Published online Apr 6, 2020. doi: 10.12998/wjcc.v8.i7.1319
Peer-review started: December 26, 2019
First decision: February 26, 2020
Revised: March 24, 2020
Accepted: March 27, 2020
Article in press: March 27, 2020
Published online: April 6, 2020
Processing time: 101 Days and 20.8 Hours
Sjögren syndrome (SS) is a chronic and systemic autoimmune disease characterized by lymphocytic infiltration of the exocrine glands. And histoplasmosis is an invasive mycosis caused by the saprophytic dimorphic fungus H. capsulatum. In patients with primary SS (PSS), disseminated histoplasmosis (DH) is extremely rare.
We report a 37-year-old female patient admitted to our hospital with exacerbating fatigue, somnolence, and pancytopenia as the main symptoms. She was eventually diagnosed with DH based on pancytopenia, splenomegaly, and findings of bone marrow smears. The atypical clinical symptoms made the diagnosis process more tortuous. Unfortunately, she died of respiratory failure on the day the diagnosis was confirmed.
We present a rare and interesting case of DH in a PSS patient. This case updates the geographic distribution of histoplasmosis in China, and expands the clinical manifestations of DH in PSS, highlighting the significance of constantly improving the understanding of PSS with DH.
Core tip: We present a rare and interesting case of disseminated histoplasmosis (DH) in a primary Sjögren syndrome (PSS) patient. This case updates the geographic distribution of histoplasmosis in China, and expands the clinical manifestations of DH in PSS. These findings improve our understanding of DH in PSS and may allow for earlier identification.
- Citation: Li JA, Cheng YY, Cui ZT, Jiang W, Zhang WQ, Du ZH, Gao B, Xie YY, Meng HM. Disseminated histoplasmosis in primary Sjögren syndrome: A case report. World J Clin Cases 2020; 8(7): 1319-1325
- URL: https://www.wjgnet.com/2307-8960/full/v8/i7/1319.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i7.1319
Sjögren syndrome (SS) is a chronic and systemic autoimmune disease characterized by lymphocytic infiltration of the exocrine glands[1]. The condition can occur as primary SS (PSS) or be secondary to another connective tissue disease. SS has a wide variety of presentations, ranging from local involvement of the exocrine glands to systemic, extraglandular involvement of multiple organs. For patients exhibiting severe organ manifestations, administration of high-dose methylprednisolone and cyclophosphamide is effective[2]. However, the use of immunosuppressive agents, or high SS activity in PSS may result in an increased risk of infection and death[3].
The clinical symptoms in SS patients with infection may be atypical, leading to a difficult diagnosis in the earlier period and delaying treatment. Histoplasmosis occurs in specific endemic areas. When it occurs in non-endemic areas, diagnosis will become even more difficult. Here, we report an extremely rare case of disseminated histoplasmosis (DH) in a female Chinese patient with PSS. This is the first case of DH reported in Jilin Province, China.
A 37-year-old woman presented to our hospital due to exacerbating fatigue and somnolence for 1 mo. Seven years prior to admission, she was admitted to a local hospital due to fatigue, darker urine color, as well as persistent dry eyes and mouth. She was diagnosed with PSS and hemolytic anemia according to these clinical symptoms, positive results of anti-Ro (SS-A) antibodies, and several lymphocytic foci in the biopsy specimen. Methylprednisolone was initially administered at a dose of 80 mg/d for 2 weeks until symptoms improved. She was then discharged on oral maintenance therapy with prednisone 40 mg/d. In the decrement course of prednisone, the above symptoms returned and she was admitted to the local hospital. After adjusting the hormone dose and adding hydroxychloroquine at a dose of 0.4 g/d, the symptoms improved. Two years ago, the routine blood indexes were basically normal, so all medications were discontinued.
One month before admission to our hospital, the patient received pulse therapy with methylprednisolone 200 mg/d and oral hydroxychloroquine at a local hospital. However, her condition was not controlled, so she was transferred to our hospital.
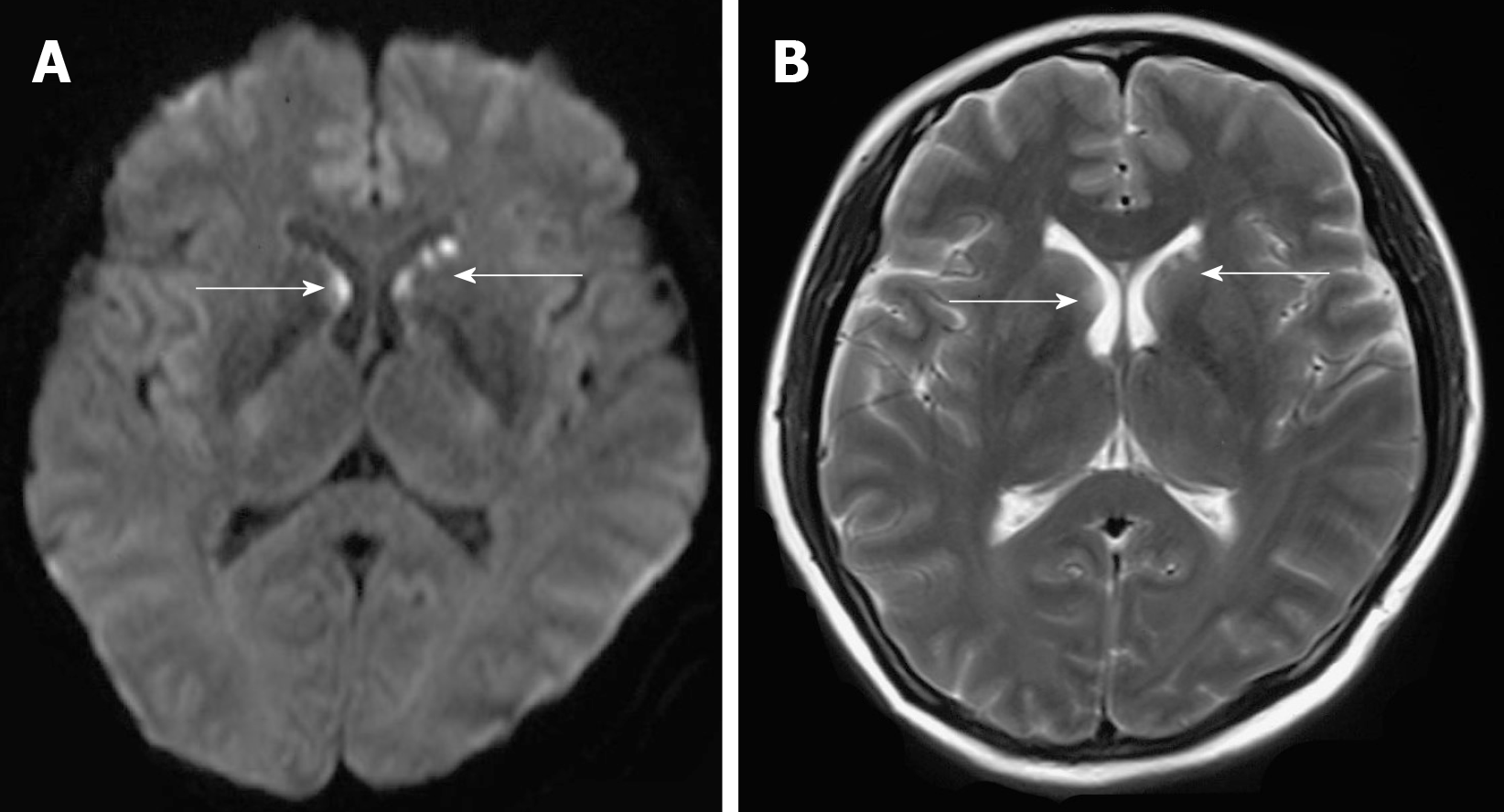
The physical examination showed drowsiness and the spleen was palpated 3 cm below the left costal margin. Myodynamia of limbs was roughly grade 4. The Chaddock sign was bilaterally positive, and the Babinski sign was bilaterally negative. Pupillary reflexes were normal. When the patient awakened, a question was not answered and instructions were not followed. Thus, the remaining neurological examination could not be performed as the patient was not cooperative. Serological examination results were as follows: Antinuclear antibodies (titer > 1:320; granular pattern); positive SS-A antibodies; pancytopenia (platelets 41 × 109 cells/L), hemoglobin 77 g/L, and leukocytes 2.97 × 109 cells/L; prolonged prothrombin time, 16.7 seconds; increased international normalized ratio, 1.41; positive rate of the direct coombs test; low complement C3 (0.25 g/L) and complement C4 (0.08 g/L); and high D-dimer level (10.62 mg/L). Additional laboratory studies revealed hypokalemia (2.99 mmol/L), hyponatremia (129.1 mmol/L), hypochloremia (97.5 mmol/L), liver dysfunction (alanine aminotransferase 113.3 U/L), and increased lactate dehydrogenase (564 U/L). Brain diffusion-weighted imaging and T2 weight imaging showed scattered abnormal signals beside the frontal horn of the lateral ventricle, particularly on the left side (Figure 1). Pancytopenia and her mental state were not improved after symptomatic treatment and transfusing fresh plasma.
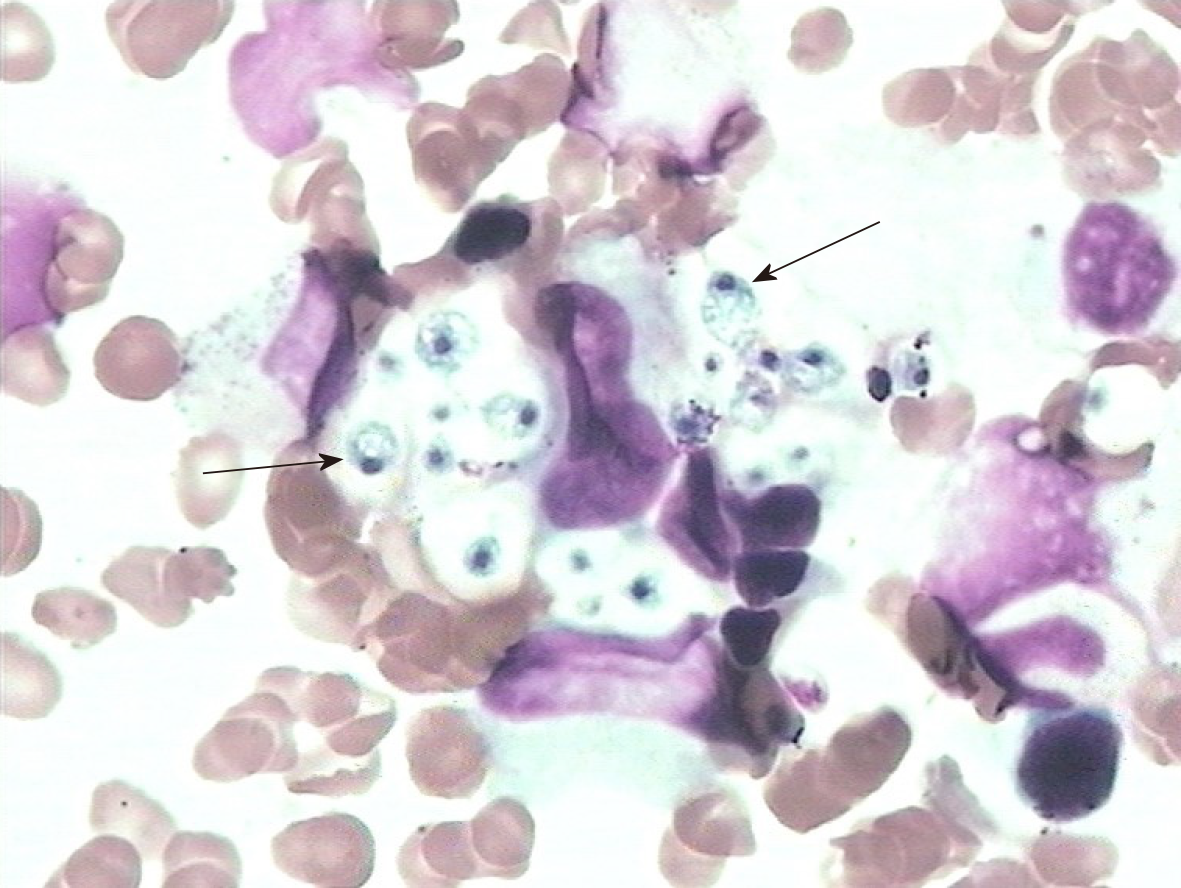
It was unlikely that the above symptoms and abnormal results could be explained solely by PSS. Thus, we reviewed the entire disease course. The patient did not develop fever, cough, or other infectious symptoms. Sputum and blood cultures were negative. Her lung computed tomography scan was normal. Serological results for human immunodeficiency virus, the tuberculin purified protein derivative test, and the T-cell spot test for tuberculosis infection were negative. Because of the progressive worsening of pancytopenia, a bone marrow biopsy was performed after obtaining written informed consent from the patient’s family. The bone marrow smear showed numerous yeast forms, indicating H. capsulatum (Figure 2, arrow), and no abnormalities were found in erythrocytes or granulocytes.
A further detailed inquiry into the medical history of this patient revealed that she was a lacquerer in Jilin Province without any other history that could increase the risk of histoplasmosis. According to the diagnostic criteria for histoplasmosis-related and endemic fungal infections standardized by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group[4], she was diagnosed with DH.
On the day she was diagnosed with DH, her consciousness suddenly worsened, and she was sent to the intensive care unit for rescue treatment before the application of amphotericin B.
Unfortunately, she died of respiratory failure on the day the diagnosis was confirmed.
SS, a systemic autoimmune disease characterized by keratoconjunctivitis sicca (dry eyes) and xerostomia (dry mouth)[5], predominantly affects middle-aged women, but can also occur in children, men, and the elderly[1]. PSS is a chronic autoimmune disease that shows various clinical manifestations such as dry eye, dry mouth, fatigue, and inflammatory musculoskeletal pain, and systemic symptoms[6] including hematologic manifestations. Patients with PSS can present with anemia, hemocytopenia, monoclonal gammopathies, and lymphoproliferative disorders, and typically respond well to immunosuppressive agents such as steroids and intravenous immunoglobulin[7].
Our patient was diagnosed seven years ago with PSS and hemolytic anemia. The condition was controlled initially with hormone administration. However, over time, in addition to the above symptoms, other problems appeared, including severe pancytopenia, coagulopathy, liver dysfunction, electrolyte disturbances, and neurological symptoms, which responded poorly to hormone administration and symptomatic treatment.
Ultimately, a bone marrow biopsy pointed to histoplasmosis, an invasive fungal infection, as the crux of the matter. So, what does this infection mean for PSS? And what are the relevant influencing factors? Infection was the second most common cause of death (18.3%, 21/115 deaths), after cardiovascular disease, in 1045 PSS patients followed for about 10 years according to a study from Spain[8]. Some researchers proposed that, if infections are regarded as complications rather than causative factors or conditions that may mimic SS in PSS, infections in PSS can be separated into two major parts: Specific and severe[9]. With specific infections, oral candidiasis, tuberculosis, and non-tuberculous mycobacterial infections are more common and attract substantial attention.
According to recent studies in Taiwan, immunosuppressive agents and a high SS activity may increase the risk of tuberculosis and non-tuberculous mycobacterial infections in PSS patients, more than the disease itself[3,10]. However, unlike other systemic autoimmune diseases, such as systemic lupus erythematosus, further research about the prevalence of severe infections (i.e., causing hospital admission or death) in PSS patients is scarce[11,12], and most information comes from clinical case studies. We report this case to enhance the clinical awareness of severe infection in PSS. Furthermore, according to existing studies, it appears that PSS treatments (i.e., immunosuppressants and glucocorticoids) are confirmed factors that affect the risk of infection. Nevertheless, additional evidence as to whether the disease itself, age of the patient, the year of first SS diagnosis, hospitalization, and other factors increase the prevalence of infection in PSS patients, especially severe infection, is needed.
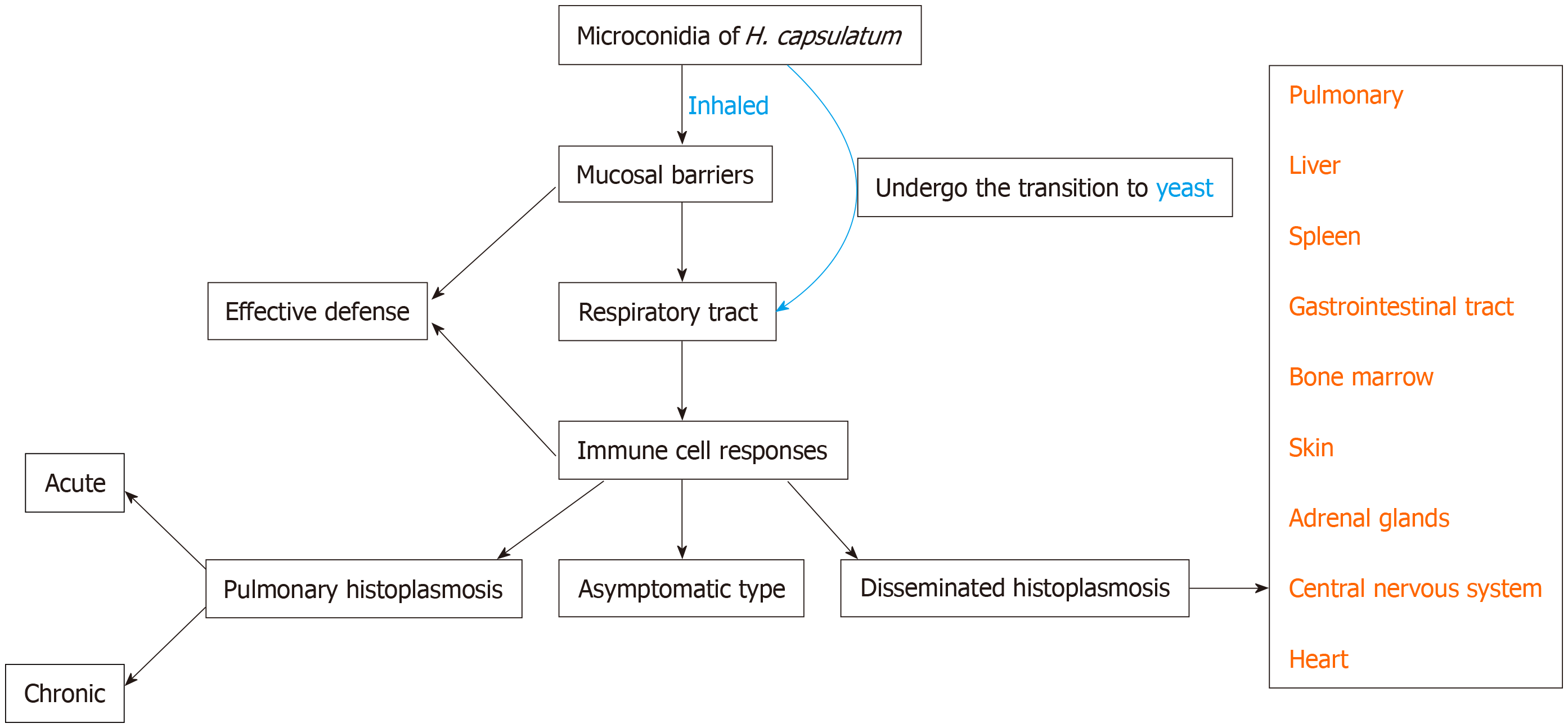
Histoplasmosis is an invasive mycosis caused by the saprophytic dimorphic fungus H. capsulatum[13]. There are two types of conidia in the mycelial form: Macroconidia (8-15 µm in diameter) and microconidia (2-5 µm in diameter). Human infection occurs after the microconidia or hyphal fragments of H. capsulatum are inhaled, travel through the respiratory system, and reach the alveoli. Then it transforms into a yeast form. Figure 3 illustrates the occurrence of histoplasmosis in a simplified flow diagram.
Histoplasmosis occurs in specific endemic areas[14], including the mid-western United States, Africa, and most of Latin America. Sporadic cases were reported in China[15], most in provinces and regions through which the Yangtze River flows, where strong winds and low sunshine levels are considered suitable for H. capsulatum growth. In contrast, Jilin Province has a semi-arid climate and four distinct seasons, and histoplasmosis had never been reported there. Our case updates the geographic distribution of histoplasmosis in China[15], indicating the need for special attention from clinicians.
According to the study of Pan et al[15] about histoplasmosis in China, the majority of cases occurred in males for both pulmonary and disseminated infection. However, whether gender is correlated with risk factors is unclear. The primary risk factors for acquiring histoplasmosis are living in or traveling to an area endemic for the fungus. Bird and bat guano are strongly associated with histoplasmosis. Exposure to these animals or their dwellings increases the risk of inhaling the fungal spores. Additionally, host factors play an important role in the occurrence of histoplasmosis[16], including human immunodeficiency virus infection, primary immunodeficiency, long-term immunosuppression (such as the use of glucocorticoids or immunosuppressants), post-organ transplantation, and being younger than 1 year or older than 50 years of age.
Although our patient had no travel experience or exposure to bird and bat guano, as a lacquerer, she worked in a humid, dusty environment for a long time. At the same time, our patient may have suffered from an unstable PSS condition along with long-term use of corticosteroids and immunosuppressants, which together contributed to abnormal immune function.
The clinical presentation of histoplasmosis can vary from the acute pulmonary to the chronic disseminated form. Based on its clinical features, there are four types of histoplasmosis: Asymptomatic (95%), acute pulmonary, chronic pulmonary, and disseminated[17]. The pathogen’s ability to evade inflammatory responses and the intensity of the host immune response determine the severity of symptoms and clinical presentation, and whether a state of latency develops with the potential for reactivation. DH is relatively rare and often presents with pyrexia of unknown cause similar to ‘disseminated tuberculosis’ involving the adrenal glands and bone marrow. According to previous studies, most patients suffering from DH experience persistent symptoms, including fever, weight loss, night sweats, cough, and shortness of breath, as well as electrolyte disturbances and abnormal liver function tests. Currently, the prevalence of these symptoms in DH cannot be reliably estimated. In some research, most people suffer from fever, including immunocompromised patients[18]. The patient in this case did not have any infectious symptoms. We speculate that this was because of a serious autoimmune disorder. In the study of Deodhar et al[17], pancytopenia, anemia, and skin lesions were more common among the immunocompromised compared to the immunocompetent group. The diagnosis was most often made by a bone marrow biopsy and fungal cultures of the bone marrow aspirate. Adrenal involvement occurred mainly among the immunocompetent group and diagnosis in that group was obtained from biopsy and adrenal tissue fungal cultures[17]. This difference suggests that it is necessary to consider the clinical features and pathophysiology in both immunocompromised and immunocompetent patients.
DH is rare in SS patients. Upon reviewing the literature related to SS with histoplasmosis, we only found one case reported in 2012[19]. That patient was a 28-year-old man suffering from fever, vomiting, and skin lesions on the trunk and face, and finally diagnosed with SS and DH after admission. Different from our case, the male patient was younger with a shorter PSS disease course and more obvious infectious symptoms, and had never used a glucocorticoid. He was successfully treated with liposomal amphotericin and itraconazole. Therefore, we suggest that the duration of the course of PSS, and the use of glucocorticoids and immuno-suppressants, may have caused the difference of clinical features and outcome in our patient by indirectly affecting immune function.
Central nervous system involvement occurs in 5%–10% of DH cases[20]. The diagnosis in most patients can be based on antigen and antibody testing of the cerebrospinal fluid and serum, as well as antigen testing of the urine. Unfortunately, the family of our patient did not permit a lumbar puncture or magnetic resonance imaging-enhanced scanning. Previous studies demonstrated that the most common clinical features of central nervous system involvement in histoplasmosis are chronic meningitis, focal brain or spinal cord lesions, stroke syndromes, encephalitis, and hydrocephalus. Thus, based on the patient's medical history and imaging features (Figure 1), the lesion observed on magnetic resonance imaging may reflect central nervous system involvement in histoplasmosis; a lesion location that is very rare.
Endemic mycosis is contingent on a compatible clinical scenario and positive culture or histopathology. H. capsulatum cultures should be handled in a biosafety level 3 laboratory. When culture or pathologic examination is not available or negative in an immunodeficient host, the clinical picture is suggestive, and mycologic laboratory tests show positive results (e.g., Histoplasma antigen positivity), the diagnosis of histoplasmosis is considered as probable[4].
Amphotericin B may be effective, but untreated DH is typically fatal. Thus, early diagnosis and antifungal therapy are crucial. Histoplasmosis should be considered if accompanied by pancytopenia, hepatosplenomegaly, and electrolyte disturbances, with or without fever, respiratory symptoms, and weight loss in immuno-compromised patients. A bone marrow biopsy and fungal culture of the bone marrow aspirate should be performed as soon as possible.
In conclusion, we present a rare and interesting case of DH in a PSS patient. This is the first case of DH reported in Jilin Province, China. This case updates the geographic distribution of histoplasmosis in China, and expands the clinical manifestations of DH in PSS. These findings improve our understanding of DH in PSS and may allow for earlier identification.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mousa HAL, Trovato GM S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Brito-Zerón P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, Sivils K, Theander E, Tzioufas A, Ramos-Casals M. Sjögren syndrome. Nat Rev Dis Primers. 2016;2:16047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 538] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 2. | Stefanski AL, Tomiak C, Pleyer U, Dietrich T, Burmester GR, Dörner T. The Diagnosis and Treatment of Sjögren's Syndrome. Dtsch Arztebl Int. 2017;114:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Chao WC, Lin CH, Liao TL, Chen YM, Hsu CY, Chen JP, Chen DY, Chen HH. The risk of nontuberculous mycobacterial infection in patients with Sjögren's syndrome: a nationwide, population-based cohort study. BMC Infect Dis. 2017;17:796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813-1821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4096] [Cited by in RCA: 3961] [Article Influence: 233.0] [Reference Citation Analysis (0)] |
| 5. | Mavragani CP, Moutsopoulos HM. Sjögren syndrome. CMAJ. 2014;186:E579-E586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Marshall LL, Stevens GA. Management of Primary Sjögren's Syndrome. Consult Pharm. 2018;33:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Choung BS, Yoo WH. Successful treatment with intravenous immunoglobulin of severe thrombocytopenia complicated in primary Sjögren's syndrome. Rheumatol Int. 2012;32:1353-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Brito-Zerón P, Kostov B, Solans R, Fraile G, Suárez-Cuervo C, Casanovas A, Rascón FJ, Qanneta R, Pérez-Alvarez R, Ripoll M, Akasbi M, Pinilla B, Bosch JA, Nava-Mateos J, Díaz-López B, Morera-Morales ML, Gheitasi H, Retamozo S, Ramos-Casals M; SS Study Group, Autoimmune Diseases Study Group (GEAS), Spanish Society of Internal Medicine (SEMI). Systemic activity and mortality in primary Sjögren syndrome: predicting survival using the EULAR-SS Disease Activity Index (ESSDAI) in 1045 patients. Ann Rheum Dis. 2016;75:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Pego-Reigosa JM, Restrepo Vélez J, Baldini C, Rúa-Figueroa Fernández de Larrinoa Í. Comorbidities (excluding lymphoma) in Sjögren's syndrome. Rheumatology (Oxford). 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Chang YS, Liu CJ, Ou SM, Hu YW, Chen TJ, Lee HT, Chang CC, Chou CT. Tuberculosis infection in primary Sjögren's syndrome: a nationwide population-based study. Clin Rheumatol. 2014;33:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Rúa-Figueroa Í, López-Longo J, Galindo-Izquierdo M, Calvo-Alén J, Del Campo V, Olivé-Marqués A, Pérez-Vicente S, Fernández-Nebro A, Andrés M, Erausquin C, Tomero E, Horcada L, Uriarte E, Freire M, Montilla C, Sánchez-Atrio A, Santos G, Boteanu A, Díez-Álvarez E, Narváez J, Martínez-Taboada V, Silva-Fernández L, Ruiz-Lucea E, Andreu JL, Hernández-Beriain JÁ, Gantes M, Hernández-Cruz B, Pérez-Venegas J, Pecondón-Español Á, Marras C, Ibáñez-Barceló M, Bonilla G, Torrente V, Castellví I, Alegre JJ, Calvet J, Marenco JL, Raya E, Vázquez T, Quevedo V, Muñoz-Fernández S, Rodríguez-Gómez M, Ibáñez J, Pego-Reigosa JM. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2017;47:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Frodlund M, Reid S, Wetterö J, Dahlström Ö, Sjöwall C, Leonard D. The majority of Swedish systemic lupus erythematosus patients are still affected by irreversible organ impairment: factors related to damage accrual in two regional cohorts. Lupus. 2019;28:1261-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Sharma R, Lipi L, Gajendra S, Mohapatra I, Goel RK, Duggal R, Mishra SR, Gautam D. Gastrointestinal Histoplasmosis: A Case Series. Int J Surg Pathol. 2017;25:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Azar MM, Zhang X, Assi R, Hage C, Wheat LJ, Malinis MF. Clinical and epidemiological characterization of histoplasmosis cases in a nonendemic area, Connecticut, United States. Med Mycol. 2018;56:896-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Pan B, Chen M, Pan W, Liao W. Histoplasmosis: a new endemic fungal infection in China? Review and analysis of cases. Mycoses. 2013;56:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Azar MM, Hage CA. Clinical Perspectives in the Diagnosis and Management of Histoplasmosis. Clin Chest Med. 2017;38:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Deodhar D, Frenzen F, Rupali P, David D, Promila M, Ramya I, Seshadri MS. Disseminated histoplasmosis: a comparative study of the clinical features and outcome among immunocompromised and immunocompetent patients. Natl Med J India. 2013;26:214-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Pérez-Lazo G, Maquera-Afaray J, Mejia CR, Castillo R. [Disseminated histoplasmosis and HIV infection: Case series in a Peruvian hospital]. Rev Chilena Infectol. 2017;34:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Rodrigo HF, Stavile RN, Deleo S. [Disseminated histoplasmosis, lymphopenia and Sjögren's syndrome]. Medicina (B Aires). 2012;72:435-438. [PubMed] |
| 20. | Wheat J, Myint T, Guo Y, Kemmer P, Hage C, Terry C, Azar MM, Riddell J, Ender P, Chen S, Shehab K, Cleveland K, Esguerra E, Johnson J, Wright P, Douglas V, Vergidis P, Ooi W, Baddley J, Bamberger D, Khairy R, Vikram HR, Jenny-Avital E, Sivasubramanian G, Bowlware K, Pahud B, Sarria J, Tsai T, Assi M, Mocherla S, Prakash V, Allen D, Passaretti C, Huprikar S, Anderson A. Central nervous system histoplasmosis: Multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine (Baltimore). 2018;97:e0245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |