Published online Mar 16, 2021. doi: 10.12998/wjcc.v9.i8.1885
Peer-review started: September 23, 2020
First decision: December 31, 2020
Revised: January 10, 2021
Accepted: January 25, 2021
Article in press: January 25, 2021
Published online: March 16, 2021
Processing time: 163 Days and 1.8 Hours
Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) involvement in the central nervous system (CNS) is particularly rare. SPTCL with CNS involvement has an exceedingly poor prognosis, and no optimum therapeutic method has been discovered. To the best of our knowledge, this is the first reported case of SPTCL invading the CNS achieving long-term remission with lenalidomide maintenance therapy.
A 63-year-old man diagnosed with SPTCL was admitted to the hospital with severe headache for 15 d after four cycles of chemotherapy. Subsequent to the treatment, the patient developed CNS involvement. Craniotomy biopsy was pathologically diagnosed as CNS T-cell lymphoma, and two courses of chemotherapy were performed postoperatively. Due to the intolerance of the side effects of chemotherapeutic drugs, the patient received lenalidomide instead. The magnetic resonance imaging of the head at the 8 mo follow-up indicated no signs of recurrence, and the vital signs were stable.
Lenalidomide deserves further investigation as a targeted drug for SPTCL cases involving the CNS.
Core Tip: Subcutaneous panniculitis-like T-cell lymphoma is a rare peripheral T-cell lymphoma that occurs primarily in the skin. We present a special case of central nervous system T-cell lymphoma secondary to subcutaneous panniculitis-like T-cell lymphoma. Lenalidomide deserves further investigation as a targeted drug for subcutaneous panniculitis-like T-cell lymphoma cases involving the central nervous system.
- Citation: Sun J, Ma XS, Qu LM, Song XS. Subcutaneous panniculitis-like T-cell lymphoma invading central nervous system in long-term clinical remission with lenalidomide: A case report. World J Clin Cases 2021; 9(8): 1885-1892
- URL: https://www.wjgnet.com/2307-8960/full/v9/i8/1885.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i8.1885
According to the World Health Organization-European Organization for Research and Treatment of Cancer in 2008, subcutaneous panniculitis-like T-cell lymphoma (SPTCL) is a rare peripheral skin T-cell lymphoma, which accounts for less than 1% of all non-Hodgkin’s lymphomas and primary skin T-cell lymphomas. It primarily involves subcutaneous adipose tissue typically derived from α/β T-cells with a favorable prognosis in the absence of hemophagocytic syndrome[1,2]. SPTCL is most commonly seen in young adult females, with a male-female ratio of 0.5[1]. Patients with SPTCL typically present with multiple subcutaneous tender nodules, which are principally found in the limbs and torso and often resembles other diseases such as lupus panniculitis[3]. The involvement of the central nervous system (CNS) in non-Hodgkin’s lymphoma is a fatal complication with a survival probability of less than 5%[4]. CNS involvement in SPTCL is fatal, and there is no consensus on the best treatment. In this paper, we describe a case of CNS T-cell lymphoma secondary to SPTCL in a 63-year-old man and summarize a literature review.
A 63-year-old man was admitted to our hospital with severe headache for 15 d.
The patient had a headache for 15 d and worsened for 2 d.
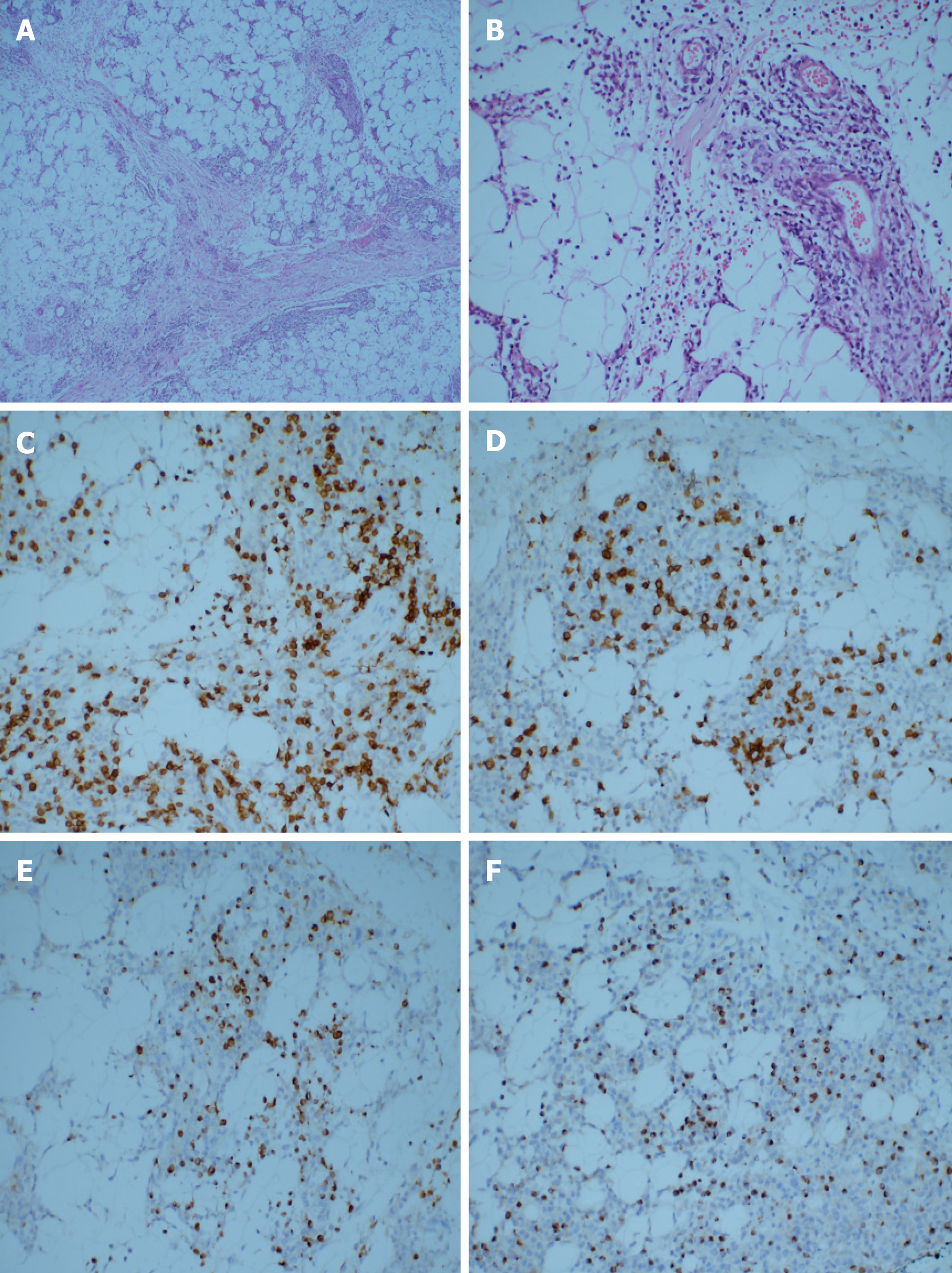
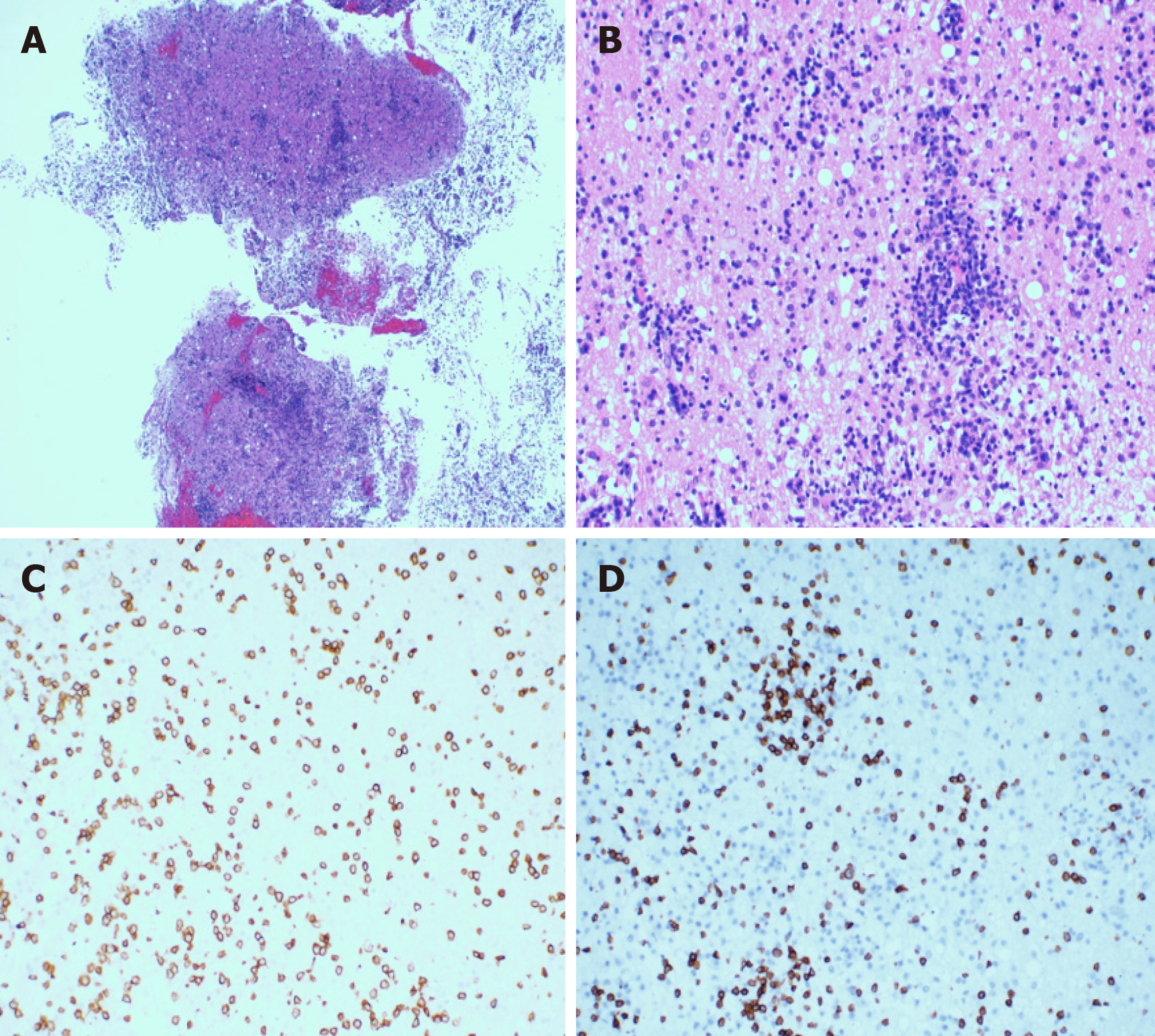
About 4 mo ago, the patient was admitted to our hospital with a complaint of “subcutaneous nodules and fever for 8 mo.” Physical examination of the patient showed subcutaneous nodules of different sizes in the extremities, positive tenderness, and normal muscle strength but decreased muscular tension. Routine blood tests showed that the number of white blood cells had decreased to 2.21 × 109/L (normal range 3.50 × 109/L to 9.50 × 109/L). He had a history of hypertension grade 2 (middle risk group). No other obvious abnormalities were found. The subcutaneous nodules of the right upper arm and right thigh of the patient were resected. The hematoxylin-eosin staining of the specimens revealed a large number of atypical lymphocytes had infiltrated the fibrous connective tissue and adipose tissue, which distributed in a typical flower-ring-like pattern around the fat cells (Figure 1A and 1B). Immuno-histochemistry revealed positive staining for CD3, CD8, Gram-B, and T-cell restricted intracellular antigen-1 but negative for CD4 and CD56 (Figure 1C-F). The Ki-67 labelling index was approximately 20% to 30%. The results of T-cell receptor (TCR) gene rearrangement showed TCR-β clonal rearrangement. The patient was diagnosed with SPTCL.
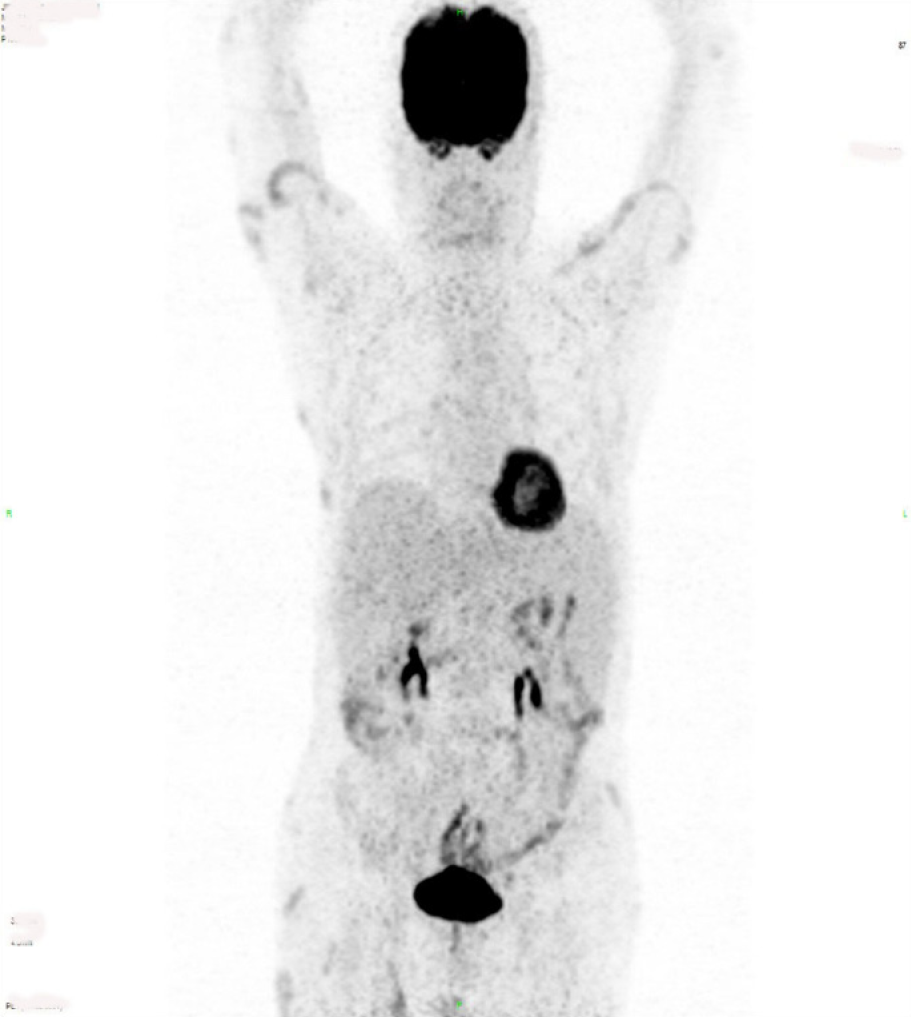
The results of the positron emission computed tomography examination indicated that the bilateral upper arm, left chest wall, bilateral abdominal wall, bilateral hip, and bilateral thigh had multiple subcutaneous lesions involving > 3 regions; the tumor was stage T3. The peripheral lymph nodes were not involved; the stage was N0. There was no visceral organ involvement; the metastasis stage was M0 (Figure 2). No bone marrow invasion was found in the bone marrow puncture. He was clinically diagnosed with SPTCL with stage T3N0M0.
The patient was treated with the cyclophosphamide, vincristine, and prednisolone (COP) regimen; each course lasted for 21 d for a total of four courses. The COP regimen included prednisone, cyclophosphamide, and vincristine. The specific doses were: Prednisone (50 mg) taken twice a day from day 1 to day 5; cyclophosphamide (1350 mg) intravenously administered by drip only on the first day; and vincristine (4 mg) administered intravenously only on the first day. At the beginning of the third chemotherapy cycle, chidamide (30 mg, orally) was added twice a week to the original COP regimen. At the end of the fourth chemotherapy cycle, the subcutaneous nodules of the extremities almost disappeared, and body temperature stabilized within the normal range. However, the white blood cells were below normal levels, and the muscle strength of the patient was still decreased.
The patient had no previous or family history of similar illnesses.
Shortly after the end of the fourth chemotherapy session, the patient had a severe headache. The patient’s physical examination was generally normal.
No other obvious abnormality was found.
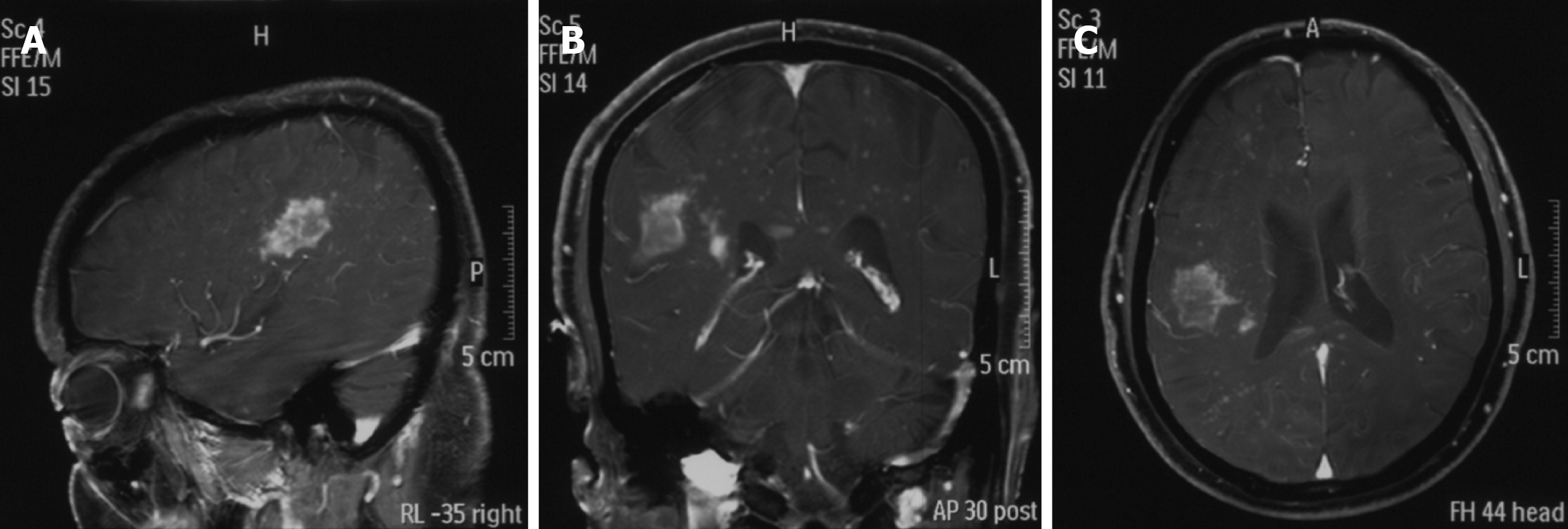
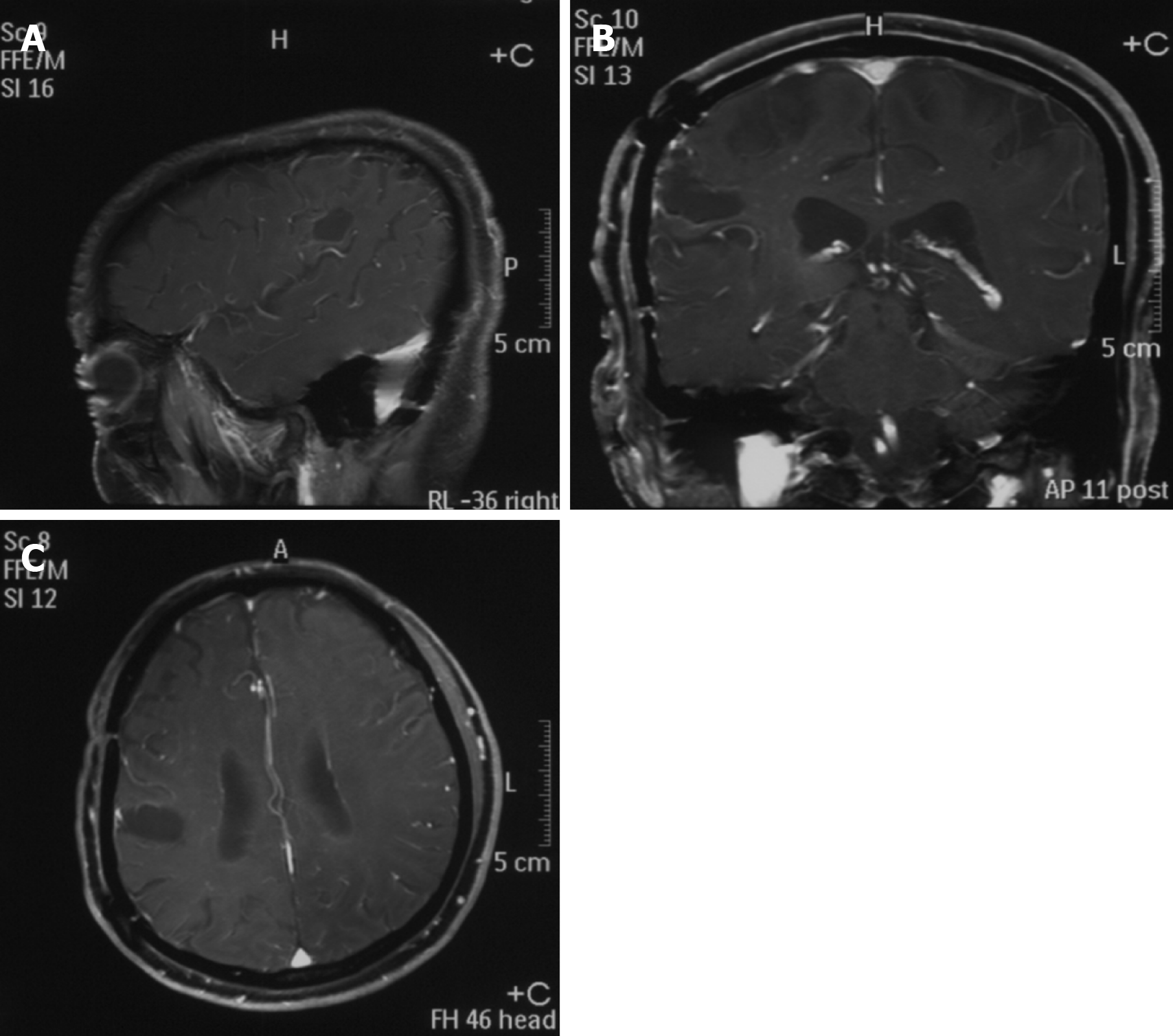
A head magnetic resonance imaging showed a large mass in the region of the right temporal lobe (Figure 3).
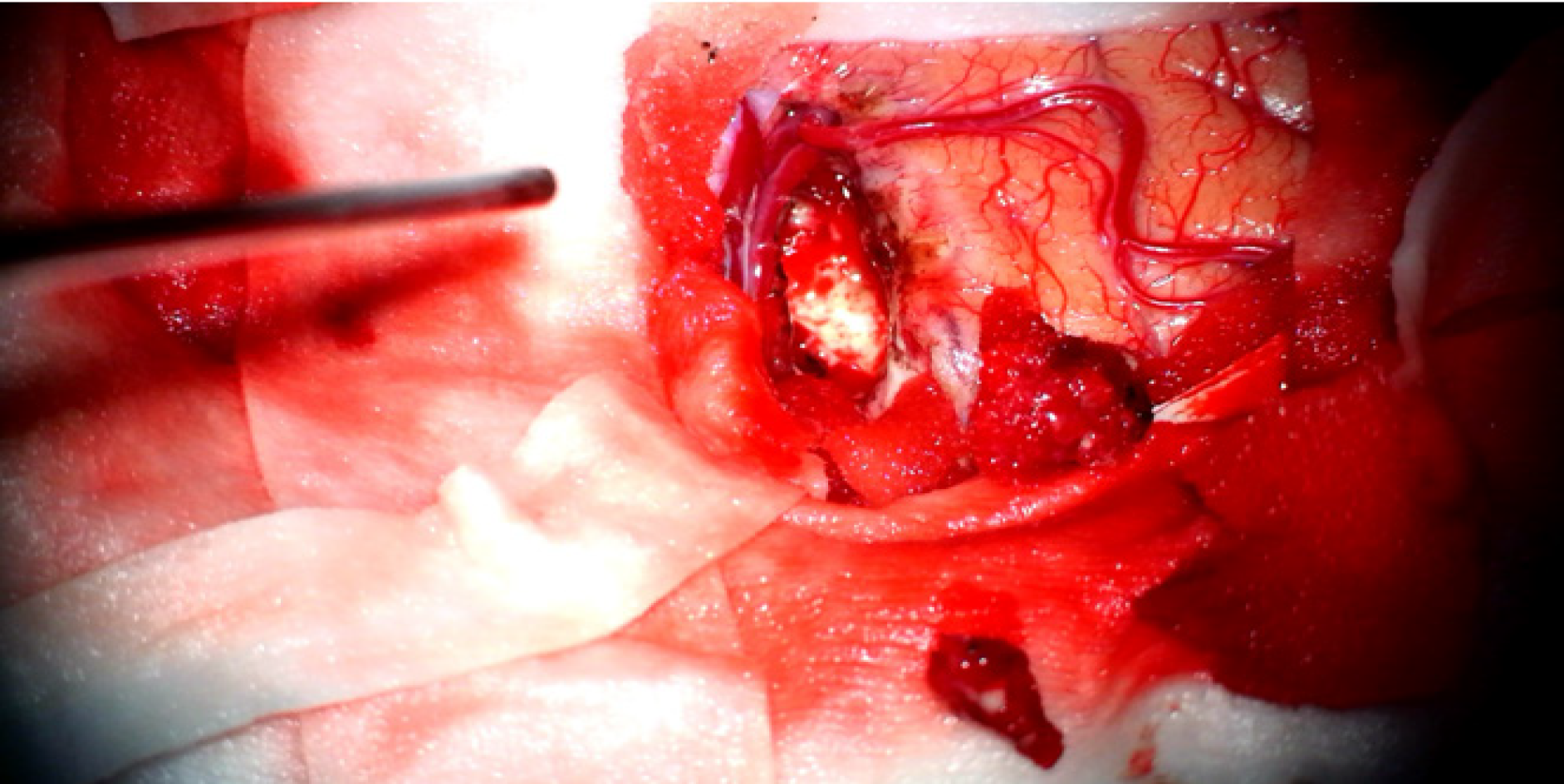
In order to alleviate the headache and further clarify the clinical diagnosis, the patient underwent a stereotactic craniotomy. A subtotal tumor resection via right temporal lobe transcortical approach was performed. Macroscopically, the lesion was gray-white in color, and soft tissue with rich blood supply blurred the boundary with the surrounding brain tissue (Figure 4). The size of the tissue was roughly 1.0 cm × 1.0 cm × 1.0 cm. Microscopically, the hematoxylin-eosin staining of the specimens revealed that the plasmocytes and lymphocytes had proliferated. These lymphocytes infiltrated the blood vessels and showed a vascular-centered growth pattern (Figure 5A and 5B). Immunohisto-chemistry revealed positive staining for CD3 and CD8 (Figure 5C and 5D). The Ki-67 labeling index was approximately 30% for T-cell lymphoma. The results of TCR gene rearrangement showed TCR-β and TCR-γ clonal rearrangement. He was diagnosed with SPTCL with invasion of the CNS.
The patient was treated with high-dose methotrexate (HD-MTX) chemotherapy regimen; each course of treatment lasted for 21 d for a total of four courses. The HD-MTX chemotherapy regimen consisted of a high dose of methotrexate. The specific method involved administration of methotrexate (6.0 g) by intravenous drip for more than 24 h. At the end of the second course, he developed serious side effects, such as a loss of appetite, mental retardation, dyspnea, slow response, decreased muscle strength, and occasional grumpy moods. A blood routine test showed leucopenia and platelet reduction.
We gave up the HD-MTX regimen and used lenalidomide (10 mg) daily for the first 21 d of each month followed by rest for the remainder of the month.
Nine months after the craniocerebral surgery, the patient was followed up. By then he had undergone nine courses of lenalidomide. The vital signs were stable, and there was no recurrence of the subcutaneous nodules at the extremities. An enhanced magnetic resonance imaging of the head showed no sign of recurrence of the tumor (Figure 6).
Given the specificity of primary cutaneous lymphomas, we selected the tumor, node, metastasis staging method proposed by the International Society for Cutaneous Lymphomas-the European Organization of Research and Treatment of Cancer in 2007[5]. The use of [18F] fluorodeoxyglucose–positron emission tomography in the early clinical diagnosis is helpful to make differential diagnoses and definite clinical stages[6].
For cutaneous T-cell lymphoma, local radiotherapy should be considered for the limited lesion scope or single lesion[1,7]. Due to the unpredictable damage to the nervous system caused by whole-brain radiotherapy and in order to prevent delayed neurotoxicity, we did not administer radiotherapy. CCAAT/enhancer binding protein is used as a reference protocol in many countries for previously untreated peripheral T-cell lymphoma patients[8,9]. Compared with primary cutaneous γ δ-T-cell lymphoma, SPTCL has relatively mild manifestations and relatively good prognosis[1,3,7,10]. Doxorubicin has side effects such as cardiotoxicity and blood system toxicity[11,12]. Recent studies have shown that chidamide is effective and acceptable in the treatment of refractory peripheral T-cell lymphoma[13,14]. Consequently, chidamide was added to the third and fourth course of COP chemotherapy to maintain the treatment.
The median time for T-cell lymphoma invading CNS was about 5.4 mo after the initial diagnosis[4]. It is not clear whether the malignant intracranial invasion is related to the TCR phenotype (which indicates the prognostic factors to some extent)[7,10]. It manifests that SPTCL patients who have undergone COP may have a recurrent involvement of the CNS, and not all patients with α/β T-cell lymphoma have a good prognosis. The patient was selected to undergo stereotactic craniotomy as active surgical treatment can quickly relieve the symptoms[15]. For this patient, radical resection was almost impossible. The median survival time after an operation is usually short. Once the stereotactic biopsy was completed, additional surgical treatment may not be helpful. HD-MTX and whole-brain radiotherapy were sufficient to treat the CNS lymphoma[15]. Patients over 60 years of age are most likely to have delayed neurotoxicity often associated with whole-brain radiotherapy. We chose only HD-MTX for the therapy. The HD-MTX regimen was abandoned because of significant side effects. The tolerance of lenalidomide is generally good, and its safety is considered acceptable and may have a positive therapeutic effect on peripheral T-cell lymphoma[16]. Considering that the patient had already used chidamide before involvement of the CNS and that the patient was intolerant to the side effects of chemotherapeutic drugs, we only used lenalidomide[13,14].
CNS T-cell tumors secondary to SPTCL are rare. To our knowledge, this is the first reported case that SPTCL invading the CNS achieved long-term remission with lenalidomide maintenance therapy after initial treatment with HD-MTX. It suggests that lenalidomide may be effective in SPTCL cases involving the CNS.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moschovi MA S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LL
| 1. | Willemze R, Jansen PM, Cerroni L, Berti E, Santucci M, Assaf C, Canninga-van Dijk MR, Carlotti A, Geerts ML, Hahtola S, Hummel M, Jeskanen L, Kempf W, Massone C, Ortiz-Romero PL, Paulli M, Petrella T, Ranki A, Peralto JL, Robson A, Senff NJ, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ; EORTC Cutaneous Lymphoma Group. Subcutaneous panniculitis-like T-cell lymphoma: Definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 420] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 2. | Gonzalez CL, Medeiros LJ, Braziel RM, Jaffe ES. T-cell lymphoma involving subcutaneous tissue. A clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 288] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Bashey S, Krathen M, Abdulla F, Sundram U, Kim YH. Romidepsin is effective in subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2012;30:e221-e225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI. Natural history of CNS relapse in patients with aggressive non-Hodgkin's lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. J Clin Oncol. 2009;27:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Kim YH, Willemze R, Pimpinelli N, Whittaker S, Olsen EA, Ranki A, Dummer R, Hoppe RT; ISCL and the EORTC. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 301] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 6. | Bennani-Baiti B, Yadav S, Flynt L, Bennani-Baiti N. Value of positron emission tomography in diagnosing subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2015;33:1216-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | López-Lerma I, Peñate Y, Gallardo F, Martí RM, Mitxelena J, Bielsa I, Velasco-Tamariz V, Yanguas-Bayona JI, Sánchez-Sambucety P, García-Patos V, Ortiz-Romero PL, Pujol RM, Estrach T. Subcutaneous panniculitis-like T-cell lymphoma: Clinical features, therapeutic approach, and outcome in a case series of 16 patients. J Am Acad Dermatol. 2018;79:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Gleeson M, Peckitt C, To YM, Edwards L, Oates J, Wotherspoon A, Attygalle AD, Zerizer I, Sharma B, Chua S, Begum R, Chau I, Johnson P, Ardeshna KM, Hawkes EA, Macheta MP, Collins GP, Radford J, Forbes A, Hart A, Montoto S, McKay P, Benstead K, Morley N, Kalakonda N, Hasan Y, Turner D, Cunningham D. CHOP vs GEM-P in previously untreated patients with peripheral T-cell lymphoma (CHEMO-T): a phase 2, multicentre, randomised, open-label trial. Lancet Haematol. 2018;5:e190-e200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124:1570-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 10. | Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1186] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 12. | Wollina U, Dummer R, Brockmeyer NH, Konrad H, Busch JO, Kaatz M, Knopf B, Koch HJ, Hauschild A. Multicenter study of pegylated liposomal doxorubicin in patients with cutaneous T-cell lymphoma. Cancer. 2003;98:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, Yu L, Ke X, Huang H, Shen Z, Fan Y, Li W, Zhao X, Qi J, Huang H, Zhou D, Ning Z, Lu X. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26:1766-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 14. | Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, Zhao W, Zhang H, Sun X, Yang H, Zhang X, Jin J, Jin Z, Li Z, Qiu L, Dong M, Huang X, Luo Y, Wang X, Wang X, Wu J, Xu J, Yi P, Zhou J, He H, Liu L, Shen J, Tang X, Wang J, Yang J, Zeng Q, Zhang Z, Cai Z, Chen X, Ding K, Hou M, Huang H, Li X, Liang R, Liu Q, Song Y, Su H, Gao Y, Liu L, Luo J, Su L, Sun Z, Tan H, Wang H, Wang J, Wang S, Zhang H, Zhang X, Zhou D, Bai O, Wu G, Zhang L, Zhang Y. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 15. | Rubenstein JL, Gupta NK, Mannis GN, Lamarre AK, Treseler P. How I treat CNS lymphomas. Blood. 2013;122:2318-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Ogura M, Imaizumi Y, Uike N, Asou N, Utsunomiya A, Uchida T, Aoki T, Tsukasaki K, Taguchi J, Choi I, Maruyama D, Nosaka K, Chen N, Midorikawa S, Ohtsu T, Tobinai K. Lenalidomide in relapsed adult T-cell leukaemia-lymphoma or peripheral T-cell lymphoma (ATLL-001): a phase 1, multicentre, dose-escalation study. Lancet Haematol. 2016;3:e107-e118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |