Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3048
Peer-review started: September 16, 2020
First decision: December 14, 2020
Revised: December 21, 2020
Accepted: December 28, 2020
Article in press: December 28, 2020
Published online: May 6, 2021
Processing time: 217 Days and 19.7 Hours
Delayed neurological sequelae (DNS) caused by carbon monoxide (CO) intoxication poses considerable treatment challenges for clinical practitioners. In this report, we used nuclear medicine imaging and the Mini-Mental State Examination (MMSE) to evaluate the effectiveness of intravascular laser irradiation of blood (ILIB) therapy for the management of DNS.
A 51-year-old woman presented to our medical center experiencing progressive bradykinesia, rigidity of limbs, gait disturbance, and cognitive impairment. Based on her neurological deficits, laboratory tests and imaging findings, the patient was diagnosed with delayed neurological sequelae of CO intoxication. She received intensive rehabilitation and ILIB therapy during 30 sessions over 2 mo after diagnosis. Brain single-photon emission computed tomography was performed both prior to and after ILIB therapy. The original hypoperfusion area in bilateral striata, bilateral frontal lobe, right parietal lobe, and bilateral cerebellum showed considerable improvement after completion of therapy. The patient’s MMSE score also increased markedly from 6/30 to 25/30. Symptoms of DNS became barely detectable, and the woman was able to carry out her daily living activities independently.
ILIB therapy could facilitate recovery from delayed neurological sequelae in patients with CO intoxication, as demonstrated by improved cerebral blood flow and functional outcomes in our patient.
Core Tip: Carbon monoxide poisoning and its associated delayed neurological sequelae remain therapeutic difficulties for physicians. We present a patient who recovered after intravascular laser irradiation of blood, as evaluated by brain single-photon emission computed tomography images and the Mini-Mental State Examination. We report this case with the aim of triggering further research, and to facilitate the recovery of patients experiencing delayed neurological sequelae.
- Citation: Liu CC, Hsu CS, He HC, Cheng YY, Chang ST. Effects of intravascular laser phototherapy on delayed neurological sequelae after carbon monoxide intoxication as evaluated by brain perfusion imaging: A case report and review of the literature. World J Clin Cases 2021; 9(13): 3048-3055
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3048.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3048
Carbon monoxide (CO) can displace oxygen in heme-containing proteins, binding with tremendous affinity, which in turn may lead to severe tissue hypoxia. In addition, it also inhibits cellular mitochondrial respiration, generates free radicals, and activates an inflammatory cascade, particularly in high-oxygen-demand organs such as the brain or heart[1]. Survivors of CO intoxication may suffer from delayed neurological sequelae (DNS) such as cognitive impairment, which profoundly affects one’s quality of life[2-4]. Currently, hyperbaric oxygen therapy (HBOT) is a reasonable treatment option for CO intoxicated patients as it facilitates faster dissociation of CO from the blood, reduces inflammation in brain tissue, and improves neurocognitive outcomes[5,6].
Intravascular laser irradiation of blood (ILIB) therapy utilizes a helium–neon laser at a wavelength of 632.8 nm (red light), which is transmitted through an optical fiber via a phlebotomy cannula. It has biomodulatory effects that control inflammation, regulate immunologic response, improve rheological behavior of blood and simulate anti-oxidant enzymatic activities[7]. ILIB is also used as an alternative treatment in various diseases such as chronic spinal cord injury, cerebral stroke, traumatic brain injury, rheumatoid arthritis, fibromyalgia, and chronic pain conditions[8-12]. However, the usefulness of ILIB for CO intoxication is not documented in the available literature.
Herein, we present the case of a patient who experienced CO intoxication and was treated with ILIB therapy. We postulate that ILIB therapy may alleviate DNS and facilitate recovery.
A 51-year-old woman was brought to the hospital due to progression of general weakness and gait ataxia over the course of the past month, which had eventually rendered her bedridden.
A month prior to this episode, she presented with general malaise, constipation, and acute urinary retention, requiring a trip to the emergency department. She was found by her family in a poorly ventilated room which was filled with smoke.
The patient had an unremarkable medical history.
The patient drank and smoked occasionally. She had no family history of disease.
The patient’s vital signs were within the normal range. A clinical neurological examination revealed prominent rigidity of the four limbs, bradykinesia and mutism. Her Glasgow Coma Scale (GCS) score was 10/15. The first impression by medical staff was toxic encephalopathy.
A blood profile revealed mildly decreased hemoglobin (12.8 g/dL) as compared to normal values. She had hypokalemia of 3.3 mEq/L without other serum electrolyte imbalances. Cerebrospinal fluid (CSF) analysis showed a normal white blood cell count (4/μL), glucose level (63 mg/dL) and a mildly elevated protein level (59 mg/dL). Surveys of drug intoxication, including diazepam, amphetamines and morphine were all inconclusive. A mildly elevated serum carboxyhemoglobin level (5%) was discovered. Results from an electrocardiogram and chest X-ray were both unremarkable.
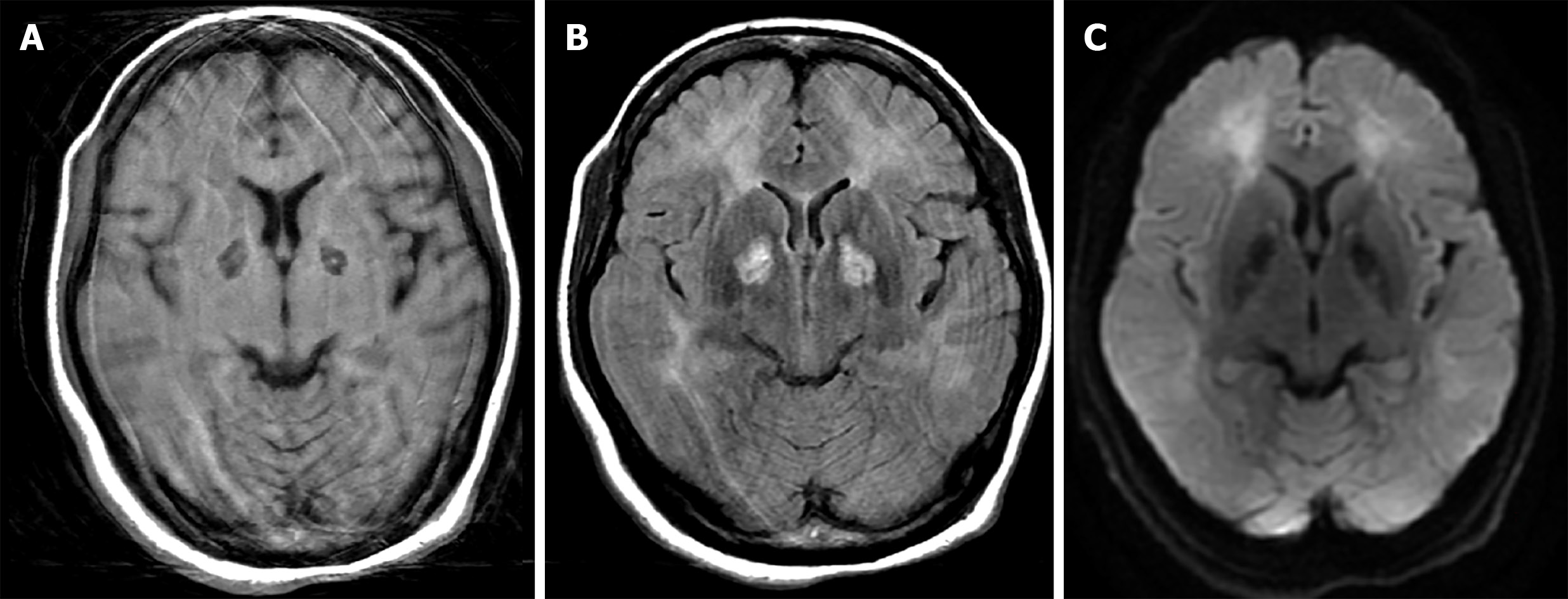
Brain computed tomography without contrast enhancement showed no intracranial hemorrhage, although hypoattenuation in bilateral globus pallidus was noted. Brain magnetic resonance images showed brain tissue necrosis with mild hemorrhage in bilateral globus pallidus (Figure 1). Other findings included diffuse leukoence
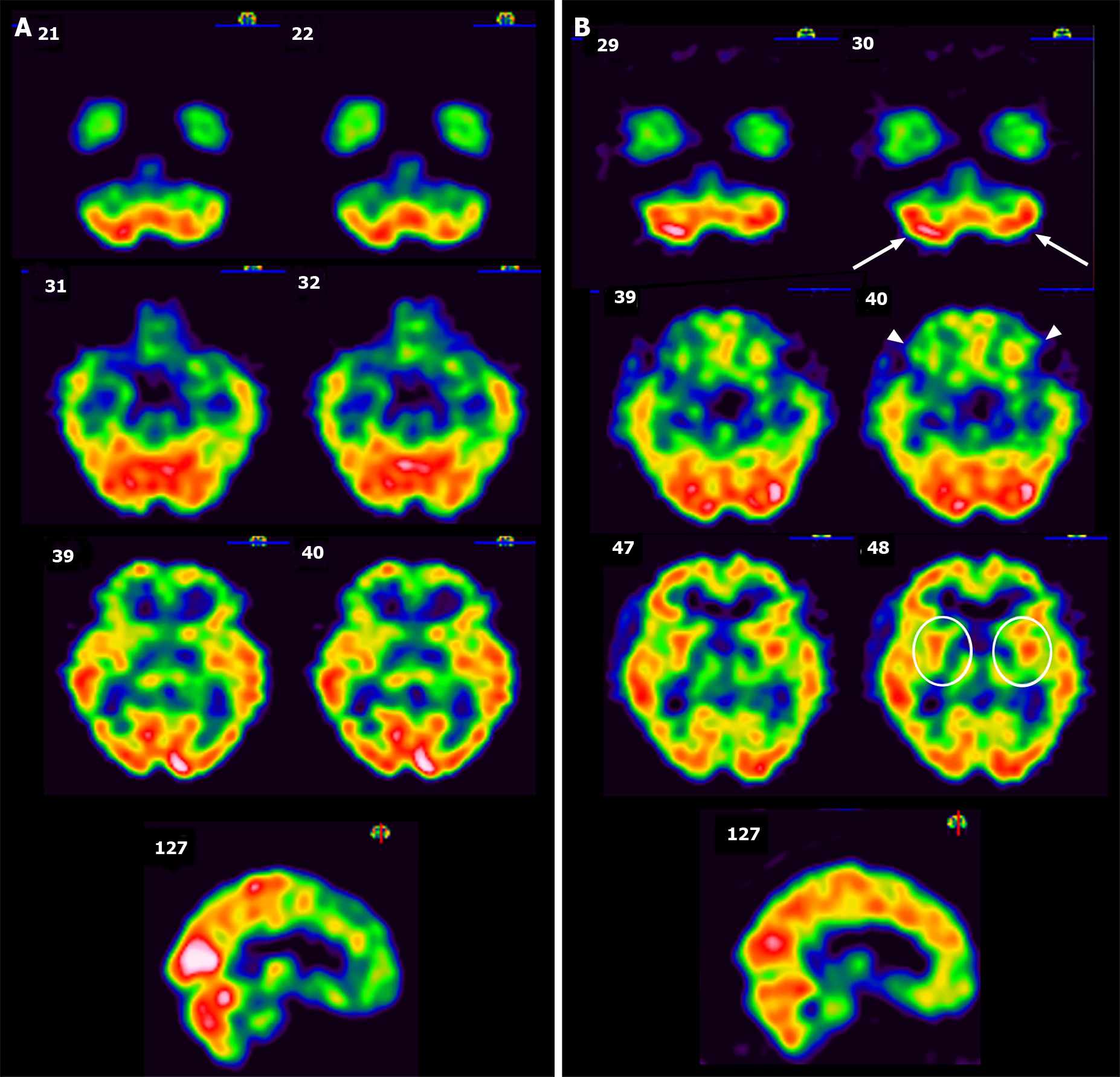
The CSF culture was clear of viruses, bacteria and fungi. The patient’s autoimmune profile was assessed due to mildly elevated serum erythrocyte sedimentation rate, but the results were normal. Other causes of encephalopathy were also investigated, such as folic acid deficiency or endocrine functional impairment, but these results were also normal. A brain imaging study using Technetium-99m ethyl cysteinate dimer single photon emission computed tomography (SPECT) was performed to evaluate regional cerebral blood flow, and the results revealed hypoperfusion in the bilateral striata, bilateral frontal, bilateral parietal, and left cerebellar regions (Figure 2A).
Based on the patient’s history of residing in a poorly ventilated smoky area, and due to delayed neurological signs, laboratory investigations and typical magnetic resonance images, she was diagnosed with CO intoxication and delayed neurological sequelae.
The patient received 10 sessions of HBOT after being transferred to the neurology ward. Unfortunately, her family was not satisfied with the therapeutic outcomes and decided on the application of ILIB therapy using a Helium–neon laser (YJ-ILIB-5, Bio-ILIB Human Energy Ltd., Taiwan), with visible red light at a wavelength of 632.8 nm. This was introduced at an accessible peripheral vein via an optical fiber 0.5 mm in diameter through a phlebotomy cannula. The amount of blood irradiated in a session was hypothesized to be close to 100% of the total blood volume, as the regional-arm-to-brain mean transit time measured using a radionuclide required less than 30 s[13]. Based on this theory, the cycle time of blood traveling round the circulatory system would not require any more than 1 h. Thus, all the blood was presumably irradiated by the laser light during each 3600-s session. We gradually increased the power output from 1.4 to 1.6 milliwatts as the sessions progressed. Several other ILIB therapy parameters were calculated as follows[8]: The irradiance was 0.72 watts (W)/cm2 to 0.82 W/cm2; total energy was 5.04 to 5.76 joules (J) and energy density was 2571.42 to 2938.78 J/cm2. Treatment sessions were performed on weekdays for 2 consecutive weeks as a single treatment course, and 3 treatment courses were carried out over a period of 2 mo, with at least 1 wk of rest between each course.
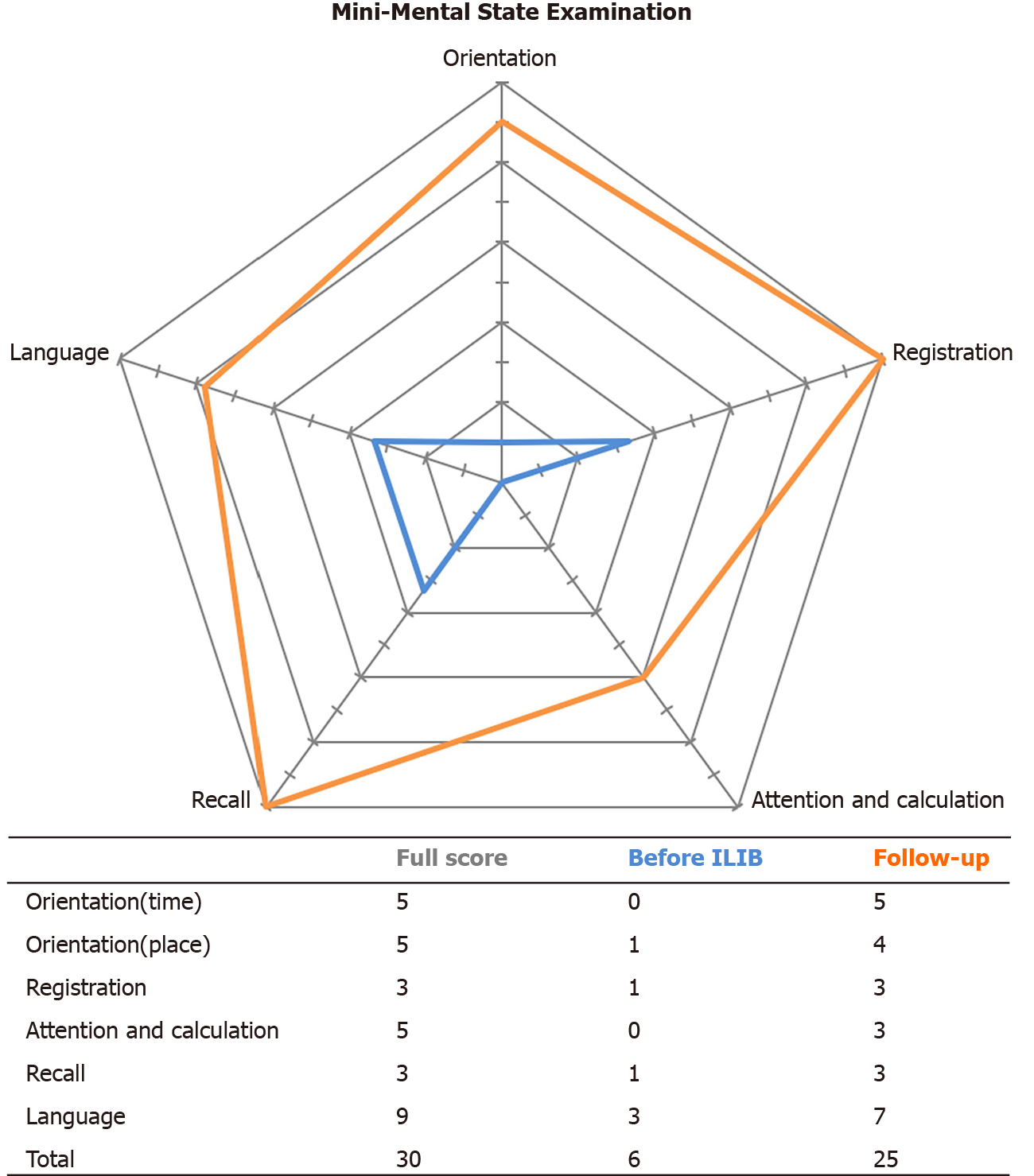
Before receiving ILIB therapy, the patient’s GCS score was 13/15, with a modified Rankin Scale of 5 and a Barthel Index of 25 out of 100. Her Mini-Mental State Examination (MMSE) was 6/30. Features of Parkinsonism, including resting tremor, bradykinesia, and postural instability were all remarkable. Rigidity of the 4 limbs interfered with all of her voluntary movements. After 3 courses of ILIB, there was marked functional recovery. Her GCS score was 15/15, with a modified Rankin Scale of 3 and a Barthel Index of 50/100. During outpatient follow-up 2 mo later, her modified Rankin Scale was 1 and Barthel Index had increased to 100/100. Her MMSE also greatly improved from 6/30 to 25/30. The remaining deficits were restricted to attention and calculation, as well as copying pentagons. Rigidity of the 4 limbs, resting tremor and bradykinesia were now barely detectable. She could walk more than 100 m and performed personal activities of daily life independently. A second brain SPECT was performed, and the results are shown in Figure 2B. Improved perfusion was observed in the bilateral striata, bilateral frontal lobe, right parietal lobe, and bilateral cerebellum. A comparison of MMSE scores before and after therapy is shown in Figure 3.
Several innovative methods of photodynamic therapy have been proposed for the treatment of CO intoxication, including extracorporeal blood illumination, total body cutaneous illumination, and delivery of light-emitting nanoparticles into the circulation; however, ILIB is not mentioned in the available literature[14]. ILIB is used in our hospital and has resulted in improvements in cognitive dysfunction, motor function, and crossed cerebellar diaschisis after acute stroke in selected cases[12,15-18]. To the best of our knowledge, this is the first report to demonstrate the effectiveness of ILIB in the treatment of CO intoxication.
Chronic or occult CO exposure, as occurred in this case, is often neglected in emergency department settings. Diagnostic difficulties stem from a lack of common diagnostic tools and nonspecific constitutional symptoms such as weakness, dizziness, headache, or GI upset[19]. Thus, appropriate treatment such as oxygen therapy is often either not provided or is delayed. Following a clinically silent period of approximately 1 mo, recurrent neuropsychiatric symptoms ensue, i.e., DNS, as in our case. Although the features of DNS may vary, they can include cognitive impairment, emotional lability, psychosis, gait disturbances, and movement disorders. It is believed that DNS manifests as ischemia-reperfusion injury involving neutrophils which become adherent to damaged vascular endothelium in the brain as a result of CO toxicity. Neutrophils release xanthine oxidase to produce excessive reactive oxygen species, initiating lipid peroxidation and causing an inflammatory response[2]. There is no consensus regarding the treatment of DNS. Several studies have found that HBOT alone or combined with steroids, N-butylphthalide, or N-acetylcysteine seemed to achieve better neurological recovery[20,21]. The anti-inflammatory and anti-oxidative nature of these medications are capable of attaining similar results to those of ILIB therapy[8].
A red light laser (632.8 nm) is the earliest available laser wavelength and widely adopted in intravenous laser therapy. It is believed to enhance adenosine triphosphate synthesis through spectral absorption of cytochrome C oxidase in the mitochondrial respiratory chain. Although theoretically green light is better absorbed by red blood cells, improves their deformability, and stimulates the activity of transmembrane sodium potassium adenosine triphosphatase, its application in vivo is lacking to date[7]. Thus, we chose red light as the therapeutic wavelength. There are at least three supposed mechanisms of ILIB therapy in treating CO intoxication. First, the laser directly dissociates CO from the blood. In murine models, Zazzeron et al[22,23] found that direct illumination of the lungs could facilitate the dissociation of CO from hemoglobin, thus increasing the concentration of exhaled CO. This finding indicates that the laser beam is capable of counteracting dissolved CO in situations involving acute intoxication or occult exposure. In the second hypothesis, ILIB affects the rheological behavior of blood, diminishes platelet aggregation, and improves the deformability of red blood cells, all of which lead to better oxygen delivery to the hypoxic or infarcted areas caused by CO intoxication. Vasodilatation also ensues as a result of increased nitric oxide released from monocytes[24]. Third, according to Huang et al[8], ILIB alleviates both mitochondrial dysfunction and oxidative stress through the regulation of mitochondria numbers and metabolic enzymatic activities. It is reasonable to suppose that enhanced antioxidant activity would help neurons become more resistant to ongoing inflammation, following ischemia-reperfusion injury induced by CO intoxication.
Neurovascular coupling of the brain is a concept which describes a close relationship between neuronal activity and cerebral blood flow responses, e.g., regional flow is increased to meet the metabolic demand[25]. Brain SPECT images have been used to portray the change in regional cerebral blood flow abnormalities during follow-up in CO-intoxicated patients[26-28]. Even after having received immediate HBOT, 12 patients with DNS still showed marked sustained hypoperfusion on SPECT imaging in the frontal and temporal areas 6 mo after poisoning, with various involvement of the parietal lobe, basal ganglia, and cerebellum, according to the study by Tsai[29]. On the contrary, in our case, after receiving ILIB, the abovementioned sustained hypoperfused areas, including the frontal lobe, parietal lobe, striata, and cerebellum, showed significant improvement. Moreover, the previously hyper
ILIB therapy alleviated DNS in our patient as evaluated by brain SPECT images and functional outcomes. Our findings warrant further comprehensive research in order to better evaluate the effectiveness of ILIB therapy for DNS, and to help facilitate patient recovery.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oren D, Zhu X S-Editor: Zhang L L-Editor: Webster JR P-Editor: Li X
| 1. | Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva S, Tejero J, Gladwin MT. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am J Respir Crit Care Med. 2017;195:596-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 426] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 2. | Lettow I, Hoffmann A, Burmeister HP, Toepper R. [Delayed neuropsychological sequelae after carbon monoxide poisoning]. Fortschr Neurol Psychiatr. 2018;86:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Sönmez BM, İşcanlı MD, Parlak S, Doğan Y, Ulubay HG, Temel E. Delayed neurologic sequelae of carbon monoxide intoxication. Turk J Emerg Med. 2018;18:167-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Jeon SB, Sohn CH, Seo DW, Oh BJ, Lim KS, Kang DW, Kim WY. Acute Brain Lesions on Magnetic Resonance Imaging and Delayed Neurological Sequelae in Carbon Monoxide Poisoning. JAMA Neurol. 2018;75:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Hampson NB, Piantadosi CA, Thom SR, Weaver LK. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am J Respir Crit Care Med. 2012;186:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 422] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 7. | Weber MH, Fußgänger-May T, Wolf T. Intravenous laser blood irradiation - Introduction of a new therapy. Deutsche Zeitschrift fur Akupunktur. 2007;50:12-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Huang SF, Tsai YA, Wu SB, Wei YH, Tsai PY, Chuang TY. Effects of intravascular laser irradiation of blood in mitochondria dysfunction and oxidative stress in adults with chronic spinal cord injury. Photomed Laser Surg. 2012;30:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Kulova LA, Burduli NM. [The influence of intravenous laser therapy on the endothelial function and the state of microcirculation in the patients presenting with rheumatoid arthritis]. Vopr Kurortol Fizioter Lech Fiz Kult. 2014;9-12. [PubMed] |
| 10. | Momenzadeh S, Abbasi M, Ebadifar A, Aryani M, Bayrami J, Nematollahi F. The intravenous laser blood irradiation in chronic pain and fibromyalgia. J Lasers Med Sci. 2015;6:6-9. [PubMed] |
| 11. | Timofeyev VT, Poryadin GV, Goloviznin MV. Laser irradiation as a potential pathogenetic method for immunocorrection in rheumatoid arthritis. Pathophysiology. 2001;8:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Yang WH, Lin SP, Chang ST. Case report: Rapid improvement of crossed cerebellar diaschisis after intravascular laser irradiation of blood in a case of stroke. Medicine (Baltimore). 2017;96:e5646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Bartolini A. Regional arm-brain mean transit time in the diagnostic evaluation of patients with cerebral vascular disease. Stroke. 1981;12:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Rose JJ, Xu Q, Wang L, Gladwin MT. Shining a Light on Carbon Monoxide Poisoning. Am J Respir Crit Care Med. 2015;192:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Liu PY, Liu CC, Liu IT, He HC, Chang ST. Advancement of the Cognitive Function on Laser Exposure after Tumor Removal in a Case with Brain Neoplasm. J Light Laser Curr Trends. 2019;2:6. |
| 16. | Sung JH, Chang ST. Reversal of Impaired Blood Flow of the Basal Ganglion from the Prior Focal Perfusion Defect in a Case of Ischemic Infarction: Observation during the Two Stages of Administration of Intravenous Laser Irradiation of Blood. J Med Stud Res. 2019;2:11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Chang JY, Liu CC, Liu IT, Chang ST. Effects of Intravascular Laser Irradiation of Blood on Cognitive Function in a Stroke Survivor with Hyperhomocysteinemia: Dual Recuperations in Thalamus and Serum Homocysteine. Biomed J Sci Tech Res. 2019;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Liu EY, Chang ST. Benefits of intravascular laser irradiation of blood on motor and sensory recovery viewing from brain function images: Portrait of a case with chronic Sjögren’s syndrome, transverse myelitis, and Guillain-Barré syndrome. Biomed J Sci Tech Res. 2019;14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wright J. Chronic and occult carbon monoxide poisoning: we don't know what we're missing. Emerg Med J. 2002;19:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Fujita M, Oda Y, Kaneda K, Kawamura Y, Nakahara T, Todani M, Yagi T, Koga Y, Tsuruta R. Variability in Treatment for Carbon Monoxide Poisoning in Japan: A Multicenter Retrospective Survey. Emerg Med Int. 2018;2018:2159147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Spina V, Tomaiuolo F, Celli L, Bonfiglio L, Cecchetti L, Carboncini MC. A Case of Carbon Monoxide-Induced Delayed Neurological Sequelae Successfully Treated with Hyperbaric Oxygen Therapy, N-Acetylcysteine, and Glucocorticoids: Clinical and Neuroimaging Follow-Up. Case Rep Neurol Med. 2019;2019:9360542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Zazzeron L, Liu C, Franco W, Nakagawa A, Farinelli WA, Bloch DB, Anderson RR, Zapol WM. Pulmonary Phototherapy for Treating Carbon Monoxide Poisoning. Am J Respir Crit Care Med. 2015;192:1191-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Zazzeron L, Liu C, Franco W, Nakagawa A, Farinelli WA, Bloch DB, Anderson RR, Zapol WM. Pulmonary Phototherapy to Treat Carbon Monoxide Poisoning in Rats. Shock. 2017;47:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Isabella APJ, Silva JTC, da Silva T, Rodrigues MFSD, Horliana ACRT, Motta LJ, Bussadori SK, Pavani C, Silva DFTD. Effect of irradiation with intravascular laser on the hemodynamic variables of hypertensive patients: Study protocol for prospective blinded randomized clinical trial. Medicine (Baltimore). 2019;98:e15111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Phillips AA, Chan FH, Zheng MM, Krassioukov AV, Ainslie PN. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab. 2016;36:647-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 26. | Chen SY, Lin CC, Lin YT, Lo CP, Wang CH, Fan YM. Reversible Changes of Brain Perfusion SPECT for Carbon Monoxide Poisoning-Induced Severe Akinetic Mutism. Clin Nucl Med. 2016;41:e221-e227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Watanabe N, Nohara S, Matsuda H, Sumiya H, Noguchi K, Shimizu M, Tsuji S, Kinuya S, Shuke N, Yokoyama K, Seto H. Statistical parametric mapping in brain single photon computed emission tomography after carbon monoxide intoxication. Nucl Med Commun. 2002;23:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Wu CI, Changlai SP, Huang WS, Tsai CH, Lee CC, Kao CH. Usefulness of 99mTc ethyl cysteinate dimer brain SPECT to detect abnormal regional cerebral blood flow in patients with acute carbon monoxide poisoning. Nucl Med Commun. 2003;24:1185-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Tsai CF, Yip PK, Chen SY, Lin JC, Yeh ZT, Kung LY, Wang CY, Fan YM. The impacts of acute carbon monoxide poisoning on the brain: Longitudinal clinical and 99mTc ethyl cysteinate brain SPECT characterization of patients with persistent and delayed neurological sequelae. Clin Neurol Neurosurg. 2014;119:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40:433-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 340] [Article Influence: 8.1] [Reference Citation Analysis (0)] |