Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.540
Peer-review started: August 6, 2020
First decision: November 14, 2020
Revised: November 28, 2020
Accepted: December 10, 2020
Article in press: December 10, 2020
Published online: January 26, 2021
Processing time: 166 Days and 23.7 Hours
Malignancy prediction remains important to preoperative diagnosis and postoperative follow-up in laryngeal neoplasm.
To evaluate the circulating immune population and develop a nomogram for prediction of malignancy in patients with laryngeal neoplasm.
A primary cohort of 156 patients was divided into laryngeal benign lesion, premalignant lesion and malignant lesion groups. Peripheral blood from patients was measured by blood routine test and flow cytometry. A nomogram was developed and applied to a validation cohort containing 55 consecutive patients.
Age, gender and seven circulating immune parameters exhibited significant differences between laryngeal benign lesion and premalignant lesion. The nomogram incorporated predictors, including gender, age, smoke index, proportions of monocytes, CD8+ T cells, CD4+ T cells, B cells and CD4/CD8+ T cell ratio. It showed good discrimination between laryngeal premalignant lesion and malignant lesion, with a C-index of 0.844 for the primary cohort. Application of this nomogram in the validation cohort (C-index, 0.804) still had good discrimination and good calibration. Decision curve analysis revealed that the nomogram was clinically useful.
This novel nomogram, incorporating both clinical risk factors and circulating immune parameters, could be appropriately applied in preoperative individualized prediction of malignancy in patients with laryngeal neoplasm.
Core Tip: Malignancy prediction remains important to preoperative diagnosis and postoperative follow-up in laryngeal neoplasm. There are continuing problems in differentiating premalignant lesions from malignant lesions of the larynx before surgery. Our finding suggested that laryngeal malignant lesions and premalignant lesions exhibit changes in the circulating immune phenotype. This circulating immune parameters-based novel nomogram could be appropriately applied in preoperative individualized prediction of malignancy in patients with laryngeal neoplasm.
- Citation: Chen M, Fang Y, Yang Y, He PJ, Cheng L, Wu HT. Circulating immune parameters-based nomogram for predicting malignancy in laryngeal neoplasm. World J Clin Cases 2021; 9(3): 540-551
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/540.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.540
Laryngeal cancer remains one of the most common tumors of the upper respiratory tract. The American Cancer Society estimated 177422 new cases of laryngeal cancer and 94771 deaths from laryngeal cancer worldwide in 2018[1]. When analyzed by tumor stage, cure rates for patients diagnosed with limited disease (T1, 2) are excellent, ranging from 80% to 90%. Unfortunately, approximately 60% of patients are still diagnosed with locally advanced (T3, 4) disease or regional nodal metastases, where survival rates are generally less than 50%[2]. Diagnostic delay is one of the main factors influencing prognosis in laryngeal cancer. According to the data from the English National Audit of Cancer Diagnosis in Primary Care of 28 cancers, laryngeal cancer had the fifth-longest delay in primary care referral[3]. Anything that can improve early diagnosis is an essential step in the right direction[4].
Laryngeal premalignant lesion is defined as an altered epithelium with an increased tendency of progression to laryngeal cancer. The altered epithelium exhibits diverse cytological and architectural changes that have traditionally been brought under the common denominator of dysplasia[5,6]. The malignant transformation rate is 14% rate[7]. There are continuing problems in differentiating premalignant lesions from malignant lesions of the larynx before surgery. Similar to other solid tumors, the exploration to define biomarkers with a discriminated impact on disease nature is a promising and diagnostic relevant research topic.
Classically, genetic markers were described in laryngeal cancer[8]. The innate and adaptive immune system in the carcinogenesis of many cancers is a topic of primary interest in the past decades[9,10]. The immune surveillance theory nominates specific functions to different leukocyte populations: Neutrophils, macrophages and CD4+ T cells induce a cytokine environment favoring chronic inflammation, whereas natural killer cells and CD8+ T cells comprise tumor suppressor populations[11]. Although those traditional groups have provided specific genetic or immune mediators in laryngeal cancer, reliable circulating immune markers with diagnostic value in the early stage are still lacking[12]. In addition, accurate predictive tools will augment the ability to diagnose laryngeal cancer.
A nomogram is a graphic representation of a statistical model specifically designed to maximize predictive accuracy[13]. In contrast to traditional models that allocate outcomes based on risk group, nomogram provides predictive information based on a combination of variables that take a more individualized outcome prediction into consideration. Several studies have indicated the superiority of multiplex predictive modeling in providing improved accuracy compared with risk group assignment techniques[14]. The nomogram is useful to surgeons in identifying patients “at risk” who should be targeted for aggressive treatment[15].
Here, frequencies of circulating immune populations in patients with laryngeal lesions were investigated. We developed and validated a nomogram that incorporated both the clinical risk factors and circulating immune parameters for individual prediction of cancer in patients with laryngeal neoplasm.
Ethical approval was obtained and informed consent was signed for this study. The primary cohort was comprised of patients who underwent microlaryngoscopic surgery after general anesthesia from January 2019 to September 2019 at the Eye, Ear, Nose, and Throat Hospital of Fudan University (Shanghai, China). Inclusion criteria included the following: Initial pathological diagnosis of laryngeal polyps or cysts, laryngeal dysplasia, and laryngeal squamous cell carcinoma; with lesions of glottis; and no previous history of laryngeal cancer. Exclusion criteria were as follows: With medical history of other malignancies or autoimmune disease; treatment with radiotherapy or chemotherapy; with use of steroid during the previous 6 mo; and blood transfusion within the previous 12 mo.
From September 2019 to January 2020, an independent validation cohort of 55 consecutive patients with laryngeal dysplasia or laryngeal squamous cell carcinoma was included using the same criteria as that for the primary cohort.
Information of participants containing gender, age, smoking status, alcohol consumption, laryngoscopic images and pathological results was extracted. Smoking status was classified as nonsmokers (with no history of smoking or having not smoked for at least 5 years) and smokers. Smoking index was calculated as number of cigarettes consumed per day multiplied by years of smoking. Alcohol consumption was classified as drinkers (with consumption of > 80 mL pure alcohol per day) and nondrinkers. Drinking index was calculated as volume of pure alcohol consumed per day multiplied by years of drinking. Based on pathological results, laryngeal benign lesion was defined as laryngeal polyps or cysts, laryngeal premalignant was defined as laryngeal dysplasia, and laryngeal malignant lesion was defined as laryngeal squamous cell carcinoma.
Routine blood examination was performed within 1 wk before surgery. Lymphocyte to monocyte ratio (LMR) was calculated as the absolute lymphocyte counts divided by the absolute monocyte counts. Neutrophil to lymphocyte ratio was calculated as the absolute neutrophil counts divided by the absolute lymphocyte counts. The determination of absolute numbers of CD45+ cell, CD3+ T cell, CD4+ T cell, CD8+ T cell, natural killer (NK) cell and B cell was monitored by 50 μL of whole blood from the patient by the TBNK multitest. After lysis of red blood cells, samples were analyzed using FACS Canto flow cytometer and FACS Canto software (both from BD Biosciences, San Jose, CA, United States). The proportions of CD3+ T cell, CD4+ T cell, CD8+ T cell, NK cell and B cell were calculated based on the CD45+ cell.
Analysis was carried out using SPSS version 20.0 (IBM Corp., Chicago, IL, United States) and R software (version 3.6.1; http://www.R-project.org) with the ggplot2, magrittr, ggpubr, glmnet, rms and Hmisc libraries added. The P values < 0.05 were considered statistically significant. Comparisons of categorical variables and quantitative variables in three groups were compared by Kruskal-Wallis test followed by Nemenyi test and one-way analysis of variance followed by least significant difference test, respectively. The optimal cut-off value of variables was defined by receiver operating characteristic curve analysis of which point closest to both maximum sensitivity and specificity. Data was converted to binary variables based on the optimal cut-off points. The least absolute shrinkage and selection operator (LASSO) method was used to select the potential predictive features from the primary cohort with laryngeal premalignant lesion and laryngeal malignant lesion. Then multivariable logistic regression analysis was performed in immune features selected from the LASSO method. Screened predictors were applied to develop a predictive model for risk of laryngeal cancer, which was presented by a nomogram. Nomogram validation contained two activities. First, Harrell’s C-index was measured to quantify the discrimination performance of this nomogram. A C-index greater than 0.75 is generally recognized as relatively good discrimination. Second, a calibration curve was depicted to evaluate the calibration of the nomogram. In addition, we evaluated the clinical utility through decision curve analysis, which quantified net benefit at different threshold probabilities in the validation data.
Clinical and immune cell data of the primary cohort were given in Table 1. Of 156 patients who met the criteria for inclusion, 50 (32.1%) patients were in the benign lesions group, 54 (34.6%) patients were in the premalignant lesions group, and 52 (33.3%) patients were in the malignant lesions group.
| Variables | Laryngeal benign lesion | Laryngeal premalignant lesion | Laryngeal malignant lesion | P value |
| Age, yr | 50.58 ± 8.66 | 59.91 ± 9.36 | 61.67 ± 9.58 | < 0.001 |
| Gender, male, n (%) | 37 (74.0%) | 52 (96.3%) | 52 (100%) | < 0.001 |
| Smokers, n (%) | 14 (28.0%) | 18 (33.3%) | 29 (55.8%) | 0.009 |
| Smoke index | 123.66 ± 245.44 | 235.74 ± 424.28 | 484.31 ± 447.77 | < 0.001 |
| Drinkers, n (%) | 6 (12.0%) | 9 (16.7%) | 14 (26.9%) | 0.140 |
| Drinker index | 1.40 ± 4.85 | 2.43 ± 7.21 | 4.37 ± 8.38 | 0.096 |
| Lymphocytes, % | 33.30 ± 9.02 | 29.89 ± 9.37 | 29.66 ± 8.57 | 0.077 |
| Monocytes, % | 6.86 ± 3.47 | 6.73 ± 1.41 | 7.80 ± 2.36 | 0.064 |
| Neutrophils, % | 57.86 ± 9.61 | 60.25 ± 11.08 | 59.95 ± 8.82 | 0.414 |
| NLR | 1.98 ± 1.02 | 2.43 ± 1.49 | 2.31 ± 1.09 | 0.158 |
| LMR | 5.29 ± 1.82 | 4.50 ± 1.33 | 4.24 ± 2.26 | 0.012 |
| CD3+ T cells, % | 68.48 ± 8.41 | 62.12 ± 12.00 | 61.82 ± 8.93 | 0.001 |
| CD8+ T cells, % | 25.77 ± 7.83 | 23.64 ± 7.82 | 23.30 ± 7.18 | 0.210 |
| CD4+ T cells, % | 39.13 ± 7.16 | 35.09 ± 8.21 | 36.11 ± 9.27 | 0.039 |
| NK cells, % | 17.35 ± 8.71 | 25.40 ± 12.65 | 24.80 ± 9.21 | < 0.001 |
| B cells, % | 13.46 ± 4.14 | 11.51 ± 4.29 | 12.31 + 6.63 | 0.157 |
| CD4/CD8+ T cell ratio | 1.71 ± 0.72 | 1.64 + 0.63 | 1.78 + 0.94 | 0.629 |
| NK/CD3+ T cell ratio | 0.27 ± 0.20 | 0.47 ± 0.45 | 0.44 ± 0.22 | 0.003 |
| NK/CD4+ T cell ratio | 0.46 ± 0.29 | 0.85 ± 0.78 | 0.79 ± 0.42 | 0.001 |
| NK/CD8+ T cell ratio | 0.75 ± 0.73 | 1.28 ± 1.35 | 1.23 ± 0.74 | 0.014 |
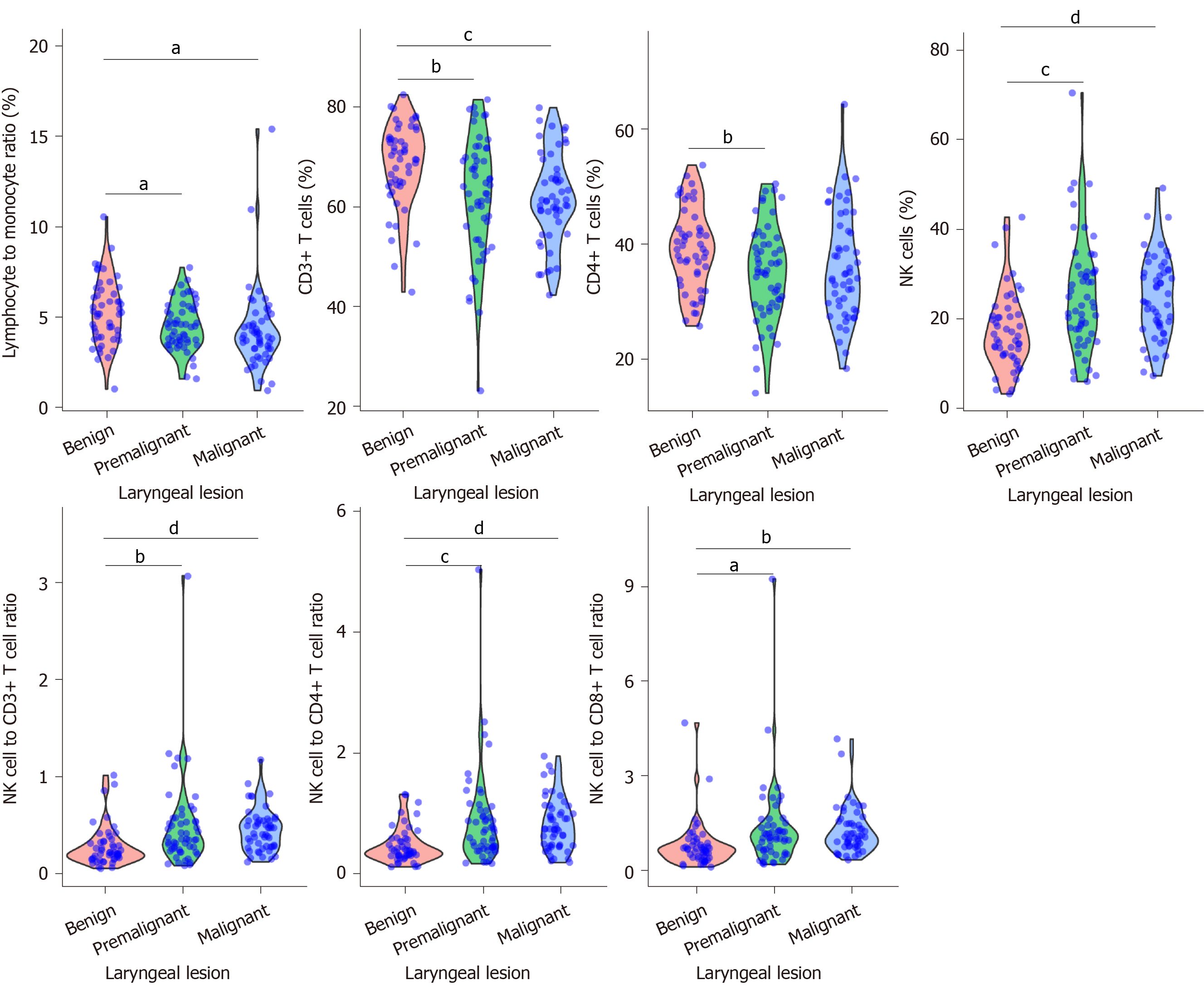
Age, gender, smoke status, and index among the three groups were significantly different (Table 1). Following pairwise comparisons, patients with laryngeal benign lesions had a significantly lower age and male proportion than those with premalignant and malignant lesions (age, benign vs premalignant, P < 0.001, benign vs malignant, P < 0.001, least significant difference test; gender, benign vs premalignant, P < 0.001, benign vs malignant, P < 0.001, Nemenyi test). Patients with laryngeal malignant lesions had significantly more smokers and a higher smoke index than those with benign and premalignant lesions (smokers, malignant vs benign, P = 0.013, malignant vs premalignant, P = 0.045, Nemenyi test; smoke index, malignant vs benign, P < 0.001, malignant vs premalignant, P = 0.001, least significant difference test).
Violin plot analysis revealed seven circulating immune cell parameters exhibited different distribution and significantly different levels between benign lesions and premalignant lesions (Figure 1). Although it showed a tendency of decreasing in LMR, CD3+ T cell proportion and CD4+ T cell proportion and a tendency of increasing in NK cell proportion, NK/CD3+ T cell ratio, NK/CD4+ T cell ratio and NK/CD8+ T cell ratio among the three groups, there were no significant differences in these levels between premalignant lesions and malignant lesions.
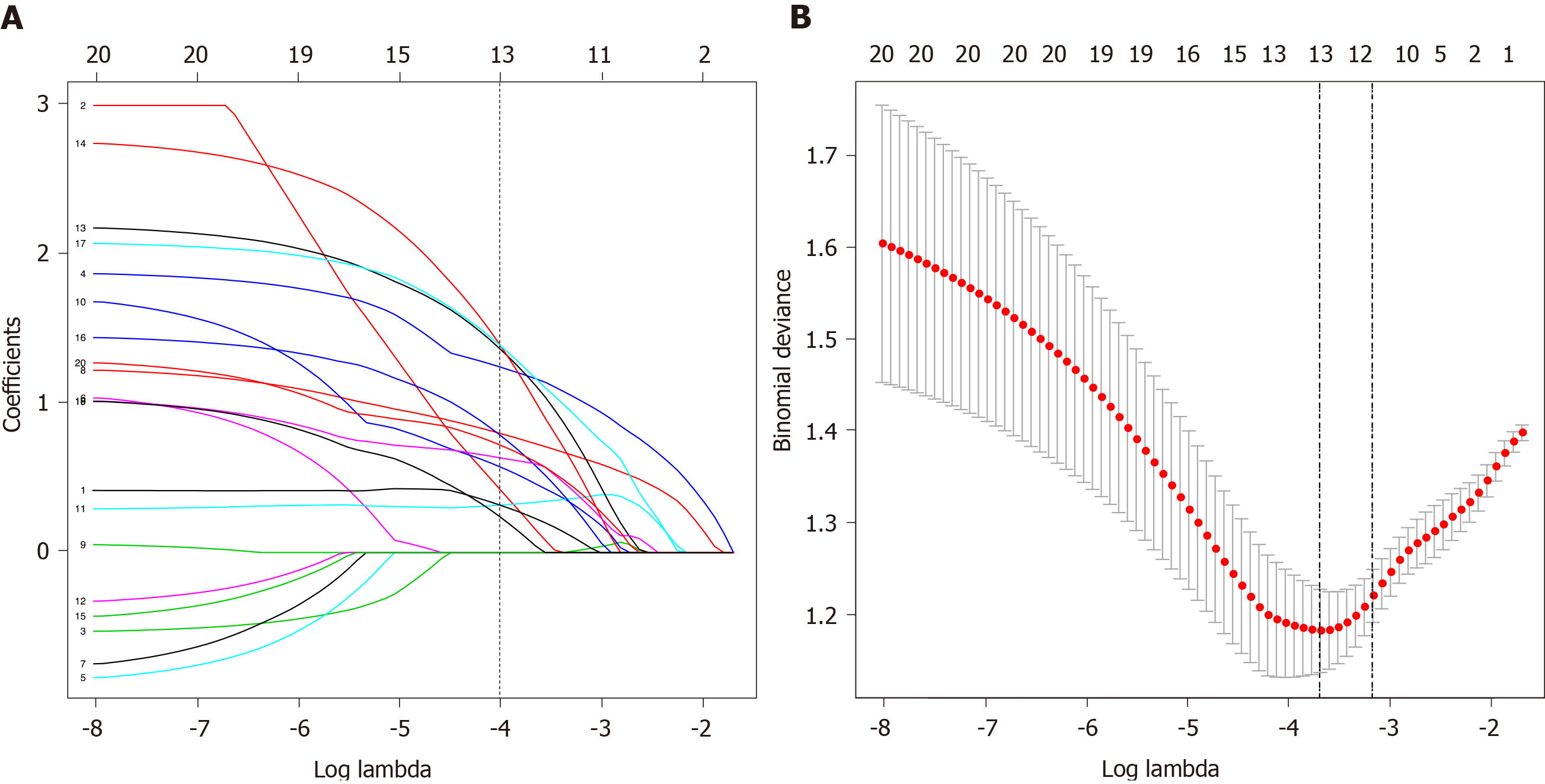
Feature selection was conducted in 54 patients with laryngeal premalignant lesions and 52 patients with laryngeal malignant lesions in the primary cohort. According to the optimal cut-off values defined by receiver operating characteristic curves (Supplementary Table 1 and Supplementary Figure 1), data were converted to binary variables. Of demographic, clinical and immune features, 20 features were lessened to 13 preliminary predictors on the basis of 106 patients in the primary cohort and were features with nonzero coefficients in the LASSO logistic regression model (Figure 2A and 2B).
These features included age, gender, smoke index, neutrophil to lymphocyte ratio, LMR, proportions of monocytes, CD8+ T cells, CD4+ T cells and B cells, CD4/CD8+ T cell ratio, NK/CD3+ T cell ratio, NK/CD4+ T cell ratio and NK/ CD8+ T cell ratio.
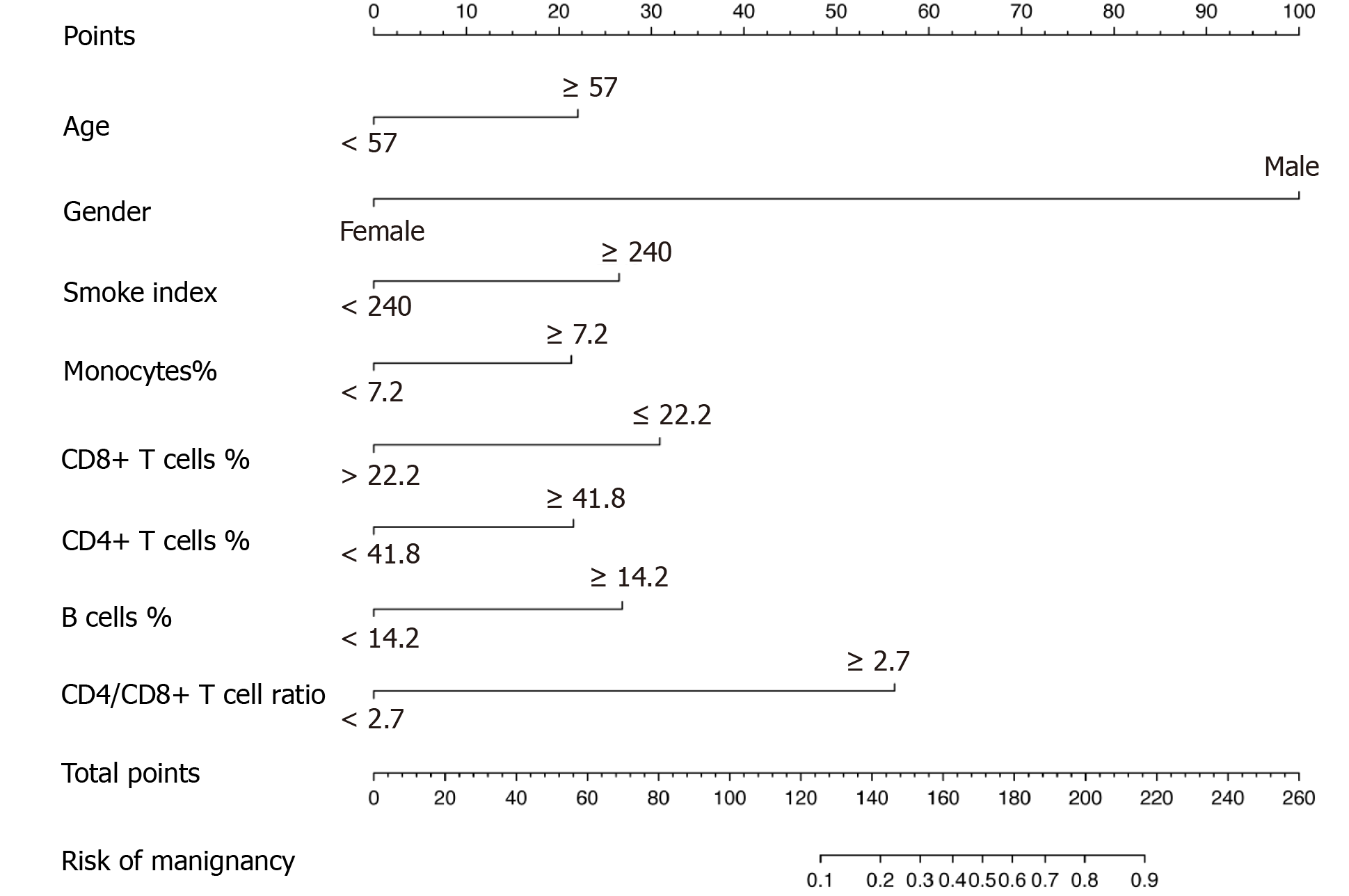
Furthermore, the multivariable logistic regression analysis identified the proportions of monocytes, CD8+ T cells, CD4+ T cells, B cells and CD4/CD8+ T cell ratio as independent immune predictors (Table 2). The model integrated the above circulating immune parameters and clinical factors (age, gender, smoke index) and was developed and presented as the nomogram (Figure 3). It yielded a C-index of 0.844 (95% confidence interval, 0.765-0.923) for the primary cohort.
| Intercept and variables |
β1 | Odds ratio (95%CI) | P value |
| Intercept | -5.500 | < 0.0001 | |
| Monocytes, % | 1.235 | 2.351 (1.258-10.066) | 0.019 |
| CD8+ T cells, % | 2.154 | 3.303 (2.562-33.856) | < 0.001 |
| CD4+ T cells, % | 2.701 | 2.730 (2.422-123.774) | < 0.001 |
| B cells, % | 1.464 | 2.315 (1.320-16.195) | 0.021 |
| CD4/CD8+ T cell ratio | 2.425 | 2.004 (1.413-247.045) | 0.045 |
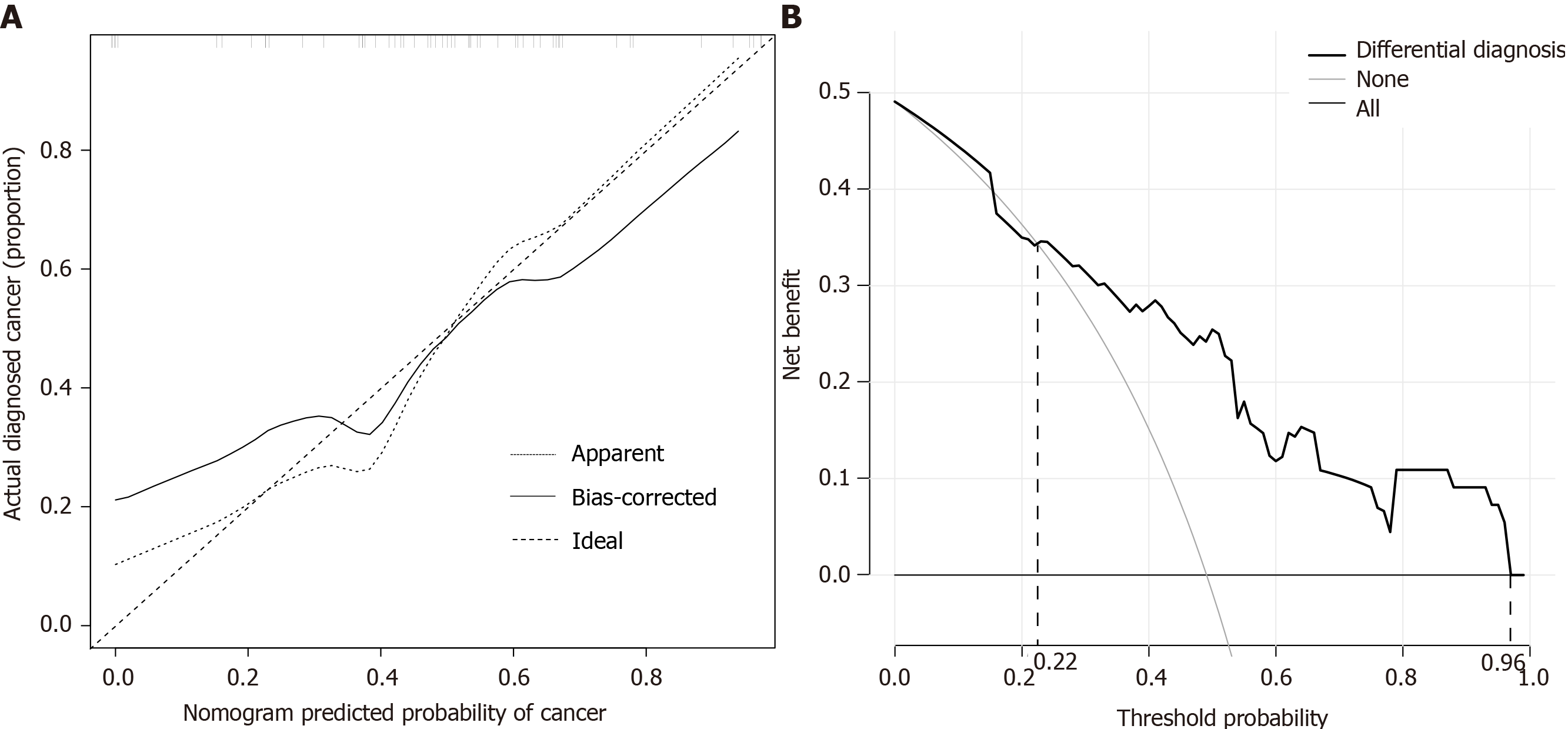
Clinical data and immune parameters of 55 patients in the validation cohort were provided in Supplementary Table 2. The calibration curve of the nomogram for the prediction of cancer risk exhibited good agreement in the validation cohort (Figure 4A). The C-index for the prediction nomogram was 0.804 (95% confidence interval, 0.688-0.920).
The decision curve analysis for cancer prediction nomogram was shown in Figure 4B. The decision curve displayed a threshold probability of > 22% and < 96%. Within this range, net benefit was comparable with the several overlaps on the basis of cancer prediction nomogram.
Laryngeal dysplasia is considered a laryngeal premalignant lesion due to its possibility to become malignant[16]. Demographic distinctions in advanced age and male predominance were found between laryngeal benign lesions and laryngeal premalignant lesions. Etiological studies of laryngeal dysplasia were restricted to external factors such as tobacco, alcohol, pepsin and microorganisms[5]. Internal factors, especially abnormal immunity, were ignored in previous studies. Abundant evidence from studies in cancer immunosurveillance suggested that variations of immune cells influence cancer development[17]. In our study, we focused on the analysis of circulating immune populations in different conditions of laryngeal lesions. Our data illustrated that circulating immune cells were involved in the stage of laryngeal lesion. These results corroborate the findings of the previous work that cancer and premalignant lesions correlate with an alteration of immune cells[18,19].
Host immune response to tumors is lymphocyte dependent. Decreased LMR and proportions of CD3+ T cells and CD4+ T cells in patients with laryngeal premalignant lesions mean relative lymphocytopenia and a deficient immune response to the tumor mediated by CD4+ T cells[20]. Opposite changes in the proportion of NK cells in the abnormal condition in premalignant lesions trigger innate immunity. The increases in NK cells to major lymphocyte subpopulation ratios were due to an increase in NK cells and decrease in lymphocytes. The altered circulating immune phenotype of laryngeal premalignant lesions was similar to the findings of studies on head and neck squamous cell carcinoma[21] and other cancers[22]. The field of immunotherapy, which targets immune cells to augment antitumor immune responses, has led to crucial clinical advances and supplied a new weapon against cancer[23]. Whether adjustment of immune abnormality in the stage of premalignant lesions could prevent or reduce the formation of tumors needs to be further studied.
In the present study, circulating immune cells exhibited different distribution patterns in laryngeal premalignant lesions and laryngeal malignant lesions. However, these levels showed no significant differences. For the construction of the determinants that could be used to establish the true premalignant and malignant character of laryngeal neoplasm, 20 candidate features were lessened to 13 preliminary predictors by testing the predictor-status correlation by the LASSO logistic regression model. This method enabled variable selection and regularization on the basis of model fitting. Multimarker screens that incorporate individual markers into panels have been generalized to recent studies. For instance, genomic markers and protein molecular markers were identified in the assessment of gastric cancer and lung cancer[24,25]. Similarly, ten immune cell parameters combined with three well-known risk factors were potential predictors for laryngeal malignant lesions. Furthermore, ten immune cell parameters were reduced to five final predictors following logistic regression analysis in the primary cohort (2:1 ratio), which demonstrated adequate discrimination between laryngeal premalignant lesions and laryngeal malignant lesions. These results support a previous study of head and neck squamous cell carcinoma that linked changes in immune cells to tumorigenesis[26]. A major strength of this study is unlike most other works, laryngeal malignant lesions were compared with premalignant lesions instead of the normal condition. In agreement with several studies focused on other premalignant lesions, including pancreatic intraepithelial neoplasia, gastric dysplasia and cervical precancerous lesions, we suggested that monocytes, T cells and B cells have possible roles for immunosurveillance of cancer[27-29].
On the basis of clinical risk factors and circulating immune cell parameters, we proposed and validated a diagnostic nomogram integrating eight items for preoperative individualized prediction malignancy risk in patients with laryngeal neoplasms. An advantage of this nomogram is that it is a weighted model, combining multipredictors and enabling appreciation of the magnitude of impact of each of the predictors on the risk of malignancy. Gender and CD4/CD8+ T cell ratio were the two major, heavily weighted factors in this model. Laryngeal cancer has one of the highest male-to-female ratios among all malignancies[30,31]. Not surprisingly, when evaluating for laryngeal cancer, gender remained a clinically relevant predictive factor. An increased CD4/CD8+ T cell ratio meant a relative reduction of CD8+ T cells, which were the principal weapons of antitumor immunity[32]. As mentioned in the literature[33,34], the CD4/CD8+ T cell ratio represented a marker of immune dysfunction and affected tumor progression. These factors may explain the correlation between CD4/CD8+ T cell ratio and risk of laryngeal cancer. It is noteworthy to mention that we first emphasized its role in the prediction risk of laryngeal cancer.
This nomogram performed well in discriminating cancer, and its discrimination ability was supported by the C-index (0.844 and 0.804 for the primary and validation cohorts, respectively). The calibration plot demonstrated a good agreement of predictive probabilities from the validation cohort. We also performed a decision curve analysis to assess the clinical utility of the nomogram by quantifying the net benefit when different threshold probabilities were considered. Thus, we found that the application of our nomogram to the present validation cohort provided an increase in the net benefit for threshold probability of > 22% and < 96%.
The nomogram is a predictive tool that is more multiplex than simple risk biomarkers and provides a tailored assessment of a given patient’s risk of malignancy. Diagnosis of laryngeal neoplasm is established by operative tissue biopsy and follow-up needed for high-risk patients. This could inform clinicians on diagnostic sampling of suspicious laryngeal lesions that reduces delay in diagnosis. It could also be used to monitor the onset and progression of laryngeal premalignant or malignant lesions in these individuals during the postoperative follow-up period. However, the association between this nomogram and tumor node metastasis (TNM) classification, the most common way of staging laryngeal malignant lesion, was not explored. We speculated that TNM classification could be predicted after the adjustments of this nomogram for the following reasons. The component parameters of the nomogram, clinical factors and immune index were related to the clinical stage of laryngeal cancer. Chen et al[35] constructed a nomogram model integrating clinical factors to evaluated lymph node metastasis of laryngeal squamous cell carcinoma. The significance of immune cells for the development of laryngeal cancer has been reported previously. In addition, the nomogram based on immune score and TNM classification for prognostic evaluation of patients with laryngeal squamous cell carcinoma was proposed[36]. For predicting TNM classification accurately, future research should be undertaken to update this nomogram by specific clinical and immune cell parameter screening.
Although we have established a novel discriminating model based on risk factors and immune cell panels, this study indicated that there are several limitations. First, this is a single center study with a small sample size. Although this nomogram is internally validated, it would be consolidated by external validation with patients assessed at multiple institutions. Second, the circulating immune cell panels were found only as biomarker results of laryngeal premalignant and malignant lesions. Confirmatory studies investigating functional mechanism for carcinogenesis are desirable. Third, tumor infiltrating lymphocytes were not considered. Recently, tumor infiltrating lymphocytes have attracted increasing interest in the field of cancer research. However, it is yet to be decided whether the nomogram that applies to the circulating immune cells combined with tumor infiltrating lymphocytes to predict risk of laryngeal cancer is more accurate. Finally, it is not clear whether the observed circulating immune panels would influence the prognostic phenomenon.
In summary, we suggest that laryngeal cancer and laryngeal premalignant lesions exhibit changes in circulating immune phenotypes. The novel nomogram, incorporating both clinical risk factors and circulating immune cell parameters, could be appropriately applied in preoperative individualized prediction of malignancy in patients with laryngeal neoplasm.
Malignancy prediction remains important to preoperative diagnosis and postoperative follow-up in laryngeal neoplasm.
There are continuing problems in differentiating premalignant lesions from malignant lesions of the larynx before surgery.
To evaluate the circulating immune population and develop a nomogram for prediction of malignancy in patients with laryngeal neoplasm.
Peripheral blood from patients with laryngeal neoplasm was analyzed by blood routine test and flow cytometry. Circulating immune population and clinical parameters were screened to develop a predictive model for risk of laryngeal cancer, which was presented by a nomogram.
The nomogram incorporated predictors, including gender, age, smoke index, proportions of monocytes, CD8+ T cells, CD4+ T cells and B cells and CD4/CD8 + T cell ratio, showed good discrimination between laryngeal premalignant lesions and malignant lesions.
This circulating immune cell parameter-based novel nomogram could be appropriately applied in preoperative individualized prediction of malignancy in patients with laryngeal neoplasm.
Future research should be undertaken to assess the external validation of the nomogram in multiple institutions. Confirmatory studies investigating functional mechanism of circulating immune populations for laryngeal carcinogenesis are desirable.
We thank the technical assistance of the peripheral blood test kindly provided by the Department of Clinical Laboratory at Eye, Ear, Nose, and Throat Hospital of Fudan University in Shanghai, China.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiba T, Stomeo F S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55840] [Article Influence: 7977.1] [Reference Citation Analysis (132)] |
| 2. | Forastiere AA, Ismaila N, Wolf GT. Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract. 2018;14:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Lyratzopoulos G, Saunders CL, Abel GA, McPhail S, Neal RD, Wardle J, Rubin GP. The relative length of the patient and the primary care interval in patients with 28 common and rarer cancers. Br J Cancer. 2015;112 Suppl 1:S35-S40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Burki TK. Symptoms associated with risk of laryngeal cancer. Lancet Oncol. 2019;20:e135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Thompson LD. Laryngeal Dysplasia, Squamous Cell Carcinoma, and Variants. Surg Pathol Clin. 2017;10:15-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Gale N, Poljak M, Zidar N. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: What is New in the 2017 WHO Blue Book for Tumours of the Hypopharynx, Larynx, Trachea and Parapharyngeal Space. Head Neck Pathol. 2017;11:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Weller MD, Nankivell PC, McConkey C, Paleri V, Mehanna HM. The risk and interval to malignancy of patients with laryngeal dysplasia; a systematic review of case series and meta-analysis. Clin Otolaryngol. 2010;35:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Wei Q, Yu D, Liu M, Wang M, Zhao M, Liu M, Jia W, Ma H, Fang J, Xu W, Chen K, Xu Z, Wang J, Tian L, Yuan H, Chang J, Hu Z, Wei L, Huang Y, Han Y, Liu J, Han D, Shen H, Yang S, Zheng H, Ji Q, Li D, Tan W, Wu C, Lin D. Genome-wide association study identifies three susceptibility loci for laryngeal squamous cell carcinoma in the Chinese population. Nat Genet. 2014;46:1110-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol. 2020;17:204-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 504] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 10. | Wellenstein MD, de Visser KE. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity. 2018;48:399-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 438] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 11. | McCully ML, Kouzeli A, Moser B. Peripheral Tissue Chemokines: Homeostatic Control of Immune Surveillance T Cells. Trends Immunol. 2018;39:734-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Trivedi S, Rosen CA, Ferris RL. Current understanding of the tumor microenvironment of laryngeal dysplasia and progression to invasive cancer. Curr Opin Otolaryngol Head Neck Surg. 2016;24:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Chen D, Chen G, Jiang W, Fu M, Liu W, Sui J, Xu S, Liu Z, Zheng X, Chi L, Lin D, Li K, Chen W, Zuo N, Lu J, Chen J, Li G, Zhuo S, Yan J. Association of the Collagen Signature in the Tumor Microenvironment With Lymph Node Metastasis in Early Gastric Cancer. JAMA Surg. 2019;154:e185249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM, Koontz BF, Hamstra DA, Feng FY, Liauw SL, Abramowitz MC, Pollack A, Anscher MS, Moghanaki D, Den RB, Stephans KL, Zietman AL, Lee WR, Kattan MW, Stephenson AJ. Contemporary Update of a Multi-Institutional Predictive Nomogram for Salvage Radiotherapy After Radical Prostatectomy. J Clin Oncol. 2016;34:3648-3654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 288] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 15. | Carmona-Bayonas A, Jiménez-Fonseca P, Lamarca Á, Barriuso J, Castaño Á, Benavent M, Alonso V, Riesco-Martínez MDC, Alonso-Gordoa T, Custodio A, Sánchez Cánovas M, Hernando Cubero J, López C, Lacasta A, Fernández Montes A, Marazuela M, Crespo G, Escudero P, Diaz JÁ, Feliciangeli E, Gallego J, Llanos M, Segura Á, Vilardell F, Percovich JC, Grande E, Capdevila J, Valle JW, García-Carbonero R. Prediction of Progression-Free Survival in Patients With Advanced, Well-Differentiated, Neuroendocrine Tumors Being Treated With a Somatostatin Analog: The GETNE-TRASGU Study. J Clin Oncol. 2019;37:2571-2580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Karatayli-Ozgursoy S, Pacheco-Lopez P, Hillel AT, Best SR, Bishop JA, Akst LM. Laryngeal dysplasia, demographics, and treatment: a single-institution, 20-year review. JAMA Otolaryngol Head Neck Surg. 2015;141:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Chang RB, Beatty GL. The interplay between innate and adaptive immunity in cancer shapes the productivity of cancer immunosurveillance. J Leukoc Biol. 2020;108:363-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Maglietta A, Maglietta R, Staiano T, Bertoni R, Ancona N, Marra G, Resta L. The Immune Landscapes of Polypoid and Nonpolypoid Precancerous Colorectal Lesions. PLoS One. 2016;11:e0159373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Singer K, Dettmer K, Unger P, Schönhammer G, Renner K, Peter K, Siska PJ, Berneburg M, Herr W, Oefner PJ, Karrer S, Kreutz M, Datz E. Topical Diclofenac Reprograms Metabolism and Immune Cell Infiltration in Actinic Keratosis. Front Oncol. 2019;9:605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16:599-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 414] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 21. | Böttcher A, Ostwald J, Guder E, Pau HW, Kramp B, Dommerich S. Distribution of circulating natural killer cells and T lymphocytes in head and neck squamous cell carcinoma. Auris Nasus Larynx. 2013;40:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, Vahrmeijer AL, van de Velde CJH, Heemskerk MHM, Hokland M, Kuppen PJK. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol Immunother. 2019;68:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 23. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3580] [Article Influence: 358.0] [Reference Citation Analysis (0)] |
| 24. | Eberlin LS, Tibshirani RJ, Zhang J, Longacre TA, Berry GJ, Bingham DB, Norton JA, Zare RN, Poultsides GA. Molecular assessment of surgical-resection margins of gastric cancer by mass-spectrometric imaging. Proc Natl Acad Sci USA. 2014;111:2436-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Richard C, Fumet JD, Chevrier S, Derangère V, Ledys F, Lagrange A, Favier L, Coudert B, Arnould L, Truntzer C, Boidot R, Ghiringhelli F. Exome Analysis Reveals Genomic Markers Associated with Better Efficacy of Nivolumab in Lung Cancer Patients. Clin Cancer Res. 2019;25:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Solomon B, Young RJ, Rischin D. Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin Cancer Biol. 2018;52:228-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 311] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 27. | Bastea LI, Liou GY, Pandey V, Fleming AK, von Roemeling CA, Doeppler H, Li Z, Qiu Y, Edenfield B, Copland JA, Tun HW, Storz P. Pomalidomide Alters Pancreatic Macrophage Populations to Generate an Immune-Responsive Environment at Precancerous and Cancerous Lesions. Cancer Res. 2019;79:1535-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Hitzler I, Kohler E, Engler DB, Yazgan AS, Müller A. The role of Th cell subsets in the control of Helicobacter infections and in T cell-driven gastric immunopathology. Front Immunol. 2012;3:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Da Silva DM, Woodham AW, Skeate JG, Rijkee LK, Taylor JR, Brand HE, Muderspach LI, Roman LD, Yessaian AA, Pham HQ, Matsuo K, Lin YG, McKee GM, Salazar AM, Kast WM. Langerhans cells from women with cervical precancerous lesions become functionally responsive against human papillomavirus after activation with stabilized Poly-I:C. Clin Immunol. 2015;161:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Majszyk D, Bruzgielewicz A, Osuch-Wójcikiewicz E, Rzepakowska A, Niemczyk K. Gender-related incidence, risk factors exposure and survival rates of laryngeal cancers - the 10-years analysis of trends from one institution. Otolaryngol Pol. 2019;73:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Leoncini E, Vukovic V, Cadoni G, Giraldi L, Pastorino R, Arzani D, Petrelli L, Wünsch-Filho V, Toporcov TN, Moyses RA, Matsuo K, Bosetti C, La Vecchia C, Serraino D, Simonato L, Merletti F, Boffetta P, Hashibe M, Lee YA, Boccia S. Tumour stage and gender predict recurrence and second primary malignancies in head and neck cancer: a multicentre study within the INHANCE consortium. Eur J Epidemiol. 2018;33:1205-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Crispin JC, Tsokos GC. Cancer immunosurveillance by CD8 T cells. F1000Res. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc. 2015;18:20052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 34. | Li F, Sun Y, Huang J, Xu W, Liu J, Yuan Z. CD4/CD8 + T cells, DC subsets, Foxp3, and IDO expression are predictive indictors of gastric cancer prognosis. Cancer Med. 2019;8:7330-7344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 35. | Chen LY, Weng WB, Wang W, Chen JF. Analyses of High-Risk Factors for Cervical Lymph Node Metastasis in Laryngeal Squamous Cell Carcinoma and Establishment of Nomogram Prediction Model. Ear Nose Throat J. 2020;145561320901613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Liao L, Chen W, Lai H, Yi X, Wang D. Prognostic nomogram based on immune scores for laryngeal squamous cell cancer. Eur Arch Otorhinolaryngol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |