cases where conventional imaging techniques cannot fully assess the tumour, enabling surgeons to adjust their approach in accordance with the molecular profile of the tumour at that time.
Aguilar et al[6] reviewed the use of liquid biopsy to monitor MRD. ctDNA profiling was shown to detect MRD, which is predictive of recurrence. The study highlighted that ctDNA could identify actionable mutations, though its utility in guiding therapy escalation for improved outcomes remains uncertain.
Conci et al[68] reviewed the current role of immunotherapy and circulating factors in the treatment of BTCs. Immunotherapy, particularly immune checkpoint inhibitors, emerged as a promising treatment for specific molecular profiles, though resistance remained a challenge. Liquid biopsy approaches, including CTCs and ctDNA, showed potential for non-invasive monitoring and earlier diagnosis (Table 5).
| No. | Title of study | Ref. | Conclusion of study |
| 1 | Simultaneous analysis of mutations and methylations in circulating cell-free DNA for hepatocellular carcinoma detection | Wang et al[66], 2022 | Shows that simultaneous mutation and methylation analysis of cfDNA improves HCC detection |
| 2 | Liquid biopsy for monitoring minimal residual disease in localized and locally-advanced non-small cell lung cancer after radical-intent treatment | Aguilar et al[6], 2024 | Evaluates liquid biopsy for detecting minimal residual disease in lung cancer, supporting its role in post-treatment monitoring |
| 3 | Current role and future perspectives of immunotherapy and circulating factors in treatment of biliary tract cancers | Conci et al[68], 2023 | Reviews the role of immunotherapy and circulating biomarkers in the treatment of biliary tract cancers |
The dynamics of liquid biopsy markers following surgical intervention may provide valuable information regarding the effectiveness of the procedure and the presence of residual disease. ctDNA levels have been reported to increase weeks to months before clinical or radiographic evidence of recurrence is apparent, suggesting that this approach could enable earlier diagnosis of recurrence and can guide early intervention to potentially improve patient outcomes[13].
Since ctDNA can help give insight into MRD there is great potential for this to play a role in improving recurrence for many cancers, most notably pancreatic and hepatobiliary cancers[9]. The longitudinal monitoring of liquid biopsy markers during follow-up may enable earlier detection of recurrence and more timely intervention, potentially improving outcomes for patients with recurrent disease. In hepatobiliary and pancreatic cancers, where treatment options for recurrent disease are often limited, the window of opportunity for effective intervention may be narrow. Earlier detection through liquid biopsy could expand this window, potentially increasing the proportion of patients eligible for local therapies for oligometastatic recurrence or ensuring that systemic therapy is initiated before disease burden becomes overwhelming.
In addition, liquid biopsy enables clinicians to evaluate the impact of adjuvant therapies by tracking tumour-specific biomarkers that can change during and after drug treatment, therefore providing a dynamic assessment of therapeutic efficacy. That provides real-time insight so we can make adjustments to the therapy based on the real-time feedback.
Amado et al[69] investigated the clearance of CTCs in HCC patients after liver transplantation or surgical resection. The study found that incomplete CTC clearance within the first month post-surgery, particularly in patients with CTC clusters, was associated with reduced survival, suggesting that CTC clearance may serve as a marker for HCC aggressiveness.
In a study of patients with hepatobiliary-pancreatic cancers, FR CTC levels significantly decreased after surgical resection, suggesting the potential utility of this marker for monitoring treatment response[2]. He et al[2] investigated the clinical significance of folate receptor-positive circulating tumor cells (FR+CTC) in HBPs. Postoperative analysis showed a significant reduction in FR+CTC levels, highlighting its potential as a non-invasive biomarker for diagnosis and surveillance.
Hata et al[70] found that postoperative ctDNA was a significant prognostic marker for predicting recurrence in PDAC patients following surgical resection. Patients with detectable postoperative ctDNA showed worse disease-free survival and overall survival, highlighting its potential as a minimal residual disease marker for assessing recurrence risk.
Jin et al[71] reviewed the clinical use of CTCs as prognostic biomarkers. They concluded that CTCs, shed from tumors into the bloodstream, can provide valuable insights into cancer progression and treatment response. Recent technological advancements enable robust characterization of CTCs, offering real-time prognostic analysis and aiding in treatment optimization by identifying drug resistance and predicting therapeutic efficacy.
Liu et al[72] discussed the role of CTCs as both a model for cancer metastasis and non-invasive biomarkers for therapeutic response. The review highlighted advances in CTC isolation, ex vivo culture, and single-cell sequencing, which enable deeper insights into metastatic progression.
Pantel et al[4] reviewed the use of blood-based analysis of cfDNA and CTCs for early cancer detection. The study emphasized the potential of liquid biopsy in detecting minimal residual disease and monitoring therapeutic responses.
Qi et al[58] investigated the significance of CTCs undergoing EMT in HCC. The study found that postoperative CTC monitoring detected recurrence earlier than clinical signs. Additionally, BCAT1, identified as a key gene in EMT, was upregulated and linked to enhanced cell proliferation and migration, making it a potential biomarker for HCC metastasis and prognosis.
von Felden et al[52] investigated the role of EpCAM-positive circulating tumor cells as a liquid biomarker for predicting hepatocellular carcinoma recurrence after curative liver resection. The study found that CTC-positive patients had a significantly higher risk of recurrence, with a shorter RFS compared to CTC-negative patients. CTC detection prior to surgery could help identify high-risk patients who may benefit from adjuvant treatment, independent of factors like incomplete resection.
Wang et al[73] identified the role of differentiation antagonizing non-protein coding RNA (DANCR) in hepatitis C virus-related HCC recurrence. The study found that DANCR was upregulated in HCC tissues and that its expression in circulating exosomes was strongly associated with HCC recurrence and mortality after curative resection. Exosomal DANCR was the most predictive biomarker for long-term outcomes, suggesting its potential as a non-invasive prognostic marker for HCC recurrence.
Wang et al[74] identified a distinct immunogenic subgroup of intrahepatic cholangiocarcinoma (ICC) in Asian patients, characterized by T cell exhaustion and neutrophil extracellular traps. Elevated levels of exosomal circ-PTPN22 and circ-ADAMTS6 were found in both tumor tissues and plasma exosomes, marking this subgroup. These circRNAs, linked to immune dysregulation in the ICC microenvironment, suggest a potential liquid biopsy biomarker for early detection and a target for immune checkpoint blockade therapy.
While definitive evidence for the impact of liquid biopsy-guided surveillance on survival outcomes in hepatobiliary and pancreatic cancers is still emerging, the conceptual framework is supported by biological principles and encouraging preliminary data (Table 6).
| No. | Title of study | Ref. | Conclusion of study |
| 1 | Clearance of circulating tumor cells in patients with hepatocellular carcinoma undergoing surgical resection or liver transplantation | Amado et al[69], 2021 | Investigates CTC clearance post-surgery or liver transplantation, indicating its potential as a prognostic factor in HCC |
| 2 | Prognostic impact of postoperative circulating tumor DNA as a molecular minimal residual disease marker in patients with pancreatic cancer undergoing surgical resection | Hata et al[70], 2023 | Suggests that postoperative ctDNA can be used as a MRD marker to predict pancreatic cancer recurrence |
| 3 | Current progress in the clinical use of circulating tumor cells as prognostic biomarkers | Jin et al[71], 2019 | Reviews the clinical applications of CTCs as prognostic biomarkers across various cancers |
| 4 | CTCs: A unique model of cancer metastases and non-invasive biomarkers of therapeutic response | Liu et al[72], 2021 | Highlights the role of CTCs as biomarkers for monitoring treatment response and cancer metastasis |
| 5 | Exosome-derived differentiation antagonizing non-protein coding RNA with risk of HCV-related hepatocellular carcinoma recurrence | Wang et al[73], 2021 | Suggests that exosomal non-protein coding RNA is associated with HCV-related HCC recurrence risk |
| 6 | Exosomal circ-PTPN22 and circ-ADAMTS6 mark T cell exhaustion and neutrophil extracellular traps in Asian intrahepatic cholangiocarcinoma | Wang et al[74], 2023 | Identifies exosomal circ-PTPN22 and circ-ADAMTS6 as markers of immune exhaustion in intrahepatic cholangiocarcinoma |
Technical and analytical: While there is great promise for liquid biopsy, there are key technical and analytical challenges that must be addressed. Sensitivity and specificity of liquid biopsy tests are one of the major challenges. Depending on the patient's tumour burden or disease, sensitivity of these tests may vary. There is also the potential for false-negative results, especially in settings where ctDNA is present at low levels or is not detectable due to tumour heterogeneity[9]. Additionally, the unreliable nature of liquid biopsy is further compounded by variation in detection methods. Different laboratories may use different technologies like PCR-based methods, NGS, and/or digital droplet PCR, which can provide differing results. Such differences in methodology can lead to pitfalls with reproducibility and replicability within and across other settings.
Liquid biopsy lacks standardized protocols, which is another major hindrance to its widespread use. Due to an absence of consensus methods for sample collection, preparation, or analysis, results are challenging to compare across different studies and clinical settings. This limitation is unfortunately compounded by the fact that clinical interpretation of results lacks standardization, particularly in determination of clinically relevant thresholds for ctDNA or other biomarkers. Therefore, despite the progressive use of liquid biopsy in research, its clinical validation is still in the preliminary phase and not yet ready for universal adaptability as a routine diagnostic or monitoring tool[9]. Food and Drug Association (FDA) has not cleared majority of these assays for routine diagnostics in oncology[66]. Therefore, clinical validation of liquid biopsies is needed to find their way into routine clinical practice.
Despite their improved accessibility and lower invasiveness compared to standard tissue biopsies, liquid biopsies are still expensive, which makes routine testing and analysis prohibitively expensive for many healthcare systems specially in under-resourced countries. Specific equipment is needed for this technology, and even if the cost of genetic testing itself has decreased over time, a considerable economic investment is required for continued monitoring and repeated testing.
Additionally, liquid biopsy may be less accessible in some areas or healthcare settings, especially in developing nations where the infrastructure for such sophisticated diagnostic technologies is not adequate. This leads to inequity in the access and availability of these tests, which restricts the benefit to a larger patient population and introduces challenges to the adoption of liquid biopsy as a new standard of care.
Integration into clinical practice: Liquid biopsy is an emerging field, and its clinical implementation has great potential, especially in the management of hepatobiliary and pancreatic cancers. If liquid biopsy is to be better integrated into existing treatment protocols, then standardized, evidence-based guidelines that define when and how these technologies should be used across the cancer care continuum will be vital.
Technological innovations: It is expected that novel liquid biopsy technology and biomarker discovery will enhance the effectiveness of the tool in the management of cancer. Emerging techniques such as digital PCR, droplet digital PCR and NGS continue to increase the sensitivity and specificity of liquid biopsy assays. In addition, the discovery of new biomarkers and their association among specific cancer subtypes would expand the application of liquid biopsy, allowing liquid biopsy to recognize more molecular alterations.
Moreover, the integration of machine learning and artificial intelligence into cancer diagnosis will improve the precision and prognosis of cancer diagnosis. Machine learning models can also help interpret data from liquid biopsies, enabling faster and more accurate treatment decisions.
Liquid biopsy represents a promising non-invasive tool for early detection, prognosis, and treatment monitoring in hepatobiliary and pancreatic cancers. The ability to detect circulating biomarkers such as ctDNA, CTCs, and exosomes allows for real-time molecular profiling, aiding in early diagnosis, risk stratification, and treatment decision-making. Preoperatively, liquid biopsy facilitates early cancer detection, tumor characterization, and personalized treatment strategies. Intraoperatively, it may provide valuable insights into surgical margin status and residual disease. Postoperatively, liquid biopsy enables monitoring of minimal residual disease and early recurrence detection, potentially improving long-term outcomes.
Despite its promise, several challenges remain, including standardization of methodologies, analytical sensitivity, and regulatory approval for clinical implementation. Future advancements in next-generation sequencing, digital PCR, and artificial intelligence-driven analysis are expected to enhance the accuracy and clinical utility of liquid biopsy. Large-scale prospective trials are warranted to validate its integration into routine oncological practice. Overall, liquid biopsy holds significant potential to transform the management of hepatobiliary and pancreatic cancers, enabling more precise and individualized patient care.
| 1. | Espejo-Cruz ML, González-Rubio S, Zamora-Olaya J, Amado-Torres V, Alejandre R, Sánchez-Frías M, Ciria R, De la Mata M, Rodríguez-Perálvarez M, Ferrín G. Circulating Tumor Cells in Hepatocellular Carcinoma: A Comprehensive Review and Critical Appraisal. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | He YG, Zheng L, Gao MF, Tang YC, Li YM, Yu KH, Li J, Huang XB. A preliminary study of the clinical significance of folate receptor-positive circulating tumor cell in the management of hepatobiliary-pancreatic cancers. Transl Cancer Res. 2020;9:6700-6709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 3. | He S, Zeng F, Yin H, Wang P, Bai Y, Song Q, Chu J, Huang Z, Liu Y, Liu H, Chen Q, Liu L, Zhou J, Hu H, Li X, Li T, Wang G, Cai J, Jiao Y, Zhao H. Molecular diagnosis of pancreatobiliary tract cancer by detecting mutations and methylation changes in bile samples. EClinicalMedicine. 2023;55:101736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Pantel K. Blood-Based Analysis of Circulating Cell-Free DNA and Tumor Cells for Early Cancer Detection. PLoS Med. 2016;13:e1002205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Wang C, Luo Q, Huang W, Zhang C, Liao H, Chen K, Pan M. Correlation Between Circulating Tumor Cell DNA Genomic Alterations and Mesenchymal CTCs or CTC-Associated White Blood Cell Clusters in Hepatocellular Carcinoma. Front Oncol. 2021;11:686365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Aguilar H, López-Roldán B, Vilalta-Lacarra A, Alkorta-Aranburu G, Claramunt R, López-Guerrero JA, Sandiego S, Gil-Bazo I. Liquid biopsy for monitoring minimal residual disease in localized and locally-advanced non-small cell lung cancer after radical-intent treatment. J Liq Biopsy. 2024;4:100145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Hassan F, O'leary D, Ita M, Corrigan M, Wang J, Redmond H. O46: Perioperative liquid biopsy may help predict the risk of recurrence in breast cancer patients. BJS. 2021;108. [DOI] [Full Text] |
| 8. | Matsudera S, Kano Y, Aoyagi Y, Tohyama K, Takahashi K, Kumaki Y, Mitsumura T, Kimura K, Onishi I, Takemoto A, Ban D, Ono H, Kudo A, Oshima N, Ogino K, Watanabe S, Tani Y, Yamaguchi T, Nakajima M, Morita S, Yamaguchi S, Takagi M, Ishikawa T, Nakagawa T, Okamoto K, Uetake H, Tanabe M, Miyake S, Tsuchioka T, Kojima K, Ikeda S. A Pilot Study Analyzing the Clinical Utility of Comprehensive Genomic Profiling Using Plasma Cell-Free DNA for Solid Tumor Patients in Japan (PROFILE Study). Ann Surg Oncol. 2021;28:8497-8505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1690] [Article Influence: 153.6] [Reference Citation Analysis (0)] |
| 10. | Martins I, Ribeiro IP, Jorge J, Gonçalves AC, Sarmento-Ribeiro AB, Melo JB, Carreira IM. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 11. | Adamo P, Cowley CM, Neal CP, Mistry V, Page K, Dennison AR, Isherwood J, Hastings R, Luo J, Moore DA, Howard PJ, Miguel ML, Pritchard C, Manson M, Shaw JA. Profiling tumour heterogeneity through circulating tumour DNA in patients with pancreatic cancer. Oncotarget. 2017;8:87221-87233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Baba S, Kawasaki T, Hirano S, Nakamura T, Asano T, Okazaki R, Yoshida K, Kawase T, Kurahara H, Oi H, Yokoyama M, Kita J, Imura J, Kinoshita K, Kondo S, Okada M, Satake T, Igawa YS, Yoshida T, Yamaguchi H, Ando Y, Mizunuma M, Ichikawa Y, Hida K, Nishihara H, Kato Y. A noninvasive urinary microRNA-based assay for the detection of pancreatic cancer from early to late stages: a case control study. EClinicalMedicine. 2024;78:102936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Chen L, Wu T, Fan R, Qian YS, Liu JF, Bai J, Zheng B, Liu XL, Zheng D, Du LT, Jiang GQ, Wang YC, Fan XT, Deng GH, Wang CY, Shen F, Hu HP, Zhang QZ, Ye YN, Zhang J, Gao YH, Xia J, Yan HD, Liang MF, Yu YL, Sun FM, Gao YJ, Sun J, Zhong CX, Wang Y, Wang H, Kong F, Chen JM, Wen H, Wu BM, Wang CX, Wu L, Hou JL, Wang HY. Cell-free DNA testing for early hepatocellular carcinoma surveillance. EBioMedicine. 2024;100:104962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 14. | Chen S, Jin Y, Wang S, Xing S, Wu Y, Tao Y, Ma Y, Zuo S, Liu X, Hu Y, Chen H, Luo Y, Xia F, Xie C, Yin J, Wang X, Liu Z, Zhang N, Zech Xu Z, Lu ZJ, Wang P. Cancer type classification using plasma cell-free RNAs derived from human and microbes. Elife. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, Schmidt CM, Yip-Schneider MT, Allen PJ, Schattner M, Brand RE, Singhi AD, Petersen GM, Hong SM, Kim SC, Falconi M, Doglioni C, Weiss MJ, Ahuja N, He J, Makary MA, Maitra A, Hanash SM, Dal Molin M, Wang Y, Li L, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Goggins MG, Hruban RH, Wolfgang CL, Klein AP, Tomasetti C, Papadopoulos N, Kinzler KW, Vogelstein B, Lennon AM. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A. 2017;114:10202-10207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 435] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 16. | Gongye X, Tian M, Xia P, Qu C, Chen Z, Wang J, Zhu Q, Li Z, Yuan Y. Multi-omics analysis revealed the role of extracellular vesicles in hepatobiliary & pancreatic tumor. J Control Release. 2022;350:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Guo DZ, Huang A, Wang YC, Zhou S, Wang H, Xing XL, Zhang SY, Cheng JW, Xie KH, Yang QC, Ma CC, Li Q, Chen Y, Su ZX, Fan J, Liu R, Liu XL, Zhou J, Yang XR. Early detection and prognosis evaluation for hepatocellular carcinoma by circulating tumour DNA methylation: A multicentre cohort study. Clin Transl Med. 2024;14:e1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 18. | Han JY, Ahn KS, Kim TS, Kim YH, Cho KB, Shin DW, Baek WK, Suh SI, Jang BC, Kang KJ. Liquid Biopsy from Bile-Circulating Tumor DNA in Patients with Biliary Tract Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Hao X, Deng SY, Wang KY, Chen L, Hou JL, Wei WW, Chen J. [Application of liquid biopsy in early screening and recurrence prediction of hepatocellular carcinoma]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Hong Y, Yang J, Liu X, Huang S, Liang T, Bai X. Deciphering extracellular vesicles protein cargo in pancreatic cancer. Biochim Biophys Acta Rev Cancer. 2024;1879:189142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Hu M, Xia X, Chen L, Jin Y, Hu Z, Xia S, Yao X. Emerging biomolecules for practical theranostics of liver hepatocellular carcinoma. Ann Hepatol. 2023;28:101137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Hu S, Liu Y, Yang Q, Chen L, Chai H, Xiao M, Qi C, Qiu W. Liquid biopsy using cell-free DNA in the early diagnosis of hepatocellular carcinoma. Invest New Drugs. 2023;41:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Kim MW, Koh H, Kim JY, Lee S, Lee H, Kim Y, Hwang HK, Kim SI. Tumor-Specific miRNA Signatures in Combination with CA19-9 for Liquid Biopsy-Based Detection of PDAC. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Koo B, Jun E, Liu H, Kim EJ, Park YY, Lim SB, Kim SC, Shin Y. A biocomposite-based rapid sampling assay for circulating cell-free DNA in liquid biopsy samples from human cancers. Sci Rep. 2020;10:14932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Lapitz A, Azkargorta M, Milkiewicz P, Olaizola P, Zhuravleva E, Grimsrud MM, Schramm C, Arbelaiz A, O'Rourke CJ, La Casta A, Milkiewicz M, Pastor T, Vesterhus M, Jimenez-Agüero R, Dill MT, Lamarca A, Valle JW, Macias RIR, Izquierdo-Sanchez L, Pérez Castaño Y, Caballero-Camino FJ, Riaño I, Krawczyk M, Ibarra C, Bustamante J, Nova-Camacho LM, Falcon-Perez JM, Elortza F, Perugorria MJ, Andersen JB, Bujanda L, Karlsen TH, Folseraas T, Rodrigues PM, Banales JM. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma. J Hepatol. 2023;79:93-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 26. | Li J, Han X, Yu X, Xu Z, Yang G, Liu B, Xiu P. Clinical applications of liquid biopsy as prognostic and predictive biomarkers in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. J Exp Clin Cancer Res. 2018;37:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Li W, Gonzalez-Gonzalez M, Sanz-Criado L, Garcia-Carbonero N, Celdran A, Villarejo-Campos P, Minguez P, Pazo-Cid R, Garcia-Jimenez C, Orta-Ruiz A, Garcia-Foncillas J, Martinez-Useros J. A Novel PiRNA Enhances CA19-9 Sensitivity for Pancreatic Cancer Identification by Liquid Biopsy. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Liu X, Liu L, Ji Y, Li C, Wei T, Yang X, Zhang Y, Cai X, Gao Y, Xu W, Rao S, Jin D, Lou W, Qiu Z, Wang X. Enrichment of short mutant cell-free DNA fragments enhanced detection of pancreatic cancer. EBioMedicine. 2019;41:345-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Luo K, Wang X, Zhang X, Liu Z, Huang S, Li R. The Value of Circulating Tumor Cells in the Prognosis and Treatment of Pancreatic Cancer. Front Oncol. 2022;12:933645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Miura Y, Ohyama H, Mikata R, Hirotsu Y, Amemiya K, Mochizuki H, Ikeda J, Ohtsuka M, Kato N, Omata M. The efficacy of bile liquid biopsy in the diagnosis and treatment of biliary tract cancer. J Hepatobiliary Pancreat Sci. 2024;31:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Nakamura K, Zhu Z, Roy S, Jun E, Han H, Munoz RM, Nishiwada S, Sharma G, Cridebring D, Zenhausern F, Kim S, Roe DJ, Darabi S, Han IW, Evans DB, Yamada S, Demeure MJ, Becerra C, Celinski SA, Borazanci E, Tsai S, Kodera Y, Park JO, Bolton JS, Wang X, Kim SC, Von Hoff D, Goel A. An Exosome-based Transcriptomic Signature for Noninvasive, Early Detection of Patients With Pancreatic Ductal Adenocarcinoma: A Multicenter Cohort Study. Gastroenterology. 2022;163:1252-1266.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 32. | Ning C, Cai P, Liu X, Li G, Bao P, Yan L, Ning M, Tang K, Luo Y, Guo H, Wang Y, Wang Z, Chen L, Lu ZJ, Yin J. A comprehensive evaluation of full-spectrum cell-free RNAs highlights cell-free RNA fragments for early-stage hepatocellular carcinoma detection. EBioMedicine. 2023;93:104645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 33. | Qu C, Wang Y, Wang P, Chen K, Wang M, Zeng H, Lu J, Song Q, Diplas BH, Tan D, Fan C, Guo Q, Zhu Z, Yin H, Jiang L, Chen X, Zhao H, He H, Wang Y, Li G, Bi X, Zhao X, Chen T, Tang H, Lv C, Wang D, Chen W, Zhou J, Zhao H, Cai J, Wang X, Wang S, Yan H, Zeng YX, Cavenee WK, Jiao Y. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy. Proc Natl Acad Sci U S A. 2019;116:6308-6312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 34. | Sok CP, Polireddy K, Kooby DA. Molecular pathology and protein markers for pancreatic cancer: relevance in staging, in adjuvant therapy, in determination of minimal residual disease, and follow-up. Hepatobiliary Surg Nutr. 2024;13:56-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Urban SK, Sänger H, Krawczyk M, Julich-Haertel H, Willms A, Ligocka J, Azkargorta M, Mocan T, Kahlert C, Kruk B, Jankowski K, Patkowski W, Krawczyk M, Zieniewicz K, Hołówko W, Krupa Ł, Rzucidło M, Gutkowski K, Wystrychowski W, Król R, Raszeja-Wyszomirska J, Słomka A, Schwab R, Wöhler A, Gonzalez-Carmona MA, Gehlert S, Sparchez Z, Banales JM, Strassburg CP, Lammert F, Milkiewicz P, Kornek M. Synergistic effects of extracellular vesicle phenotyping and AFP in hepatobiliary cancer differentiation. Liver Int. 2020;40:3103-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Xing X, Cai L, Ouyang J, Wang F, Li Z, Liu M, Wang Y, Zhou Y, Hu E, Huang C, Wu L, Liu J, Liu X. Proteomics-driven noninvasive screening of circulating serum protein panels for the early diagnosis of hepatocellular carcinoma. Nat Commun. 2023;14:8392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 37. | Xiong Y, Xie CR, Zhang S, Chen J, Yin ZY. Detection of a novel panel of somatic mutations in plasma cell-free DNA and its diagnostic value in hepatocellular carcinoma. Cancer Manag Res. 2019;11:5745-5756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Xu C, Jun E, Okugawa Y, Toiyama Y, Borazanci E, Bolton J, Taketomi A, Kim SC, Shang D, Von Hoff D, Zhang G, Goel A. A Circulating Panel of circRNA Biomarkers for the Noninvasive and Early Detection of Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2024;166:178-190.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 39. | Yadav DK, Bai X, Yadav RK, Singh A, Li G, Ma T, Chen W, Liang T. Liquid biopsy in pancreatic cancer: the beginning of a new era. Oncotarget. 2018;9:26900-26933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Yan TB, Huang JQ, Huang SY, Ahir BK, Li LM, Mo ZN, Zhong JH. Advances in the Detection of Pancreatic Cancer Through Liquid Biopsy. Front Oncol. 2021;11:801173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019;18:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 269] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 42. | Yi K, Wang Y, Rong Y, Bao Y, Liang Y, Chen Y, Liu F, Zhang S, He Y, Liu W, Zhu C, Wu L, Peng J, Chen H, Huang W, Yuan Y, Xie M, Wang F. Transcriptomic Signature of 3D Hierarchical Porous Chip Enriched Exosomes for Early Detection and Progression Monitoring of Hepatocellular Carcinoma. Adv Sci (Weinh). 2024;11:e2305204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Yi K, Zhang Z, Chen P, Xi X, Zhao X, Rong Y, Long F, Zhang Q, Zhang Y, Gao M, Liu W, Liu BF, Zhu Z, Wang F. Tidal microfluidic chip-based isolation and transcriptomic profiling of plasma extracellular vesicles for clinical monitoring of high-risk patients with hepatocellular carcinoma-associated precursors. Biosens Bioelectron. 2025;276:117228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Yokose T, Kabe Y, Matsuda A, Kitago M, Matsuda S, Hirai M, Nakagawa T, Masugi Y, Hishiki T, Nakamura Y, Shinoda M, Yagi H, Abe Y, Oshima G, Hori S, Nakano Y, Honda K, Kashiro A, Morizane C, Nara S, Kikuchi S, Shibahara T, Itonaga M, Ono M, Minegishi N, Koshiba S, Yamamoto M, Kuno A, Handa H, Sakamoto M, Suematsu M, Kitagawa Y. O-Glycan-Altered Extracellular Vesicles: A Specific Serum Marker Elevated in Pancreatic Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Zhao G, Jiang R, Shi Y, Gao S, Wang D, Li Z, Zhou Y, Sun J, Wu W, Peng J, Kuang T, Rong Y, Yuan J, Zhu S, Jin G, Wang Y, Lou W. Circulating cell-free DNA methylation-based multi-omics analysis allows early diagnosis of pancreatic ductal adenocarcinoma. Mol Oncol. 2024;18:2801-2813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Mehdorn AS, Gemoll T, Busch H, Kern K, Beckinger S, Daunke T, Kahlert C, Uzunoglu FG, Hendricks A, Buertin F, Wittel UA, Sunami Y, Röcken C, Becker T, Sebens S. Biomarkers in Liquid Biopsies for Prediction of Early Liver Metastases in Pancreatic Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 47. | Boons G, Vandamme T, Peeters M, Beyens M, Driessen A, Janssens K, Zwaenepoel K, Roeyen G, Van Camp G, Op de Beeck K. Cell-Free DNA From Metastatic Pancreatic Neuroendocrine Tumor Patients Contains Tumor-Specific Mutations and Copy Number Variations. Front Oncol. 2018;8:467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Hata T, Mizuma M, Iseki M, Takadate T, Ishida M, Nakagawa K, Hayashi H, Morikawa T, Motoi F, Unno M. Circulating tumor DNA as a predictive marker for occult metastases in pancreatic cancer patients with radiographically non-metastatic disease. J Hepatobiliary Pancreat Sci. 2021;28:648-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Hu H, Song H, Han B, Zhao H, He J. Tumor-Educated Platelet RNA and Circulating Free RNA: Emerging Liquid Biopsy Markers for Different Tumor Types. Front Biosci (Landmark Ed). 2024;29:80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 50. | Umemoto K, Sunakawa Y, Ueno M, Furukawa M, Mizuno N, Sudo K, Kawamoto Y, Kajiwara T, Ohtsubo K, Okano N, Matsuhashi N, Itoh S, Matsumoto T, Shimizu S, Otsuru T, Hasegawa H, Okuyama H, Ohama H, Moriwaki T, Ohta T, Odegaard JI, Nakamura Y, Bando H, Yoshino T, Ikeda M, Morizane C. Clinical significance of circulating-tumour DNA analysis by metastatic sites in pancreatic cancer. Br J Cancer. 2023;128:1603-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, White C, Lowe C, Sherba JJ, Hartmanshenn C, O'Neill KM, Balter ML, Fritz ZR, Androulakis IP, Schloss RS, Yarmush ML. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 2018;6:79-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 280] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 52. | von Felden J, Schulze K, Krech T, Ewald F, Nashan B, Pantel K, Lohse AW, Riethdorf S, Wege H. Circulating tumor cells as liquid biomarker for high HCC recurrence risk after curative liver resection. Oncotarget. 2017;8:89978-89987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 53. | Tsumura T, Doi K, Marusawa H. Precision Medicine of Hepatobiliary and Pancreatic Cancers: Focusing on Clinical Trial Outcomes. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Liu F, Hao X, Liu B, Liu S, Yuan Y. Bile liquid biopsy in biliary tract cancer. Clin Chim Acta. 2023;551:117593. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Shen N, Zhang D, Yin L, Qiu Y, Liu J, Yu W, Fu X, Zhu B, Xu X, Duan A, Chen Z, Wang X, Cao X, Zhao T, Zhou Z, Yu L, Qin H, Fang Z, Li JY, Liu Y, Xiong L, Yuan B, Li F, Zhang Y. Bile cellfree DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol Rep. 2019;42:549-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Li Y, Wu J, Li E, Xiao Z, Lei J, Zhou F, Yin X, Hu D, Mao Y, Wu L, Wenjun L. TP53 mutation detected in circulating exosomal DNA is associated with prognosis of patients with hepatocellular carcinoma. Cancer Biol Ther. 2022;23:439-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Pelizzaro F, Cardin R, Penzo B, Pinto E, Vitale A, Cillo U, Russo FP, Farinati F. Liquid Biopsy in Hepatocellular Carcinoma: Where Are We Now? Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Qi LN, Xiang BD, Wu FX, Ye JZ, Zhong JH, Wang YY, Chen YY, Chen ZS, Ma L, Chen J, Gong WF, Han ZG, Lu Y, Shang JJ, Li LQ. Circulating Tumor Cells Undergoing EMT Provide a Metric for Diagnosis and Prognosis of Patients with Hepatocellular Carcinoma. Cancer Res. 2018;78:4731-4744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 59. | Qiao Z, Wang E, Bao B, Tan X, Chen H, Wang D, Yuan L. Diagnostic and prognostic value of circulating exosomal glypican-1 in pancreatic cancer: a meta-analysis. Lab Med. 2024;55:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 60. | Wang J, Shi R, Yin Y, Luo H, Cao Y, Lyu Y, Luo H, Zeng X, Wang D. Clinical significance of small extracellular vesicles in cholangiocarcinoma. Front Oncol. 2024;14:1334592. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Wang K, Wang X, Pan Q, Zhao B. Liquid biopsy techniques and pancreatic cancer: diagnosis, monitoring, and evaluation. Mol Cancer. 2023;22:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 62. | Zhang N, Yang X, Piao M, Xun Z, Wang Y, Ning C, Zhang X, Zhang L, Wang Y, Wang S, Chao J, Lu Z, Yang X, Wang H, Zhao H. Biomarkers and prognostic factors of PD-1/PD-L1 inhibitor-based therapy in patients with advanced hepatocellular carcinoma. Biomark Res. 2024;12:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 63. | Zhao L, Song J, Sun Y, Ju Q, Mu H, Dong X, Ding J, Liu Y, Wang X, Sun L, Wu J, Jiao Y, Lu S, Zhao X. Tumor-derived proliferative CTCs and CTC clusters predict aggressiveness and early recurrence in hepatocellular carcinoma patients. Cancer Med. 2023;12:13912-13927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 64. | Qi X, Chen S, He H, Wen W, Wang H. The role and potential application of extracellular vesicles in liver cancer. Sci China Life Sci. 2021;64:1281-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Shu L, Li X, Liu Z, Li K, Shi A, Tang Y, Zhao L, Huang L, Zhang Z, Zhang D, Huang S, Lian S, Sheng G, Yan Z, Zhang Z, Xu Y. Bile exosomal miR-182/183-5p increases cholangiocarcinoma stemness and progression by targeting HPGD and increasing PGE2 generation. Hepatology. 2024;79:307-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 66. | Wang P, Song Q, Ren J, Zhang W, Wang Y, Zhou L, Wang D, Chen K, Jiang L, Zhang B, Chen W, Qu C, Zhao H, Jiao Y. Simultaneous analysis of mutations and methylations in circulating cell-free DNA for hepatocellular carcinoma detection. Sci Transl Med. 2022;14:eabp8704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 67. | Yin L, Duan A, Zhang W, Li B, Zhao T, Xu X, Yang L, Nian B, Lu K, Chen S, Li Z, Liu J, Duan Q, Liu D, Chen H, Cui L, Chang Y, Kuang Y, Zhang D, Wang X, Zhang Y. Identification of whole-genome mutations and structural variations of bile cell-free DNA in cholangiocarcinoma. Genomics. 2024;116:110916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 68. | Conci S, Catalano G, Roman D, Zecchetto C, Lucin E, De Bellis M, Tripepi M, Guglielmi A, Milella M, Ruzzenente A. Current Role and Future Perspectives of Immunotherapy and Circulating Factors in Treatment of Biliary Tract Cancers. Int J Med Sci. 2023;20:858-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 69. | Amado V, González-Rubio S, Zamora J, Alejandre R, Espejo-Cruz ML, Linares C, Sánchez-Frías M, García-Jurado G, Montero JL, Ciria R, Rodríguez-Perálvarez M, Ferrín G, De la Mata M. Clearance of Circulating Tumor Cells in Patients with Hepatocellular Carcinoma Undergoing Surgical Resection or Liver Transplantation. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 70. | Hata T, Mizuma M, Motoi F, Ohtsuka H, Nakagawa K, Morikawa T, Unno M. Prognostic impact of postoperative circulating tumor DNA as a molecular minimal residual disease marker in patients with pancreatic cancer undergoing surgical resection. J Hepatobiliary Pancreat Sci. 2023;30:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 71. | Jin KT, Chen XY, Lan HR, Wang SB, Ying XJ, Abdi SM, Wang W, Hu ZM, Mou XZ. Current progress in the clinical use of circulating tumor cells as prognostic biomarkers. Cancer Cytopathol. 2019;127:739-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Liu J, Lian J, Chen Y, Zhao X, Du C, Xu Y, Hu H, Rao H, Hong X. Circulating Tumor Cells (CTCs): A Unique Model of Cancer Metastases and Non-invasive Biomarkers of Therapeutic Response. Front Genet. 2021;12:734595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Wang SC, Li CY, Chang WT, Cheng WC, Yen CH, Tu WY, Lin ZY, Lin CC, Yeh ML, Huang CF, Huang JF, Dai CY, Chuang WL, Chen YL, Yu ML. Exosome-derived differentiation antagonizing non-protein coding RNA with risk of hepatitis C virus-related hepatocellular carcinoma recurrence. Liver Int. 2021;41:956-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Wang X, Wang G, Wu Z, Dong Y, Shi Y, Yang F, Chen X, Wang J, Du S, Xu H, Zheng Y. Exosomal circ-PTPN22 and circ-ADAMTS6 mark T cell exhaustion and neutrophil extracellular traps in Asian intrahepatic cholangiocarcinoma. Mol Ther Nucleic Acids. 2023;31:151-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |