Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1661
Peer-review started: September 14, 2020
First decision: December 14, 2020
Revised: December 18, 2020
Accepted: January 6, 2021
Article in press: January 6, 2021
Published online: March 6, 2021
Primary retroperitoneal liposarcoma (PRPLS) is a rare soft tissue tumor with nonspecific clinical symptoms; it has different computed tomography (CT) image features according to pathological types. Some patients with a single tumor have been previously reported in the literature. We present an exceptional case of a PRPLS patient with multiple large tumors exhibiting different patterns of appearance on CT and confirmed as atypical lipomatous tumor/well-differentiated liposarcoma by postoperative pathology.
A 64-year-old man presented with abdominal distension for 1 year. The patient was diagnosed with PRPLS based on physical examination, laparotomy, ultrasonography, CT scan, and surgery. Both of the tumors were completely resected through surgery and confirmed as atypical lipomatous tumor/well-differentiated liposarcoma by postoperative pathology. The postoperative course was uneventful without recurrence or metastasis, as demonstrated by abdominal-pelvic CT during an 18 mo follow-up.
Multiple large Well-differentiated liposarcomas with different patterns of appearance on CT image can occur simultaneously in the same patient, to which more attention should be paid to make an effective differential diagnosis.
Core Tip: Primary retroperitoneal liposarcoma (PRPLS) is a rare soft tissue tumor with nonspecific clinical symptoms; it has different computed tomography (CT) image features according to pathological types. We present an exceptional case of a PRPLS patient with multiple large tumors exhibiting different patterns of appearance on CT and confirmed as atypical lipomatous tumor/well-differentiated liposarcoma by postoperative pathology. This suggested that more attention should be paid to an effective differential diagnosis of PRPLS with different patterns of appearance on CT.
- Citation: Xie TH, Ren XX, Fu Y, Ha SN, Liu LT, Jin XS. Multiple well-differentiated retroperitoneal liposarcomas with different patterns of appearance on computed tomography: A case report. World J Clin Cases 2021; 9(7): 1661-1667
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1661.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1661
Primary retroperitoneal liposarcoma (PRPLS) originates from soft tissue in the retroperitoneum and is rarely observed clinically[1-4]. Since the clinical symptoms are usually nonspecific and its anatomical location is the retroperitoneal space, reaching an early definitive diagnosis of PRPLS is usually difficult[5]. Complete resection by surgery is the main mode of treatment for this disease[6]. Owing to the complex pathology of this disease, the effectiveness of chemoradiotherapy is limited[7,8]. The prognosis of this disease depends on the pathological type; however, all types are linked to a high local recurrence rate[9,10]. Therefore, it is important to promptly detect the disease, administer appropriate treatment, and regularly monitor the disease progression after operation[10,11]. Herein, we report a case with multiple PRPLS managed at our hospital and review the currently literature on this disease.
A 64-year-old man was referred to the Department of General Surgery, Affiliated Hospital of Hebei University (Baoding, China) in July 2018, with a complaint of abdominal distension for 1 year.
Apart from abdominal distension, the patient was asymptomatic, without distinct medical, surgical, or medication history.
A physical examination revealed the presence of abdominal bulge with a flexible mass (dimensions: 24 cm × 20 cm) located in the abdomen. This mass had a regular shape, clear boundary, and was palpable with a rubbery medium-hard texture. There was no pain upon application of pressure on the mass.
The laboratory examinations, including the assessment of tumor markers (alpha fetoprotein, carcinoembryonic antigen, carbohydrate antigen (CA) 125, CA15-3, CA19-9, and CA72-4), as well as routine blood, urine, and stool tests, did not reveal abnormalities.
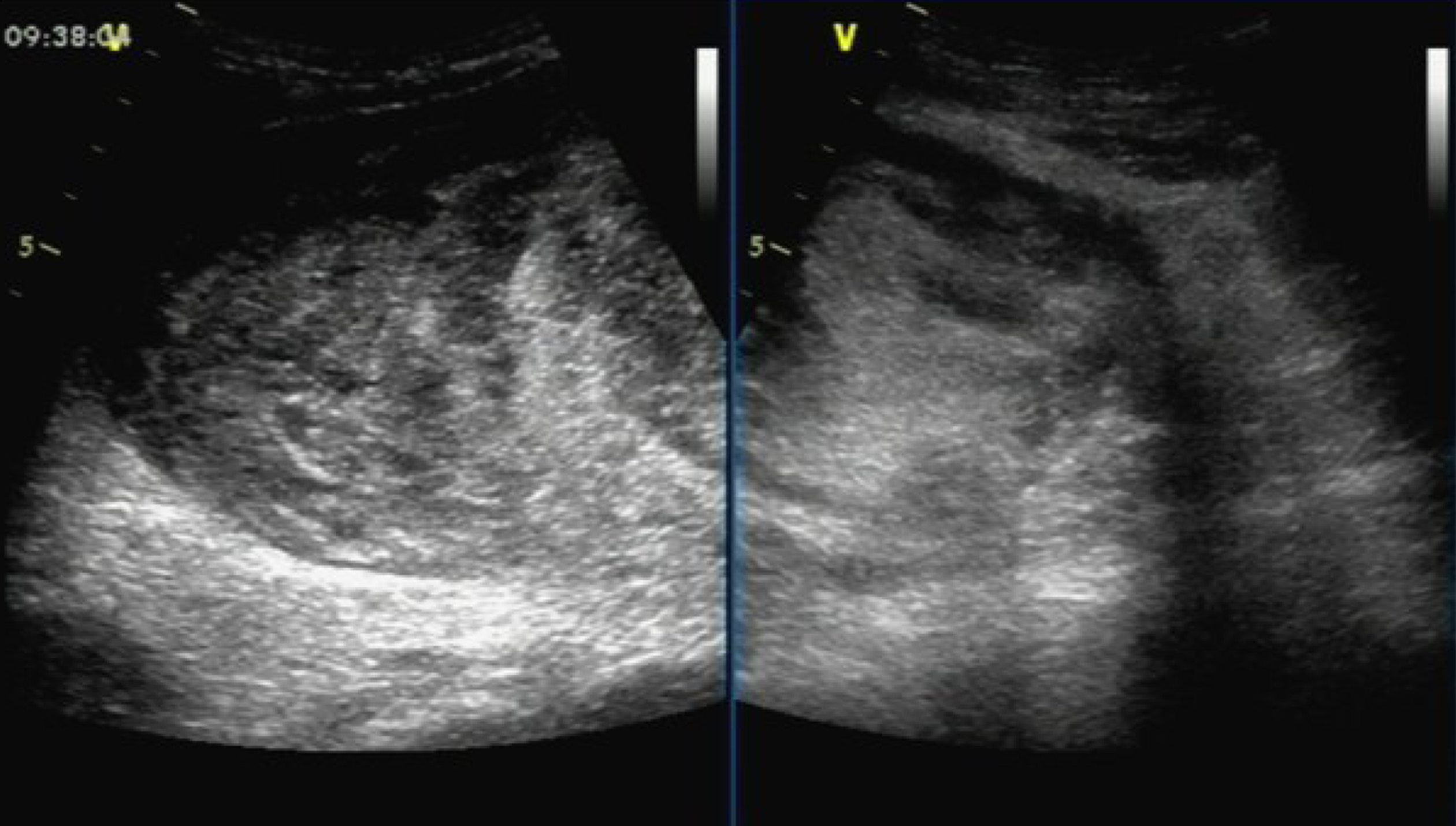
Ultrasound examination showed a solid hypoechoic mass (dimensions: 18.5 cm × 15.5 cm × 8.8 cm) in the right middle abdomen. The upper and lower margins reached the subcostal region, and the subumbilicus, respectively. The boundary was clear, the morphology was irregular, and the internal echo was not uniform. Marked echo interheterogenicity was observed in the center, and blood flow signals were detected around and within it through color Doppler flow imaging; in the lower abdomen, there were several echo groups with strong echo intensity, and the boundary was unclear. The dimensions of the largest mass in the right and left lower abdomen were approximately 8.3 cm × 5.0 cm and 7.9 cm × 4.4 cm, respectively; both had a vague boundary and an irregular shape (Figure 1).
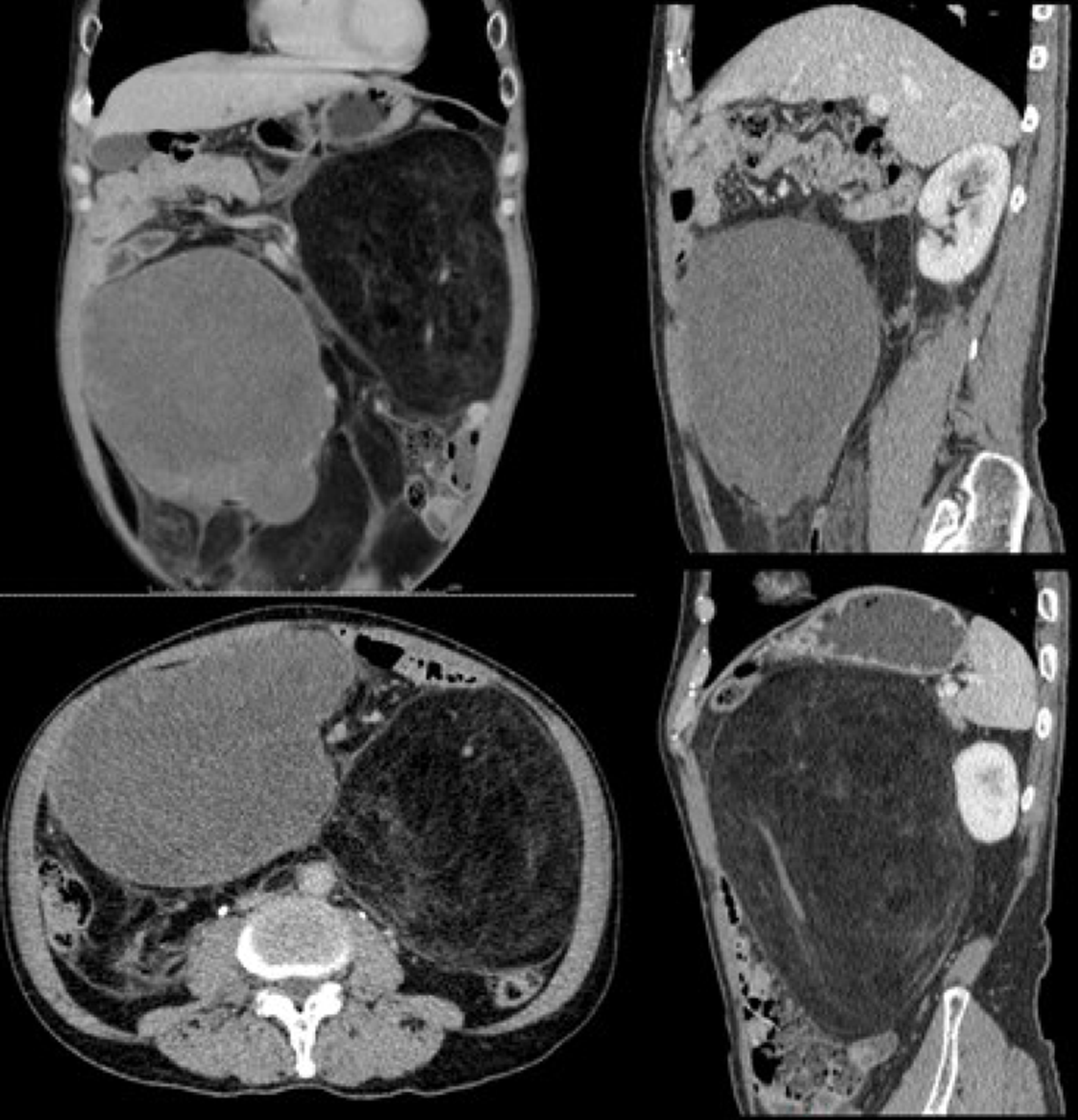
Abdominal-pelvic computed tomography (CT) revealed multiple low-density masses in the abdominal cavity. The dimensions of the largest mass in the left abdominal cavity were approximately 14.7 cm × 13.2 cm, and the mass had a clear boundary and convoluted vascular course. After performing contrast-enhanced scans, the mass did not exhibit significant enhancement with a -65 HU value. Another mixed-density mass was observed in the right abdominal cavity, with dimensions of approximately 15.4 cm × 10.6 cm; following contrast-enhanced scans, its CT values during the three phases were approximately 28/31/50 HU (Figure 2).
PRPLS was diagnosed based on the reported clinical symptoms, auxiliary examination, and postoperative pathology.
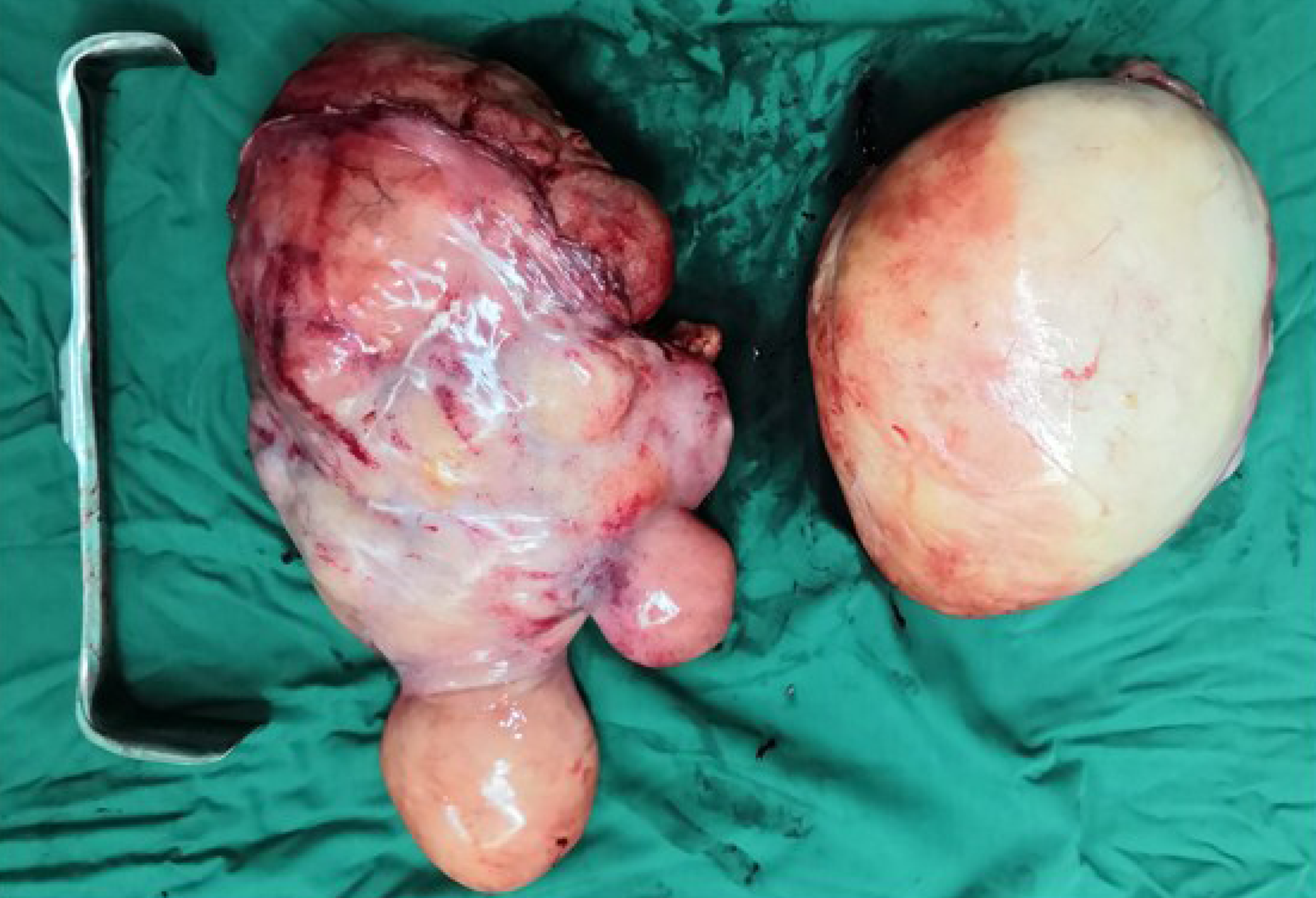
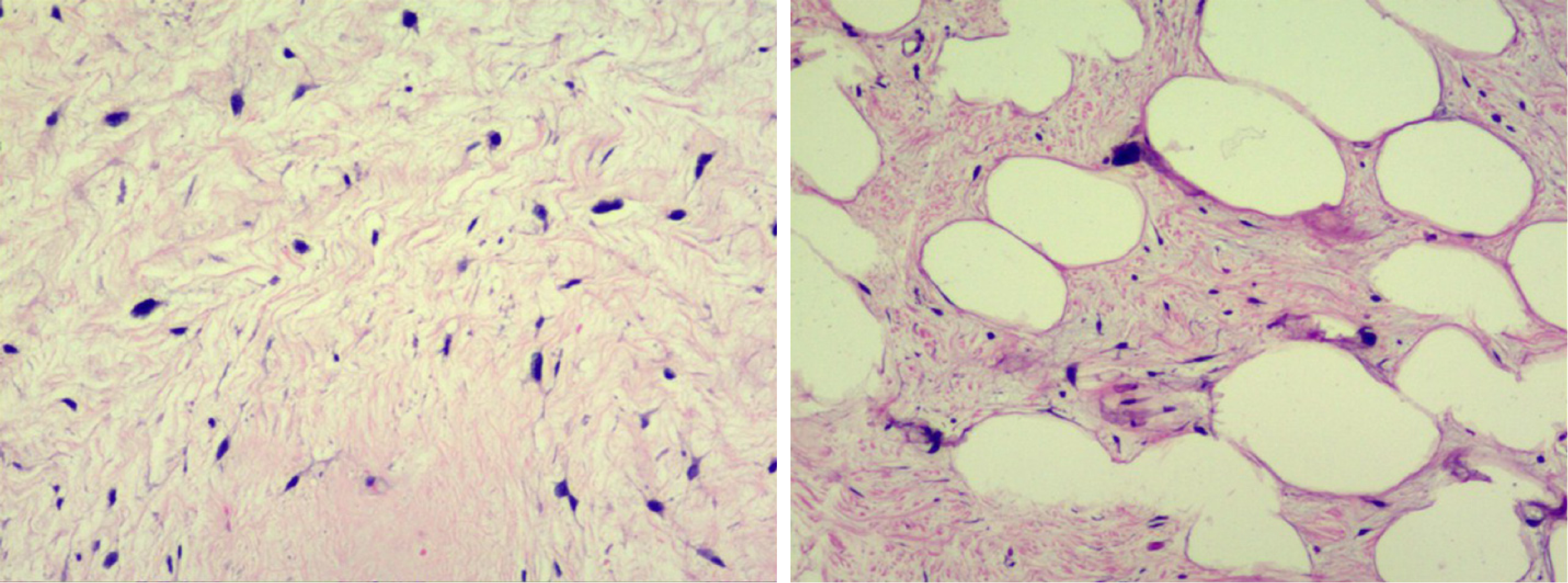
During surgery, we observed that both tumors were located in the retroperitoneal space near the mesenteric root with a clear limit out of the surrounding tissue. One tumor (35 cm × 22 cm × 20 cm) on the right side of the retroperitoneum had a lobulated shape. The other tumor (25 cm × 18 cm × 17 cm) on the left side of the retroperitoneum had a regular shape (Figure 3). Both tumors had been identified as atypical lipomatous tumor/well-differentiated liposarcoma by postoperative pathology. Moreover, microscopically, they were characterized by a leaf-like pattern aggregate of mature adipocytes separated by fibrous septa (Figure 4).
The patient recovered smoothly without severe complications. There was no recurrence or metastasis found by the abdominal-pelvic CT during the follow-up at 3, 6, 12, and 18 mo.
Liposarcoma originates from primitive mesenchymal cells and usually occurs in the retroperitoneum, with approximately 35% of cases arising from perinephric fat[1]. PRPLS accounts for approximately 45% of soft tissue sarcoma cases[2,3] with a prevalence of 0.3-0.4 cases per 100000 inhabitants[4]. According to the morphological characteristics and cytogenetic variation, PRPLS is categorized by the World Health Organization into five subtypes: Well-differentiated; dedifferentiated; myxoid; pleomorphic; and mixed-type[12]. The first two types are the most common in PRPLS[13], while the rarer subtypes (i.e., myxoid and pleomorphic) occur less often in the retroperitoneum[14].
Owing to the special position of PRPLS, the patients do not have obvious clinical manifestations in the early stages of the disease. Moreover, the tumors are often detected only after they are palpable in the abdomen. Since PRPLS has no characteristic symptoms, the complaints of patients commonly relate to discomfort, bellyache, bloating, and direct invasion or compression of other adjacent organs (e.g., the stomach, intestines, kidney, bladder, or great vessels). In this case, the patient presented with a complaint of abdominal bloating.
There are no significant laboratory abnormalities observed in the early stages[5]. The preferred diagnostic method for PRPLS is examination through CT[15], which is also the essential reference standard in tumor staging and preoperative evaluation[7,15]. The typical CT imaging of PRPLS includes legibility of low-density tissue as fat component with large size and non-enhancement during the arterial phase. In a small number of cases, the kidney is usually displaced by mass compression or surrounded by the mass. This report shows two different patterns of appearance of PRPLS on CT, which have the same pathological type: Atypical lipomatous tumor/well-differentiated liposarcoma. One was observed in the left abdominal cavity, and demonstrated a fat-like density with a -65 HU value, a clear boundary, and a convoluted vascular course. Moreover, it had no significant enhancement after contrast-enhanced scanning. The other larger mass was a mixed-density mass observed in the right abdominal cavity, after contrast-enhanced scanning; its CT values during the three phases were approximately 28/31/50 HU.
In some cases, PRPLS was detected accidentally during a regular health checkup by ultrasound and color Doppler, which are preferred imaging methods to distinguish vascular lesions and soft tissue sarcoma because they can provide detailed information about vascular anatomy[16,17]. Recently, with the development of contrast-enhanced ultrasound technique that can effectively diagnose abdominal parenchymal diseases and vascular lesions in real time and without the use of ionizing radiation[18-21], it may provide a new perspective and possibility for researchers on the diagnosis of PRPLS.
At present, surgical treatment remains one of the primary modes of therapy for PRPLS[6]. However, for patients who underwent macroscopically complete resection of PRPLS, a high recurrence rate remains following operation[9]. PRPLS grows in a special location; hence, the tumor often has a large volume at the time of diagnosis. Enlargement of the tumor in the retroperitoneal space may drastically change the normal anatomy of the surrounding organs and great vessels through compression. This effect poses difficulties to surgons regarding the protection of the important organs and great vessels during surgery[22,23].
PRPLS is rarely confirmed with metastasis through the lymph gland. Thus, it is not essential to perform regional lymphadenectomy. The recurrence rate can be reduced by completely removing the tumor and the surrounding suspicious adipose tissue[24]. In patients in whom the tumor has invaded adjacent organs or cannot be easily separated from great vessels, combined devisceration is an effective approach to reducing the risk of intraoperative hemorrhage and increasing the possibility of intact excision[24].
Postoperative recurrence of PRPLS, especially local recurrence, is relatively common. Previous studies have shown that the 5-, 8-, and 10-year crude cumulative incidences of local recurrence were 25.9%, 31.3%, and 35%[10]. Different treatment policies influence the local recurrence outcomes of well-differentiated and dedifferentiated PRPLS without impacting overall survival, whereas deaths are related to locoregional recurrences[10]. Resection of recurrent tumors is associated with prolonged patient survival[11].
Given that the possibility of cure after recurrence is low, a relatively conservative approach is taken for the resection of recurrent PRPLS after an extended primary procedure. The goal is to excise the recurrent tumor directly invading organs, rather than performing a wider excision, as performed for PRPLS[25].
PRPLS is a group of rare diseases, and associated with high recurrence rates, thus, surgical treatment and careful long-term clinical follow-up are warranted. It is a remarkable fact that multiple large well-differentiated liposarcomas with different patterns of appearance on CT image can occur simultaneously in the same patient, to which more attention should be paid to make an effective differential diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corvino A, Kimura Y S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Hasegawa T, Seki K, Hasegawa F, Matsuno Y, Shimodo T, Hirose T, Sano T, Hirohashi S. Dedifferentiated liposarcoma of retroperitoneum and mesentery: varied growth patterns and histological grades--a clinicopathologic study of 32 cases. Hum Pathol. 2000;31:717-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol. 2014;11:187-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12135] [Cited by in F6Publishing: 12706] [Article Influence: 1588.3] [Reference Citation Analysis (2)] |
| 4. | Mettlin C, Priore R, Rao U, Gamble D, Lane W, Murphy P. Results of the national soft-tissue sarcoma registry. J Surg Oncol. 1982;19:224-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Yang J, Zhao Y, Zheng CH, Wang Q, Pang XY, Wang T, Ma JJ. Huge retroperitoneal liposarcoma with renal involvement requires nephrectomy: A case report and literature review. Mol Clin Oncol. 2016;5:607-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Garcia-Ortega DY, Villa-Zepeda O, Martinez-Said H, Cuellar-Hübbe M, Luna-Ortiz K. Oncology outcomes in Retroperitoneal sarcomas: Prognostic factors in a Retrospective Cohort study. Int J Surg. 2016;32:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Nathenson MJ, Barysauskas CM, Nathenson RA, Regine WF, Hanna N, Sausville E. Surgical resection for recurrent retroperitoneal leiomyosarcoma and liposarcoma. World J Surg Oncol. 2018;16:203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Datta J, Ecker BL, Neuwirth MG, Geha RC, Fraker DL, Roses RE, Karakousis GC. Contemporary reappraisal of the efficacy of adjuvant chemotherapy in resected retroperitoneal sarcoma: Evidence from a nationwide clinical oncology database and review of the literature. Surg Oncol. 2017;26:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Gyorki DE, Choong PFM, Slavin J, Henderson MA. Importance of preoperative diagnosis for management of patients with suspected retroperitoneal sarcoma. ANZ J Surg. 2018;88:274-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, Van Coevorden F, Rutkowski P, Callegaro D, Hayes AJ, Honoré C, Fairweather M, Cannell A, Jakob J, Haas RL, Szacht M, Fiore M, Casali PG, Pollock RE, Raut CP. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients From the Multi-institutional Collaborative RPS Working Group. Ann Surg. 2016;263:1002-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 332] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 11. | Fairweather M, Gonzalez RJ, Strauss D, Raut CP. Current principles of surgery for retroperitoneal sarcomas. J Surg Oncol. 2018;117:33-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Fletcher CD. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology. 2014;64:2-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 320] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 13. | van Houdt WJ, Zaidi S, Messiou C, Thway K, Strauss DC, Jones RL. Treatment of retroperitoneal sarcoma: current standards and new developments. CurrOpin Oncol. 2017;29:260-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Vijay A, Ram L. Retroperitoneal liposarcoma: a comprehensive review. Am J Clin Oncol. 2015;38:213-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Messiou C, Moskovic E, Vanel D, Morosi C, Benchimol R, Strauss D, Miah A, Douis H, van Houdt W, Bonvalot S. Primary retroperitoneal soft tissue sarcoma: Imaging appearances, pitfalls and diagnostic algorithm. Eur J Surg Oncol. 2017;43:1191-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Corvino A, Catalano O, Corvino F, Sandomenico F, Setola SV, Petrillo A. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound? J Ultrasound. 2016;19:197-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Corvino A, Sandomenico F, Corvino F, Campanino MR, Verde F, Giurazza F, Tafuri D, Catalano O. Utility of a gel stand-off pad in the detection of Doppler signal on focal nodular lesions of the skin. J Ultrasound. 2020;23:45-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Corvino A, Catalano O, de Magistris G, Corvino F, Giurazza F, Raffaella N, Vallone G. Usefulness of doppler techniques in the diagnosis of peripheral iatrogenic pseudoaneurysms secondary to minimally invasive interventional and surgical procedures: imaging findings and diagnostic performance study. J Ultrasound. 2020;23:563-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Corvino A, Sandomenico F, Setola SV, Corvino F, Pinto F, Catalano O. Added value of contrast-enhanced ultrasound (CEUS) with Sonovue® in the diagnosis of inferior epigastric artery pseudoaneurysm: report of a case and review of literature. J Ultrasound. 2019;22:485-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Corvino A, Catalano O, Corvino F, Sandomenico F, Petrillo A. Diagnostic Performance and Confidence of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Cystic and Cysticlike Liver Lesions. AJR Am J Roentgenol. 2017;209:W119-W127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Corvino A, Sandomenico F, Setola SV, Corvino F, Tafuri D, Catalano O. Morphological and dynamic evaluation of complex cystic focal liver lesions by contrast-enhanced ultrasound: current state of the art. J Ultrasound. 2019;22:251-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Strauss DC, Hayes AJ, Thway K, Moskovic EC, Fisher C, Thomas JM. Surgical management of primary retroperitoneal sarcoma. Br J Surg. 2010;97:698-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Rutkowski P, Trepka S, Ptaszynski K, Kołodziejczyk M. Surgery quality and tumor status impact on survival and local control of resectable liposarcomas of extremities or the trunk wall. Clin OrthopRelat Res. 2013;471:860-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Bonvalot S, Miceli R, Berselli M, Causeret S, Colombo C, Mariani L, Bouzaiene H, Le Péchoux C, Casali PG, Le Cesne A, Fiore M, Gronchi A. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol. 2010;17:1507-1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 25. | MacNeill AJ, Miceli R, Strauss DC, Bonvalot S, Hohenberger P, Van Coevorden F, Rutkowski P, Callegaro D, Hayes AJ, Honoré C, Fairweather M, Cannell A, Jakob J, Haas RL, Szacht M, Fiore M, Casali PG, Pollock RE, Raut CP, Gronchi A, Swallow CJ. Post-relapse outcomes after primary extended resection of retroperitoneal sarcoma: A report from the Trans-Atlantic RPS Working Group. Cancer. 2017;123:1971-1978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |