Published online Mar 26, 2020. doi: 10.12998/wjcc.v8.i6.1065
Peer-review started: November 22, 2019
First decision: December 12, 2019
Revised: February 19, 2020
Accepted: February 28, 2020
Article in press: February 28, 2020
Published online: March 26, 2020
Processing time: 124 Days and 13.7 Hours
Graft-vs-host disease (GVHD) is a major cause of mortality after allogeneic hematopoietic stem cell transplantation. Some patients have steroid-refractory (SR) GVHD.
To evaluate the effect and safety of ruxolitinib add-on in the treatment of patients with SR acute (a) and chronic (c) GVHD.
We retrospectively analyzed 38 patients administered ruxolitinib add-on to standard immunosuppressive therapy for SR-aGVHD or SR-cGVHD following allogeneic hematopoietic stem cell transplantation. Ruxolitinib was administered 5-10 mg/d depending on disease severity, patient status, and the use of anti-fungal drugs. Overall response rate, time to best response, malignancy relapse rate, infection rate, and treatment-related adverse events were assessed.
The analysis included 10 patients with SR-aGVHD (grade III/IV, n = 9) and 28 patients with SR-cGVHD (moderate/severe, n = 24). For the SR-aGVHD and SR-cGVHD groups, respectively: Median number of previous GVHD therapies was 2 (range: 1-3) and 2 (1-4); median follow-up was 2.5 (1.5-4) and 5 (1.5-10) mo; median time to best response was 1 (0.5-2.5) and 3 (1-9.5) mo; and overall response rate was 100% (complete response: 80%) and 82.1% (complete response: 10.7%) with a response observed in all GVHD-affected organs. The malignancy relapse rates for the SR-aGVHD and SR-cGVHD groups were 10.0% and 10.7%, respectively. Reactivation rates for cytomegalovirus, Epstein-Barr virus, and varicella-zoster virus, respectively, were 30.0%, 10.0%, and 0% for the SR-aGVHD group and 0%, 14.3%, and 7.1% for the SR-cGVHD group.
Ruxolitinib add-on was effective and safe as salvage therapy for SR-GVHD.
Core tip: This study aimed to investigate the effect and safety of ruxolitinib add-on in the treatment of patients with steroid-refractory (SR) acute (a) and chronic (c) graft-vs-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation. An important finding of this retrospective case series was that the use of ruxolitinib as a salvage therapy for SR-GVHD resulted in an overall response rate of 100% in patients with SR-aGVHD (complete response: 80%) and 82.1% in patients with SR-cGVHD (complete response: 10.7%). In addition, this was achieved with a lower dose (5-10 mg/d) of ruxolitinib than previous reports, indicating that the dose of ruxolitinib could be lowered when used in combination with antifungal drugs (CYP2C9/CYP3A4 inhibitors).
- Citation: Dang SH, Liu Q, Xie R, Shen N, Zhou S, Shi W, Liu W, Zou P, You Y, Zhong ZD. Ruxolitinib add-on in corticosteroid-refractory graft-vs-host disease after allogeneic stem cell transplantation: Results from a retrospective study on 38 Chinese patients. World J Clin Cases 2020; 8(6): 1065-1073
- URL: https://www.wjgnet.com/2307-8960/full/v8/i6/1065.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i6.1065
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is currently the only approach to cure malignant tumors of the blood system. Unfortunately, the application of allo-HSCT is restricted by graft-vs-host disease (GVHD), an important complication that severely affects quality of life and is the main cause of death after transplantation[1,2]. Currently, the standard treatments for GVHD are immunosuppressive agents (such as glucocorticoids, cyclosporine, tacrolimus, and mycophenolate mofetil) and monoclonal antibodies targeted against immune cells. The efficacies of these therapies are poor for some patients, especially those with steroid-refractory (SR) GVHD (SR-GVHD)[3,4]. Furthermore, long-term use of immunosuppressive agents at large doses significantly reduces the graft-vs-leukemia (GVL) effects and the ability of the body to fight infections, increasing the risks of relapse, complications, and morbidity[5,6]. Thus, alternatives to immunosuppressive agents are being sought as novel therapies for GVHD.
Ruxolitinib is an inhibitor of JAK1/2-STAT signaling, which is widely involved in immune responses, inflammatory processes, cell proliferation, and differentiation[7,8]. Ruxolitinib exerts a unique anti-GVHD action while retaining the GVL effect[9]. Several previous studies in the United States and Europe have reported that ruxolitinib shows clinical benefit when used as a first-line or salvage therapy in patients with SR-GVHD[10-13]. However, there are limited data on the effects of ruxolitinib in patients with GVHD in China.
This study evaluated the clinical effectiveness and adverse effects of ruxolitinib by retrospectively analyzing 38 patients in China with refractory GVHD after allo-HSCT, who were treated with ruxolitinib add-on to standard immunosuppressive agents.
This single-center, retrospective case series included consecutive patients with SR-GVHD who received ruxolitinib at the Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, between May 2017 and March 2018. This study was approved by the institutional review board of the Union Hospital of Tongji Medical College. Informed consent was waived due to the retrospective study design.
The inclusion criteria were: (1) A confirmed diagnosis of cancer of the blood system; (2) Treated using allo-HSCT, with corticosteroids administered as the first-line immunosuppressive therapy; (3) Developed acute SR GVHD (aGVHD) after transplantation (defined as any grade progression within 3 d of the start of 40 mg/d corticosteroid therapy or failure to improve by at least 1 grade within 7 d of corticosteroid therapy)[5] or chronic GVHD (cGVHD) that was refractory to corticosteroids (defined as active cGVHD despite ≥ 4 wk of treatment with ≥ 0.25 mg/kg/d (15-20 mg/d) prednisone or equivalent during the past 12 mo, and up to two previous lines of cGVHD treatment with stable concurrent immunosuppression during the previous 4 wk)[6]; and (4) Were treated for GVHD with ruxolitinib. The diagnosis of aGVHD was made using the modified Glucksberg standards[14], and the diagnosis of cGVHD was made using the 2015 version of the National Institutes of Health (NIH) standards[15].
The exclusion criteria were: (1) Coexistence of SR-GVHD and uncontrolled infection; (2) Platelet count < 25 × 109/L; (3) Complications such as relapse of the primary malignancy or progressive disease that might influence the outcome or decrease the effectiveness of treatment for cGVHD; (4) Pregnant or breastfeeding women; (5) Poor compliance with ruxolitinib therapy, defined as the administration of ruxolitinib for < 1 wk, irregular administration of ruxolitinib, or discontinuation of ruxolitinib without consulting the doctor; and (6) Concurrent administration of anti-GVHD therapies other than those that the patient was refractory.
All patients received ruxolitinib orally as an add-on immunosuppressive therapy. The standard practice at our hospital is that ruxolitinib is added to the refractory treatment before any other new agents are introduced, and this practice is inspired by Caucasian patients[10,16,17]. The dose of ruxolitinib administered was 5 mg twice a day for those with grade III/IV aGVHD or moderate/severe cGVHD and no decreases in red blood cell count, hemoglobin level, white blood cell count, or platelet count; 5 mg once a day for those with grade I/II aGVHD or mild cGVHD or decreases in red blood cell count, hemoglobin level, white blood cell count, or platelet count; and 5-10 mg once a day in those receiving antifungal therapy such as itraconazole, voriconazole, or posaconazole[18]. The dose of ruxolitinib was decreased gradually 2 wk after the best overall response, and eventually, the drug was discontinued.
The grading of aGVHD used the modified Glucksberg standards[14]. The overall grading of cGVHD and the severity scores for various organs were determined using the 2015 version of the NIH standards[15]. The joint and fascia manifestations of GVHD were scored using the Passive Range of Motion scale[19] for joint mobility. The overall grade and the scores for the various organ sites were re-evaluated each month after the initiation of ruxolitinib therapy. Treatment responses were evaluated every 3 mo and classified as complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD). For patients with SR-aGVHD, CR was defined as complete resolution of GVHD, PR as a decrease in the Glucksberg score of at least one grade at one or more sites without deterioration at the other sites (a response had to last for at least 3 wk), SD as a response between CR and PR, and PD as an increase in the Glucksberg score at any site or the involvement of a new site that required medical intervention to control the disease condition. For patients with SR-cGVHD, the effect evaluation used the 2015 version of the NIH chronic GVHD response evaluation standards[20]. The overall response rate (ORR) was defined as CR + PR. Follow-up was censored at disease relapse (preemptive treatment performed for morphologic relapse or molecular genetic relapse), non-relapse-related death (e.g., due to infection), or progression of GVHD (PD).
Cytopenia was graded as 0-5 based on the Common Terminology Criteria for Adverse Events v3.0[21]. Clinically relevant ruxolitinib-related adverse events (AEs) were defined to be those of grade 2 or higher, after the exclusion of other causes.
Only descriptive statistical analysis was performed. The data evaluated included remission of GVHD, time to best overall response, relapse of the primary hematologic disease, infection, and ruxolitinib-related AEs.
A total of 42 patients with SR-GVHD were treated with ruxolitinib during the study period, including 10 patients with SR-aGVHD and 32 patients with SR-cGVHD. A total of four patients (all with SR-cGVHD) were subsequently excluded due to poor adherence to ruxolitinib therapy with no improvement in symptoms of GVHD (n = 2) or concomitant use of mycophenolate mofetil (n = 2). Therefore, a total of 38 patients (10 with SR-aGVHD and 32 with SR-cGVHD) were included in the final analysis, and their baseline clinical characteristics are presented in Table 1.
| Parameter | aGVHD, n = 10 | cGVHD, n = 28 |
| Age in yr, median (range) | 35 (19–55) | 30 (14–55) |
| Sex, n (%) | ||
| Female | 3 (30.0) | 14 (50.0) |
| Male | 7 (70.0) | 14 (50.0) |
| Disease, n (%) | ||
| AML | 5 (50.0) | 11 (39.3) |
| ALL | 2 (20.0) | 8 (28.6) |
| CML | 0 (0) | 6 (21.4) |
| MDS | 2 (20.0) | 3 (10.7) |
| CMML | 1 (10.0) | 0 (0) |
| Donor type, n (%) | ||
| Matched related | 8 (80.0) | 15 (53.6) |
| Partially mismatched related | 2 (20.0) | 8 (28.6) |
| Unrelated | 0 (0) | 5 (17.9) |
| Types of transplantation, n (%) | ||
| PBSC | 6 (60.0) | 22 (78.6) |
| BM-HSC | 2 (20.0) | 1 (3.6) |
| PBSC + BM-HSC | 2 (20.0) | 5 (17.9) |
| Median number of infused nucleated cells as /kg | 11.1 × 108 | 15.62 × 106 |
| Median number of infused CD34+ cells as /kg | 5.99 × 106 | 6.05 × 106 |
| aGVHD of grade 3 or higher, n (%) | 9 (90.0) | - |
| Moderate or severe cGVHD, n (%) | - | 24 (85.7) |
| Multiple organ involvement, n (%) | 3 (30.0) | 22 (78.6) |
| Median number of immunosuppressive agents1 | 2 | 2 |
| aGVHD stage 3–4 or cGVHD NIH 2–3, n (%) | ||
| Skin | 5 (50.0) | 19 (67.9) |
| Digestive tract | 3 (30.0) | 6 (21.4) |
| Liver | 2 (20.0) | 4 (14.3) |
| Eyes | - | 12 (42.9) |
| Oral cavity | - | 11 (39.3) |
| Lungs | - | 6 (21.4) |
| Fascia | - | 8 (28.6) |
The primary disease before transplantation included acute myeloid leukemia, acute lymphocytic leukemia, chronic myeloid leukemia, chronic myelomonocytic leukemia, and myelodysplastic syndrome.
The majority of patients with aGVHD (90.0%) had disease of grade 3 or higher, and most of the patients with cGVHD (85.7%) had moderate or severe disease (Table 1). Multiple organ involvement was found in 30.0% of patients with aGVHD and 78.6% of patients with cGVHD. GVHD severity in each organ system is summarized in Table 1.
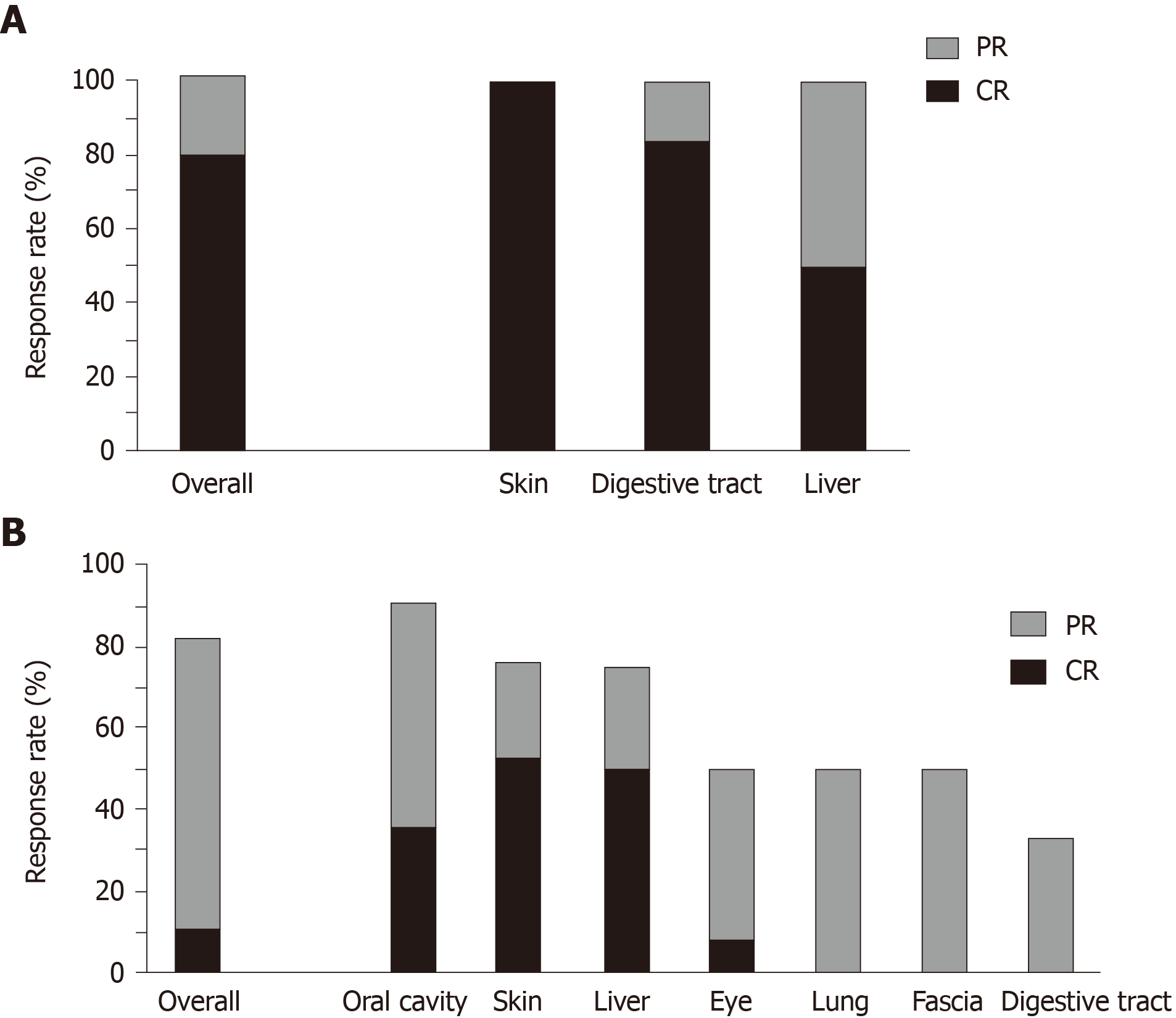
The median follow-up time was 2.5 mo (range, 1.5-4.0 mo) in patients with aGVHD and 5 mo (range, 1.5-10.0 mo) in patients with cGVHD. Figure 1 shows the effectiveness of ruxolitinib in the treatment of GVHD. For patients with aGVHD, the ORR of ruxolitinib was 100%, and the treatment response rate (CR + PR) for each organ system (skin, digestive tract, and liver) was also 100% (Figure 1A). For patients with cGVHD, the ORR of ruxolitinib was 82.1%, and the treatment response rates (CR + PR) for the various organ systems ranged from 33.3% (digestive tract) to 90.9% (oral cavity; Figure 1B). Only one (3.6%) subject with cGVHD experienced disease progression while on ruxolitinib therapy. The median time to best overall response was 1.0 (range, 0.5-2.5) mo in patients with aGVHD and 3.0 (range, 1.0-9.5) mo in patients with cGVHD. The median time to best overall response in the various organ systems ranged from 25-32 d in patients with aGVHD and 42-259 d in patients with cGVHD (Table 2). For patients with cGVHD, the most rapid responses were observed in the liver, skin, and oral cavity (Table 2). The rate of immunosuppressive agent discontinuation was 80.0% (8/10) in patients with aGVHD and 75% (21/28) in patients with cGVHD. Two of the patients with aGVHD who discontinued ruxolitinib after achieving CR subsequently developed cGVHD.
| Parameter | aGVHD, n = 10 | cGVHD, n = 28 |
| Overall response rate, n (%) | 10 (100) | 22 (82.1) |
| Median follow-up time in mo, median (range) | 2.5 (1.5–4) | 5 (1.5–10) |
| Time to best overall response in mo, median (range) | 1.0 (0.5–2.5) | 3.0 (1.0–9.5) |
| Time to best response for each organ system in d, median (range) | ||
| Skin | 28 (14–28) | 77 (7–147) |
| Digestive tract | 25 (14–60) | 259 (70–308) |
| Liver | 32 (21–42) | 42 (28–56) |
| Eye | - | 112 (42–182) |
| Oral cavity | - | 80 (28–168) |
| Lung | - | 91 (49–140) |
| Fascia | - | 91 (56–266) |
| Discontinuation of immunosuppressive agents after ruxolitinib, n (%) | 8 (80.0) | 21 (75.0) |
AEs reported during treatment with ruxolitinib are shown in Table 3. In patients with aGVHD, the rate of relapse of the primary blood disease was 10.0% (1/10). Five patients with aGVHD (50.0%) developed a lung infection during treatment with ruxolitinib that improved with antifungal therapy, while an additional patient (10.0%) developed a lung infection with a more protracted course. Two of the four patients with aGVHD who did not develop a lung infection were administered antifungal drugs at prophylactic doses, and two did not receive antifungal drugs. Three patients with aGVHD (30.0%) developed positivity for cytomegalovirus (CMV) DNA, and one patient (10.0%) became positive for Epstein-Barr virus (EBV) DNA. All 10 patients with aGVHD exhibited varying degrees of cytopenia before treatment with ruxolitinib. Two of the 10 patients with aGVHD (20.0%) exhibited grade 1 cytopenia after therapy with ruxolitinib.
| Parameter | aGVHD, n = 10 | cGVHD, n = 28 |
| Activation of cytomegalovirus | 3 (30.0) | 0 (0) |
| Activation of Epstein-Barr virus | 1 (10.0) | 4 (14.3) |
| Infection with herpes-zoster virus | 0 (0) | 2 (7.1) |
| Relapse of malignant blood disease | 1 (10.0) | 3 (10.7) |
The rate of relapse of the primary blood disease was 10.7% (3/28) in patients with cGVHD. Two patients with cGVHD discontinued antifungal therapy, but lung infection did not occur. The rate of infection with herpes-zoster virus was 7.1% (2/28), and the activation rate of EBV was 14.3% (4/28); all patients recovered after active treatment. No patients developed CMV activation during therapy with ruxolitinib. One patient suffered from a decrease in hemoglobin level that could not be explained by other reasons; no other patients developed cytopenia. One patient developed symptoms of nausea, vomiting, and systemic pain 2 d after initiation of ruxolitinib therapy, but no symptoms were experienced when ruxolitinib was re-administered after having been discontinued for 1.5 mo. One patient developed a mild rash 1 d after starting ruxolitinib therapy, but no treatment was needed, and the rash disappeared 1 wk later.
Although glucocorticoids, often in combination with other immunosuppressive agents, are used as a first-line therapy for GVHD, many patients develop refractory GVHD[3,4]. Furthermore, some patients with cGVHD require long-term use of immunosuppressive therapy that can lead to treatment-related complications such as infection, organ toxicity, and relapse of malignant diseases[5,6,22]. Ruxolitinib has been suggested as a possible novel therapy for GVHD with less toxicity than standard immunosuppressive agents.
An important finding of this retrospective case series was that the use of ruxolitinib add-on as a salvage therapy for SR-GVHD resulted in an ORR of 100% in patients with SR-aGVHD (CR rate of 80%) and 82.1% in patients with SR-cGVHD (CR rate of 10.7%). Furthermore, a response was observed in all GVHD-affected organs. The malignancy relapse rates were around 10% for both groups, and the reactivation rates for CMV, EBV, and varicella-zoster virus were ≤ 30%. Importantly, there were no treatment-related AEs of grade 2 or higher. Taken together, our findings provide further evidence that ruxolitinib may show good treatment effects and low toxicity as a salvage therapy for SR-aGVHD and SR-cGVHD after allo-HSCT.
The results of our study showed that oral administration of ruxolitinib was effective in the treatment of SR-GVHD. For patients with SR-aGVHD, ruxolitinib had a rapid onset of action (median time to best overall clinical response of 1 mo) and produced a prolonged treatment response with a CR rate of 80%, a PR rate of 20%, and an ORR of 100%. Our findings are broadly in agreement with previous studies in Europe. Assouan et al[12] reported an ORR of 70% (CR rate: 50%; PR rate: 20%) after a median time of 31 d. Zeiser et al[10] determined an ORR of 81.5% (CR rate: 46.3%; PR rate: 35.2%) after a median of 1.5 wk and observed particularly impressive responses in the intestines, skin, and liver. It was notable that comparable if not better treatment responses were observed in our cohort than in those described by Zeiser et al[10] and Assouan et al[12] despite our dosage of ruxolitinib (5-10 mg/d) being lower than those in these other studies (10-20 mg/d). One possible reason for this is that the patients in our study were given azole antifungal drugs to prevent fungal infections after transplantation. Azole antifungal agents are known inhibitors of two key drug-metabolizing enzymes, CYP2C9 and CYP3A4[23], which are involved in the metabolism of ruxolitinib[24].
For patients with SR-cGVHD in the present study, the ORR was 82.1%, with a median time to the best overall response of 3 mo. Furthermore, ruxolitinib was effective for cGVHD at several different sites with the highest remission rates in the oral cavity, skin, and liver. These results are comparable to those of a previous study in Europe, which found an ORR of 85.4% and a median time to response of 3 wk, with responses seen in all organ systems[10]. An investigation in the United States determined that the ORR was 99% for the oral cavity, 82% for the skin, 99% for the liver, 92% for the gastrointestinal tract, 100% for the musculoskeletal system, 80% for the lungs, 75% for scleroderma, and 100% for the eyes[13]. Another study in the United States reported a lower ORR of 47%, with an organ response in the skin, mouth, eyes, lungs, and joints/fascia of 25%, 60%, 26%, 10%, and 41%, respectively[11].
The response to ruxolitinib in our study was poor for patients with scleroderma, which may be because the follow-up was short. A previous investigation reported that ruxolitinib therapy of 12 patients with scleroderma resulted in a softening of the skin in eight patients but no decrease in the area of affected skin[25]. The response of the lungs to ruxolitinib in patients with SR-cGVHD has varied, with a response rate of 80% (all PRs) reported by Khoury et al[13] and only 10% reported by Modi et al[11]. We observed a PR rate of 50% for the lungs with a median time to remission of 13 wk. In view of the apparent inconsistencies between studies, additional research is needed to clarify the response rates to ruxolitinib in the lungs.
The relapse rates in our study were 10% for SR-aGVHD and 10.7% for SR-cGVHD. Among the four patients with relapse observed in our study, three were still alive at the end of the follow-up period and were continuing to take ruxolitinib, whereas one died due to relapse that occurred 2 mo after discontinuation of ruxolitinib. Previous studies of patients administered ruxolitinib have reported relapse rates for hematologic malignancies of 9.3%–10.0% for SR-aGVHD[12,26] and 2.2%–2.4% for SR-cGVHD[26].
The activation rates of CMV and EBV were 30% and 10%, respectively, in patients with SR-aGVHD in our study. This is comparable to a previously published value of 33.3% for CMV, although data for EBV were not reported[26]. Our cohort of patients with SR-cGVHD exhibited a herpes-zoster infection rate of 7.1% and an EBV activation rate of 14.3% (no CMV activation was observed). A previous investigation described a CMV activation rate of 14.6% in patients with SR-cGVHD[26]. Based on our data, we conclude that treatment with ruxolitinib did not lead to a notable increase in the risk of serious infection.
Importantly, no severe ruxolitinib-related adverse events were observed in our study, with no incidences of clinically relevant cytopenia or treatment-related bleeding complications. One patient developed a mild skin reaction that resolved without discontinuation of ruxolitinib. Another patient developed nausea, vomiting, and systemic pain 2 d after the administration of ruxolitinib, but symptoms were not experienced when ruxolitinib was restarted after a 1 mo period of discontinuation. The low occurrence of AEs in the present study might be related to the low dose of ruxolitinib used, despite the concomitant use of strong CYP3A4 inhibitors.
This study has some limitations. First, this was a retrospective study and so may have been prone to selection bias and reporting bias. Second, this was a single-center study with a small sample size; hence, the generalizability of the findings is not known. Third, the follow-up period was less than 1 year, so longer-term outcomes were not assessed. Long-term outcomes, including overall survival, cumulative recurrence, and patient-reported outcomes, will be investigated in future studies. Fourth, a comparator group was not included. Finally, combination therapies should be explored in the future.
In conclusion, ruxolitinib add-on may be an effective and safe therapy for SR-aGVHD and SR-cGVHD in patients in China. Prospective multicenter studies are merited to confirm and extend our findings.
Graft-vs-host disease (GVHD) is a major cause of mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Steroids are an important component of the strategy against GVHD, but some patients have steroid-refractory (SR) GVHD. Ruxolitinib is an inhibitor of JAK1/2-STAT signaling and exerts a unique anti-GVHD action.
There are limited data on the effects of ruxolitinib in patients with GVHD in China. Determining the clinical benefits of ruxolitinib in patients with GVHD could help improve their management.
This study evaluated the clinical effectiveness and adverse effects of ruxolitinib by retrospectively analyzing 38 patients in China with refractory GVHD after allo-HSCT who were treated with ruxolitinib add-on to standard immunosuppressive agents.
This single-center, retrospective case series included consecutive patients with SR-GVHD who received ruxolitinib at the Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (China), between May 2017 and March 2018. All patients received 5-10 mg ruxolitinib per d orally as an add-on immunosuppressive therapy. Treatment responses were evaluated every 3 mo. The grading of acute (a) GVHD used the modified Glucksberg standards. The overall grading of chronic (c) GVHD and the severity scores for various organs were determined using the 2015 version of the National Institutes of Health standards.
The analysis included 10 patients with SR-aGVHD (grade III/IV, n = 9) and 28 patients with SR-cGVHD (moderate/severe, n = 24). The median number of previous GVHD therapies was 2 (range: 1-3) and 2 (1-4) for the SR-aGVHD and SR-cGVHD groups, respectively. During a median follow-up of 2.5 (1.5-4) and 5 (1.5-10) mo, the objective response rates were 100% (complete response, 80%) for aGVHD and 82% for cGVHD. Nevertheless, there was a risk of malignancy relapse, with rates of 10.0% and 10.7% for the SR-aGVHD and SR-cGVHD groups, respectively. The reactivation rates for cytomegalovirus, Epstein-Barr virus and varicella-zoster virus were 30.0%, 10.0% and 0% for the SR-aGVHD group and 0%, 14.3% and 7.1% for the SR-cGVHD group.
Ruxolitinib induces clinical benefits in patients with GVHD, with a good profile of complications and adverse effects. Therefore, it could be hypothesized that ruxolitinib improves the quality of life of patients with SR-GVHD compared with the standard immunosuppressive regimens. Of note, the dosage of ruxolitinib (5-10 mg/d) used here was lower than those in these other studies (10-20 mg/d). This might be because of concomitant azole anti-fungal, which could increase the half-life of ruxolitinib by competing for the same cytochromes.
Ruxolitinib has been suggested as a possible novel therapy for GVHD with less toxicity than standard immunosuppressive agents. Future studies and clinical trials should look into the optimization of the ruxolitinib regimens for the individualized management of GVHD.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rizzieri DA, Sakellari I S-Editor: Zhou JJ L-Editor: Filipodia E-Editor: Qi LL
| 1. | Hu Y, Cui Q, Luo C, Luo Y, Shi J, Huang H. A promising sword of tomorrow: Human γδ T cell strategies reconcile allo-HSCT complications. Blood Rev. 2016;30:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Kurosawa S, Oshima K, Yamaguchi T, Yanagisawa A, Fukuda T, Kanamori H, Mori T, Takahashi S, Kondo T, Kohno A, Miyamura K, Umemoto Y, Teshima T, Taniguchi S, Yamashita T, Inamoto Y, Kanda Y, Okamoto S, Atsuta Y. Quality of Life after Allogeneic Hematopoietic Cell Transplantation According to Affected Organ and Severity of Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23:1749-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Harris GH, Fixman EC, Stermitz FR, Castedo L. (-)-delta-N-normethylskytanthine from Tecoma arequipensis. J Nat Prod. 1988;51:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 480] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 4. | Olson WP, Faith MR. Human serum albumin as a cosolvent for parenteral drugs. J Parenter Sci Technol. 1988;42:82-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 158] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Yalniz FF, Hefazi M, McCullough K, Litzow MR, Hogan WJ, Wolf R, Alkhateeb H, Kansagra A, Damlaj M, Patnaik MM. Safety and Efficacy of Infliximab Therapy in the Setting of Steroid-Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23:1478-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Petty TL, Ashbaugh DG. The adult respiratory distress syndrome. Clinical features, factors influencing prognosis and principles of management. Chest. 1971;60:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 162] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Aittomäki S, Pesu M. Therapeutic targeting of the Jak/STAT pathway. Basic Clin Pharmacol Toxicol. 2014;114:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Becker H, Engelhardt M, von Bubnoff N, Wäsch R. Ruxolitinib. Recent Results Cancer Res. 2014;201:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Solcia E, Sampietro R, Capella C. Differential staining of catecholamines, 5-hydroxytryptamine and related compounds in aldehyde-fixed tissues. Histochemie. 1969;17:273-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, Spoerl S, Ditschkowski M, Ecsedi M, Sockel K, Ayuk F, Ajib S, de Fontbrune FS, Na IK, Penter L, Holtick U, Wolf D, Schuler E, Meyer E, Apostolova P, Bertz H, Marks R, Lübbert M, Wäsch R, Scheid C, Stölzel F, Ordemann R, Bug G, Kobbe G, Negrin R, Brune M, Spyridonidis A, Schmitt-Gräff A, van der Velden W, Huls G, Mielke S, Grigoleit GU, Kuball J, Flynn R, Ihorst G, Du J, Blazar BR, Arnold R, Kröger N, Passweg J, Halter J, Socié G, Beelen D, Peschel C, Neubauer A, Finke J, Duyster J, von Bubnoff N. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29:2062-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 423] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 11. | Modi B, Hernandez-Henderson M, Yang D, Klein J, Dadwal S, Kopp E, Huelsman K, Mokhtari S, Ali H, Malki MMA, Spielberger R, Salhotra A, Zain J, Cotliar J, Parker P, Forman S, Nakamura R. Ruxolitinib as Salvage Therapy for Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2019;25:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Assouan D, Lebon D, Charbonnier A, Royer B, Marolleau JP, Gruson B. Ruxolitinib as a promising treatment for corticosteroid-refractory graft-versus-host disease. Br J Haematol. 2018;181:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Klímová M, Harvánková D, Sixtová E. [Terminal progressive scleroderma in a twelve-year-old girl]. Cesk Pediatr. 1967;22:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Kajinuma H. [Glucagon]. Horumon To Rinsho. 1971;19:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1768] [Cited by in RCA: 2129] [Article Influence: 212.9] [Reference Citation Analysis (0)] |
| 16. | Decoster C, Mockel J, Van Sande J, Unger J, Dumont JE. The role of calcium and guanosine 3':5'-monophosphate in the action of acetylcholine on thyroid metabolism. Eur J Biochem. 1980;104:199-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Mayer K, Trubestein G. [On the causes and pathogenesis of so-called late epilepsy]. Med Welt. 1968;37:1951-1956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Highlights of prescribing information. Jakafi (ruxolitinib) tablets, for oral use. Available from: Https://www.Accessdata.Fda.Gov/drugsatfda_docs/label/2011/202192lbl.Pdf. Accessed 10-31-2019. |
| 19. | Carpenter PA. How I conduct a comprehensive chronic graft-versus-host disease assessment. Blood. 2011;118:2679-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | O'Keeffe R, Brooksbank BW. Determination of 3-methoxy-4-hydroxyphenylethylene glycol, a noradrenaline metabolite, in cerebrospinal fluid and urine. Clin Chem. 1973;19:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 323] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 21. | Common terminology criteria for adverse events v3.0 (ctcae). Available from: Https://ctep.Cancer.Gov/protocoldevelopment/electronic_applications/docs/ctcaev3.Pdf. 2006. |
| 22. | Stewart BL, Storer B, Storek J, Deeg HJ, Storb R, Hansen JA, Appelbaum FR, Carpenter PA, Sanders JE, Kiem HP, Nash RA, Petersdorf EW, Moravec C, Morton AJ, Anasetti C, Flowers ME, Martin PJ. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Brüggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, Burger DM. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48:1441-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 24. | Buisson G, Kassis M, Belot MW, Huberman MM, Merville R, Pompians L, Miniac, Roux R, Solas J. [Preprosthetic surgery]. Dent Cadmos. 1970;38:1293-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Hurabielle C, Sicre de Fontbrune F, Moins-Teisserenc H, Robin M, Jachiet M, Coman T, Dhedin N, Cassius C, Chasset F, de Masson A, Michonneau D, Bagot M, Bergeron A, Socié G, Peffault de Latour R, Bouaziz JD. Efficacy and tolerance of ruxolitinib in refractory sclerodermatous chronic graft-versus-host disease. Br J Dermatol. 2017;177:e206-e208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Raatikainen R, Silvennoinen R. [Constipation after delivery: double-blind comparative study of 2 laxative preparations]. Katilolehti. 1974;79:421-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |