Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4873
Peer-review started: March 11, 2021
First decision: March 25, 2021
Revised: April 6, 2021
Accepted: April 28, 2021
Article in press: April 28, 2021
Published online: June 26, 2021
Processing time: 92 Days and 1.7 Hours
Early diagnosis and appropriate antibiotic treatment are important to survival of Listeria monocytogenes (L. monocytogenes) bacteremia. Penicillin tends to be the most commonly used antibiotic. However, there are limited data on antibiotic use in elderly patients with serious complications. We describe the clinical presentation, antibiotic therapy, and traceability of L. monocytogenes in a centenarian with a history of eating frozen food.
A 102-year-old man suffered from high fever with chill after hematochezia. Tentative diagnoses were lower gastrointestinal hemorrhage and localized peritonitis. Meropenem and ornidazole were the empirical therapy. The patient did not respond and developed multiple system dysfunction even after teicoplanin was added to the therapy. L. monocytogenes was identified from blood cultures on day 5 of admission. The patient had a history of consuming frozen dumplings. Meropenem/ornidazole/teicoplanin were replaced with merope
More awareness of listeriosis should be raised. Linezolid might be an option for listeriosis in elderly people with serious complications.
Core Tip: Appropriate antibiotic treatment is important to survival of Listeria monocytogenes bacteremia. Penicillin tends to be the most commonly used antibiotic. However, there are limited data on antibiotic use in elderly patients with serious complications. Linezolid might be valuable for treatment of the L. monocytogenes bacteremia. Geriatricians should be suspicious of listeriosis when patients do not respond to broad-spectrum antibiotics, and when patients have a history of frozen food consumption. Healthy eating habits and food processing methods should be prioritized in elderly people.
- Citation: Zhang ZY, Zhang XA, Chen Q, Wang JY, Li Y, Wei ZY, Wang ZC. Listeria monocytogenes bacteremia in a centenarian and pathogen traceability: A case report. World J Clin Cases 2021; 9(18): 4873-4880
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4873.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4873
Listeria monocytogenes (L. monocytogenes) is a facultative anaerobic, Gram-positive pathogenic bacte
The incidence of human listeriosis is low[6] but the mortality rate is high, especially in elderly people[5]. The rareness and long incubation period[7] of listeriosis make prompt diagnosis and tracing the source difficult. Appropriate antibiotic treatment and early diagnosis are of importance to survival[8]. Penicillin tends to be the most commonly used antibiotic[5]. However, there are limited data on antibiotic use in elderly patients with serious complications.
To raise awareness of early diagnosis and antibiotic therapy of listeriosis, we report a case of L. monocytogenes bacteremia in a 102-year-old man with serious conditions who did not respond to initial broad-spectrum antibiotic therapy. An extensive source tracing of the L. monocytogenes infection was performed by Beijing Centers for Disease Control and Prevention (CDC). We searched PubMed from 2012 to 2019 for articles with key words “Listeria monocytogenes,” “bacteremia,” and “septicemia.” The articles were limited to human infection. Fourteen case reports are summarized in Supplementary Table 1.
A 102-year-old man was admitted with hematochezia, high fever of 39.4 °C, chills, and stupor.
Patient’s symptoms started 7 d before admission and worsened over the last 24 h. He suffered from dark red bloody stool three episodes for 7 d. The total amount of stool was about 400 g. He also had symptoms of fatigue and anepithymia with subsequent stupor. Other symptoms such as amaurosis, syncope, nausea, vomiting, and abdominal pain were unpresented. Five days prior to onset, he had consumed quick-frozen dumplings stored in the refrigerator.
His medical history included hypertension and stage 3 chronic kidney disease, which were well controlled. The patient took amlodipine 5 mg once a day until he
There was no history of hereditary disease. No family members had similar symp
The patient was febrile with a temperature of 39.4 °C, blood pressure was 149/65 mmHg, and pulse 94 beats/min. He became stuporous with Glasgow Coma Scale score 7/15. There was no nuchal rigidity, rash, and lymphadenectasis. The abdomen was distended in the right lower quadrant.
White blood count was 13270/μL with 89.8% neutrophils, hemoglobin 8.4 g/dL, and platelet count 243000/μL. Fecal occult blood was positive and there were no white blood cells in the stools. C-reactive protein and procalcitonin level were 173 mg/L (normal range, 1-8 mg/L) and 0.734 ng/mL (normal range, 0-0.5 ng/mL), respectively. Biochemical examination upon admission, and days 5 and 28 is summarized in Table 1, indicating deterioration of liver, kidney and myocardial damage and coagulation within 1 wk of admission.
| Upon admission | On day 5 | On day 28 | |
| WBC (/μL) | 13270 | 18350 | 6130 |
| Neut (%) | 89.8 | 90.1 | 71.6 |
| CRP (mg/L) | 173 | 180 | 14 |
| PCT (ng/mL) | 0.734 | 0.905 | 0.042 |
| AST (IU/L) | 25 | 93 | 36 |
| T-Bil (μmol/L) | 14.22 | 20.43 | 15.53 |
| ALT (IU/L) | 35 | 51 | 38 |
| CRE (μmol/L) | 92 | 121 | 82 |
| BUN (mmol/L) | 12.45 | 19.48 | 9.68 |
| TnI (ng/mL) | 0.001 | 0.253 | 0.003 |
| NTproBNP (pg/mL) | 10031 | 11560 | 5642 |
| INR | 1.12 | 1.33 | 1.15 |
| PT (s) | 13.2 | 16.8 | 14.9 |
| APTT (s) | 45.1 | 54.2 | 45.9 |
| Fib (g/L) | 4.61 | 5.41 | 3.17 |
| D-dimmer (μg/mL) | 4.62 | 5.63 | 3.15 |
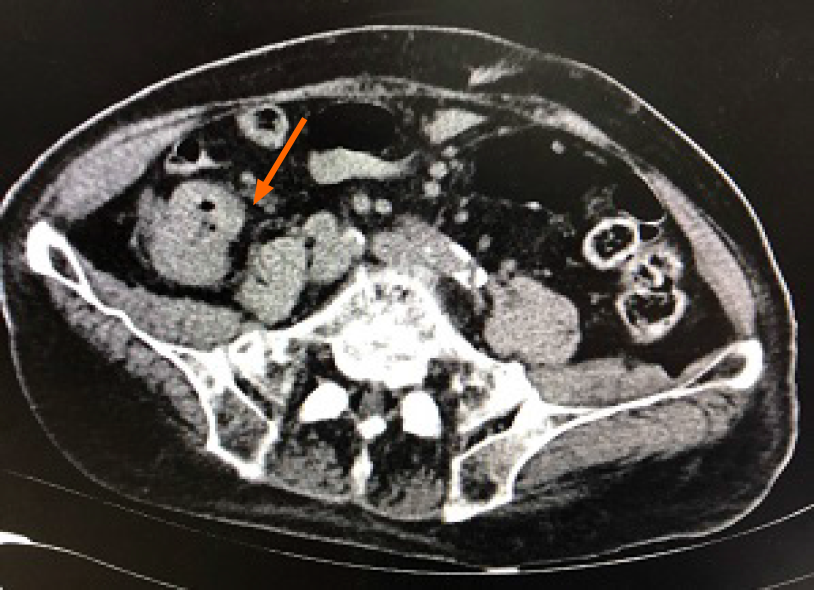
Echocardiography showed a depressed left ventricular ejection fraction (41%) and no evidence of vegetation or endocarditis. Abdominal ultrasound examination showed a tubular hypoechoic structure approximately 8.7 cm × 3.3 cm with unclear boundary, and uneven internal echo in the right lower quadrant. Computed tomography scan of the abdomen and pelvis without contrast was performed, which showed mural thickening and exudation surrounding the ascending colon, suggesting that inflammation might be present (Figure 1).
Four sets of blood cultures were taken using BD BACTEC 92F aerobic and 93F anaerobic media on admission. Blood culture was performed by a BACTEC system (Becton Dickinson, Sparks, MD, United States). On day 5 of admission, L. monocytogenes was identified from blood cultures. Antimicrobial susceptibility testing was performed using the dilution method. The isolate was susceptible to erythromycin (32 mm inhibition zone), gentamicin (24 mm inhibition zone), levofloxacin (25 mm inhibition zone), linezolid (32 mm inhibition zone), penicillin (30 mm inhibition zone), trimethoprim/sulfamethoxazole (29 mm inhibition zone), and vancomycin (21 mm inhibition zone) was intermediate to ampicillin/sulbactam and there was resistance to clindamycin and oxacillin. Meropenem was not tested.
Food and environmental samples from the patient’s home were collected by the CDC. One deep-frozen dumpling sample from the refrigerator was positive for L. monocytogenes. The L. monocytogenes isolate identified by the hospital was also sent to the CDC laboratory in Beijing. The strain was serotyped using multiplex polymerase chain reaction. Multilocus sequence typing was performed on the isolate by amplification and sequencing of internal fragments of seven housekeeping genes[9]. Sequencing was performed on the ABI 3770 automatic sequencer. L. monocytogenes isolates from the patient and dumpling sample revealed different serotype and sequence types (STs): 1/2b and ST5 from the patient and 1/2c and ST9 from the dumpling.
The patient was finally diagnosed with L. monocytogenes bacteremia, localized peritonitis, sepsis, multiple system dysfunction (respiration, liver, central nervous system, renal, and heart), and disseminated intravascular coagulation.
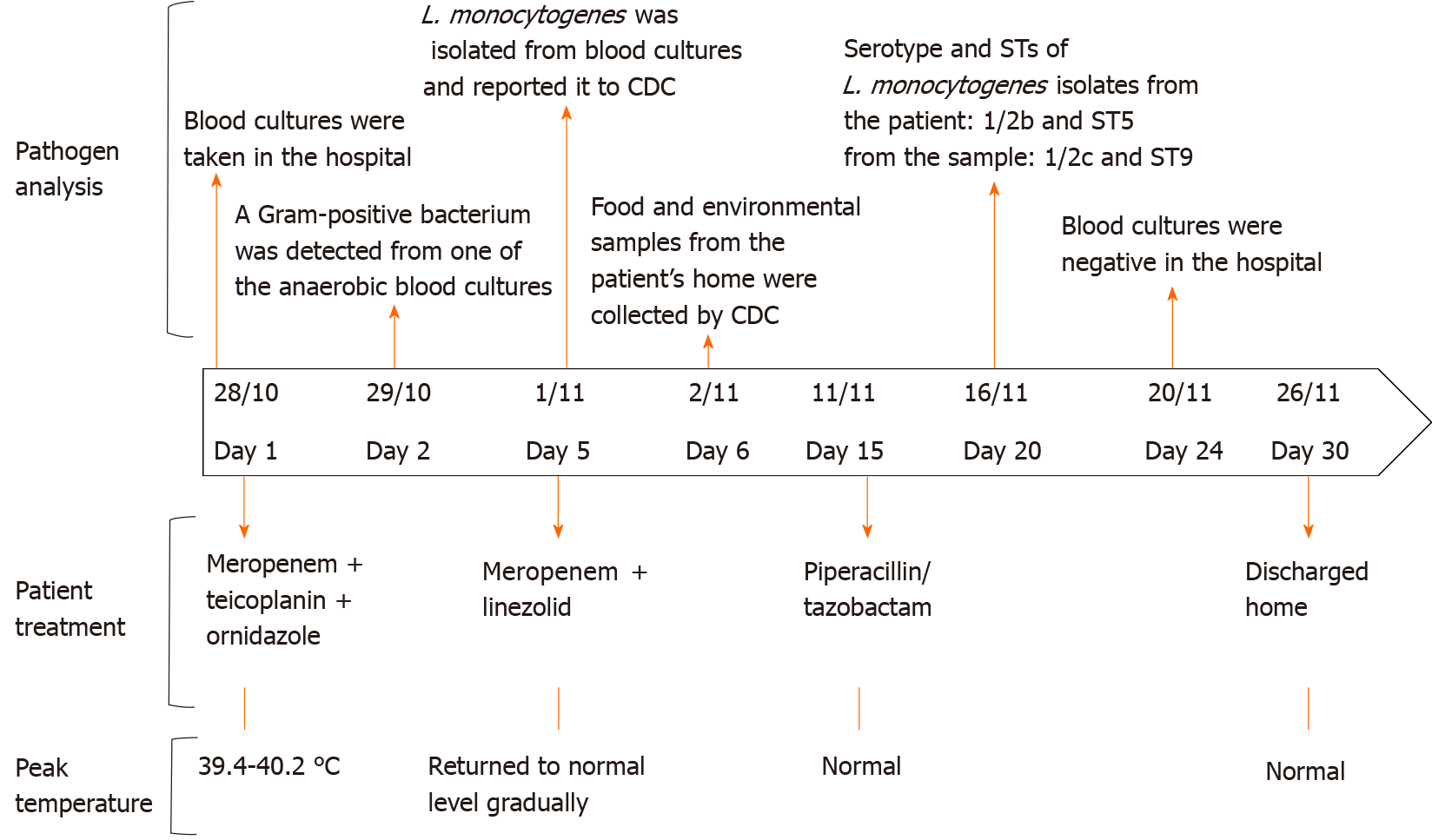
As the patient was in critical condition and had abdominal infection commonly caused by Gram-negative or anaerobic bacteria, he was treated empirically with intravenous meropenem (0.5 g every 8 h), teicoplanin (0.4 g every 12 h), and ornidazole (0.5 g every 12 h). The patient did not respond to the initial antibiotic treatment as high fever and stupor persisted. On day 2, a Gram-positive bacterium was detected in one of the anaerobic blood cultures (time to positivity 27.92 h). On day 3, the patient presented with Biot’s breathing and oxygen saturation on room air of 88% (partial pressure of oxygen/fraction of inspired oxygen ratio: 276 mmHg). Oxygen therapy was admi
On day 5, L. monocytogenes was identified from the blood cultures. We did not choose penicillin to replace meropenem to avoid antibiotic step-down in a critical condition. Vancomycin or sulfamethoxazole/trimethoprim was not administered for serious complications and renal insufficiency. Gram-positive L. monocytogenes was susceptible to linezolid (32 mm inhibition zone); thus, intravenous teicoplanin and ornidazole were stopped and subsequently meropenem and linezolid (0.6 g every 12 h) were initiated.
The temperature gradually returned to normal. Starting from day 8, the patient was afebrile and conscious. He could answer questions correctly and no neurological sequelae developed. The abdominal signs relieved gradually. The patient received meropenem and linezolid for 10 d, and then piperacillin/tazobactam (4.5 g, every 8 h) as step-down therapy was administered for a total of 2 wk. The patient received intravenous antibiotic treatment for 4 wk upon discharge, and blood cultures were negative. Antibiotics used are summarized in Figure 2.
Abdominal ultrasound performed before discharge showed normal findings. Fecal occult blood was negative and liver, kidney, and coagulation functions were normal (Table 1). The patient was discharged in fair condition and there was no sign of relapse during follow-up after discharge. No family members suffered from listeriosis. He eventually died of Klebsiella pneumoniae after 8 mo.
We report a case of L. monocytogenes bacteremia in a 102-year-old man with serious complications that was successfully treated by meropenem, linezolid, and piperacillin/tazobactam. Geriatricians should be suspicious of listeriosis when infected patients do not respond to broad-spectrum antibiotics and when the patients have history of frozen food consumption. Linezolid might be valuable for the treatment of L. monocytogenes bacteremia. L. monocytogenes strains from the patient and refrigerated dumpling belonged to different serotype and STs. Thus, the L. monocytogenes-contaminated dumpling was not the source of the patient’s infection. To the best of our knowledge, this is the oldest patient with L. monocytogenes bacteremia.
Listeriosis has an estimated incidence of 2-15 cases per million per year in developed countries[6]. There is currently no accurate incidence of listeriosis in China. The number of patients in Mainland China is higher than that reported in the previous decade[1]. A systematic review[1] extracted 136 articles about listeriosis in Mainland China. A total of 562 listeriosis patients were reported during 2011-2017 including 227 (40.4%) nonperinatal patients, 231 (41.1%) perinatal patients, and 104 (18.5%) nonclustered patients. Beijing CDC received 49 clinical L. monocytogenes infectious case reports (27 pregnancy-associated and 22 nonpregnancy-associated infections) between 2014 and 2016[9]. L. monocytogenes causes severe and life-threatening diseases such as meningitis and bacteremia, with an estimated lethality of 10%-30%[2,5,12]. Mortality rates increase among those reporting a delay in diagnosis and treatment and in those with severe comorbidity[5].
In this study, our patient’s advanced age, nonresponse to broad-spectrum antibiotics, poor dietary history and mucosal injury caused by localized peritonitis, were the clues to suspicion of L. monocytogenes infection. As described in Supplementary Table 1, 7 of 14 case reports were patients aged > 65 years. Three cases were suspected to have come from possibly contaminated food sources, but none was traced by further analysis. L. monocytogenes is naturally and intrinsically resistant to first-generation quinolones, fosfomycin and cephalosporins[13]. Penicillin, amoxicillin and ampicillin are recommended as first-line drugs for L. monocytogenes infection by current expert opinions[5]. Cotrimoxazole can be administered as an alternative treatment for listeriosis. Linezolid and vancomycin are valuable drugs in the treatment of listeriosis. Linezolid is an oxazolidinone with in vitro activity against L. monocytogenes. Its elevated cerebrospinal fluid and intracellular concentrations seem adequate for the treatment of neurolisteriosis and bacteremia in animal models[14]. The Multicentric Observational National Study on Listeriosis and Listeria (MONALISA) study reported five cases (1%) treated with linezolid for a median duration of 10-15 d[8]. Meropenem in the treatment of listeriosis is still a matter of current debate. Meropenem displays a markedly low minimum inhibitory concentration in vitro, even lower than that of ampicillin against L. monocytogenes[15]. However, data on the efficacy of meropenem in clinical cases of listeriosis are scarce. Meropenem therapy failure in L. monocytogenes infection was reported on the basis of case reports[16]. A Danish retrospective study showed that definitive therapy with meropenem was associated with significantly higher 30-d mortality[17]. The current patient failed to respond to initial meropenem therapy until linezolid was added, indicating that linezolid is a valuable antibiotic for the treatment of the L. monocytogenes bacteremia. Meropenem, a broad-spectrum antibiotic, in combination therapy plays a role for the treatment of abdominal infection in the current patient. To avoid the long-term use of watch and reserve group antibiotics[18], piperacillin/tazobactam was administered as step-down therapy.
Pre-existing gastrointestinal disease was reported as a risk factor for L. monocytogenes infection of the gastrointestinal tract[19]. L. monocytogenes possesses different virulence factors such as internalin (InI) B, InIC, InIJ and the Listeria-mucin-binding invasion A that binds mucins[20-22]. The binding of L. monocytogenes to mucins via virulence factors may allow the bacterium to penetrate the mucus and facilitate bacterial adhesion or invasion of the host cells. L. monocytogenes evolved sophisticated mechanisms to cross the intestinal epithelial cells by different routes[23]: Listeria adhesion protein-mediated L. monocytogenes translocation, InlA/E-cadherin-mediated L. monocytogenes transcytosis, and M cell-mediated L. monocytogenes translocation occurs in the Peyer’s patches. Within the host cell, L. monocytogenes destroys the phagolysosome membrane and gains access to the cytoplasm by listeriolysin O[24]. After invasion through the gastrointestinal tract, L. monocytogenes spread from cell to cell and may disseminate hematogenously. In the current patient, we speculate that the infection was probably acquired from mucosal injury caused by inflammation, which led to bacteremia and subsequent septicemia.
The long incubation period and food diversity make it difficult to trace the source of listeriosis. In this study, L. monocytogenes isolate from deep-frozen dumplings in the patient’s home and blood cultures had different serotype and STs and was ruled out as the cause of infection. Mandatory declaration and surveillance of L. monocytogenes is urgently needed. From May 2015 to March 2016 in Italy, the source of an outbreak due to L. monocytogenes was identified as cheese through epidemiological and microbiological surveillance[25]. In England, crab meat was identified as the most plausible vehicle of infection by retrospective whole genome sequencing and epidemiological information[26].
This case report had some limitations. First, L. monocytogenes was identified after 5 d. We did not use characteristic tumbling motility of L. monocytogenes microscopically as a cheap test to obtain a presumptive diagnosis. Second, lumbar puncture was not performed, thus neurolisteriosis could not be ruled out. Third, the endoscopy was not performed for the advanced age of the patient and refusal of the relatives. Normal findings from abdominal ultrasound before discharge and relieved abdominal signs indicated the increased likelihood of abdominal infection. However, the exact cause of lower gastrointestinal bleeding is not clear. Finally, meropenem and piperacil
Although clinical data are currently limited, our patient seemed to benefit from linezolid therapy without linezolid-associated thrombocytopenia during 10 d of treatment. Linezolid might be a reasonable treatment option for L. monocytogenes bacteremia in elderly patients with serious complications. More suspicion of invasive listeriosis should be raised when infected elderly patients have a history of frozen food consumption history and do not respond to broad-spectrum antibiotics. Healthy eating habits and food processing methods should be prioritized in elderly people.
We thank the relatives of the patient for providing consent to publish this case report. We thank for the sampling staffs from Xicheng CDC in Beijing.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mayr J, Ong LT S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Fan Z, Xie J, Li Y, Wang H. Listeriosis in mainland China: A systematic review. Int J Infect Dis. 2019;81:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | de Noordhout CM, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, Havelaar A, Speybroeck N. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:1073-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 432] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 3. | Kishnani PM, Tiwari AA, Gautam V, Sharma M, Barbuddhe SB, Doijad SP, Chakraborty T, Nayak AR, Bhartiya NM, Daginawala HF, Singh LR, Kashyap RS. Draft Genome Sequence of Listeria monocytogenes Strain CIIMS-PH-1, a Serovar 4b Isolate from Infant Septicemia. Genome Announc. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Barocci S, Mancini A, Canovari B, Petrelli E, Sbriscia-Fioretti E, Licci A, D'Addesa S, Petrini G, Giacomini M, Renzi A, Migali A, Briscolini S. Listeria monocytogenes meningitis in an immunocompromised patient. New Microbiol. 2015;38:113-118. [PubMed] |
| 5. | Pagliano P, Arslan F, Ascione T. Epidemiology and treatment of the commonest form of listeriosis: meningitis and bacteraemia. Infez Med. 2017;25:210-216. [PubMed] |
| 6. | Vera A, González G, Domínguez M, Bello H. [Main virulence factors of Listeria monocytogenes and its regulation]. Rev Chilena Infectol. 2013;30:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Goulet V, King LA, Vaillant V, de Valk H. What is the incubation period for listeriosis? BMC Infect Dis. 2013;13:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Charlier C, Perrodeau É, Leclercq A, Cazenave B, Pilmis B, Henry B, Lopes A, Maury MM, Moura A, Goffinet F, Dieye HB, Thouvenot P, Ungeheuer MN, Tourdjman M, Goulet V, de Valk H, Lortholary O, Ravaud P, Lecuit M; MONALISA study group. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis. 2017;17:510-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 343] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 9. | Zhang X, Niu Y, Liu Y, Lu Z, Wang D, Cui X, Chen Q, Ma X. Isolation and Characterization of Clinical Listeria monocytogenes in Beijing, China, 2014-2016. Front Microbiol. 2019;10:981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17221] [Article Influence: 1913.4] [Reference Citation Analysis (2)] |
| 11. | National Center for Clinical Medicine of Geriatric Diseases. Chinese expert consensus on diagnosis and treatment of infection-induced senile multiple organ dysfunction syndrome. Zhonghua Laonian Duoqiguan Jibing Zazhi. 2018;17:3-15. [DOI] [Full Text] |
| 12. | European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018;16:e05500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 540] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 13. | Wilson A, Gray J, Chandry PS, Fox EM. Phenotypic and Genotypic Analysis of Antimicrobial Resistance among Listeria monocytogenes Isolated from Australian Food Production Chains. Genes (Basel). 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Callapina M, Kretschmar M, Dietz A, Mosbach C, Hof H, Nichterlein T. Systemic and intracerebral infections of mice with Listeria monocytogenes successfully treated with linezolid. J Chemother. 2001;13:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Carryn S, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Activity of beta-lactams (ampicillin, meropenem), gentamicin, azithromycin and moxifloxacin against intracellular Listeria monocytogenes in a 24 h THP-1 human macrophage model. J Antimicrob Chemother. 2003;51:1051-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Stepanović S, Lazarević G, Jesić M, Kos R. Meropenem therapy failure in Listeria monocytogenes infection. Eur J Clin Microbiol Infect Dis. 2004;23:484-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Thønnings S, Knudsen JD, Schønheyder HC, Søgaard M, Arpi M, Gradel KO, Østergaard C; Danish Collaborative Bacteraemia Network (DACOBAN). Antibiotic treatment and mortality in patients with Listeria monocytogenes meningitis or bacteraemia. Clin Microbiol Infect. 2016;22:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | World Health Organization. World Health Organization Model List of Essential Medicines. 21st list. Geneva: World Health Organization, 2019: 8. |
| 19. | Barbuddhe SB, Chakraborty T. Listeria as an enteroinvasive gastrointestinal pathogen. Curr Top Microbiol Immunol. 2009;337:173-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Lindén SK, Bierne H, Sabet C, Png CW, Florin TH, McGuckin MA, Cossart P. Listeria monocytogenes internalins bind to the human intestinal mucin MUC2. Arch Microbiol. 2008;190:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Popowska M, Krawczyk-Balska A, Ostrowski R, Desvaux M. InlL from Listeria monocytogenes Is Involved in Biofilm Formation and Adhesion to Mucin. Front Microbiol. 2017;8:660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Mariscotti JF, Quereda JJ, García-Del Portillo F, Pucciarelli MG. The Listeria monocytogenes LPXTG surface protein Lmo1413 is an invasin with capacity to bind mucin. Int J Med Microbiol. 2014;304:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Drolia R, Bhunia AK. Crossing the Intestinal Barrier via Listeria Adhesion Protein and Internalin A. Trends Microbiol. 2019;27:408-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Nguyen BN, Peterson BN, Portnoy DA. Listeriolysin O: A phagosome-specific cytolysin revisited. Cell Microbiol. 2019;21:e12988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Duranti A, Sabbatucci M, Blasi G, Acciari VA, Ancora M, Bella A, Busani L, Centorame P, Cammà C, Conti F, De Medici D, Di Domenico M, Di Marzio V, Filippini G, Fiore A, Fisichella S, Gattuso A, Gianfranceschi M, Graziani C, Guidi F, Marcacci M, Marfoglia C, Neri D, Orsini M, Ottaviani D, Petruzzelli A, Pezzotti P, Rizzo C, Ruolo A, Scavia G, Scuota S, Tagliavento G, Tibaldi A, Tonucci F, Torresi M, Migliorati G, Pomilio F. A severe outbreak of listeriosis in central Italy with a rare pulsotype associated with processed pork products. J Med Microbiol. 2018;67:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Elson R, Awofisayo-Okuyelu A, Greener T, Swift C, Painset A, Amar CFL, Newton A, Aird H, Swindlehurst M, Elviss N, Foster K, Dallman TJ, Ruggles R, Grant K. Utility of Whole Genome Sequencing To Describe the Persistence and Evolution of Listeria monocytogenes Strains within Crabmeat Processing Environments Linked to Two Outbreaks of Listeriosis. J Food Prot. 2019;82:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |