Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5149
Peer-review started: July 14, 2020
First decision: August 8, 2020
Revised: August 12, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: November 6, 2020
Processing time: 108 Days and 2.5 Hours
A hybrid operating room (Hybrid-OR) is a surgical theatre that combines a conventional operating room with advanced medical imaging devices. There are still plenty of limitations when endovascular treatment or microsurgical treatment is used individually to treat large or giant carotid-ophthalmic aneurysms.
To explore and summarize the technical features and effectiveness of the application of a Hybrid-OR in managing major intracranial carotid-ophthalmic aneurysms.
The Department of Neurosurgery treated 12 cases of large or giant intracranial carotid-ophthalmic aneurysms between March 2013 and December 2019 in a Hybrid-OR. All cases were treated with clipping and parent vessel reconstruction.
With the assistance of the Hybrid-OR, the rate of incomplete intraoperative aneurysm clipping decreased from 25% (3/12) to 0%, while the rate of vessel stenosis decreased from 16.7% (2/12) to 8.35% (1/12). In terms of thromboembolic events, ischemic infarction complication occurred in only one patient, and none of the patients experienced embolic infarction complications. All 12 patients were followed for an average of 3 years, and no aneurysms recurred. The postoperative recovery was evaluated with the modified Rankin Scale (mRS): 11 patients showed no symptoms (mRS = 0), 1 patient showed slight disability (mRS 1-2), and none of the patients had severe disability (mRS = 5) or died (mRS = 6).
The Hybrid-OR provides new ideas for the surgical clipping of large or giant intracranial carotid-ophthalmic aneurysms and decreases the rate of intraoperative vessel stenosis and unsuccessful clipping.
Core Tip: We retrospectively analyzed 12 cases of large or giant intracranial carotid-ophthalmic aneurysms treated in a hybrid operating room (Hybrid-OR) at our hospital. We aimed to explore and summarize the technical features and effectiveness of a Hybrid-OR in treating major intracranial carotid-ophthalmic aneurysms. The Hybrid-OR provides new ideas for the surgical clipping of large or giant intracranial carotid-ophthalmic aneurysms and decreases the rate of intraoperative vessel stenosis and unsuccessful clipping.
- Citation: Zhang N, Xin WQ. Application of hybrid operating rooms for clipping large or giant intracranial carotid-ophthalmic aneurysms. World J Clin Cases 2020; 8(21): 5149-5158
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5149.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5149
Intracranial carotid-ophthalmic aneurysms refer to aneurysms that originate from the distal dural ring (DDR) of the internal carotid artery (ICA) at the top of the cavernous sinus to the origin of the posterior communicating artery (PComA). They are known as C6 aneurysms or para-clinoid aneurysms, which account for approximately 5% of intracranial aneurysms[1,2]. The rates of fatality and disability are high because of the location of growth, complexity and specificity of ophthalmic segment aneurysms[1,2]. Although endovascular and micro-neurosurgical technologies have been developing rapidly, ophthalmic segment aneurysms, especially large (diameter = 10-25 mm) and giant (diameter > 25 mm) aneurysms, are still considered a challenge for surgeons.
The hybrid operating room (Hybrid-OR) is known as the “one-stop” operating room. In a broad sense, it is an operating room equipped with multiple medical examination devices and operation assisting devices. For instance, intraoperative magnetic resonance imaging (MRI) and navigation devices and other treatment equipment are available so that imagological examinations, tracking, navigation assistance and multiple surgical approaches can be performed in combination in one operating room. In a narrow sense, Hybrid-OR is a endovascular-surgical combined operating room retaining not only the advantages of traditional open operations (visibility and convenience for operating and navigating) but also the advantages of endovascular operations (less trauma and multiple endovascular assisting approaches).
Hybrid operations were originally defined by Angelini et al[3] in 1996 and were first used for cardiac surgery. Recently, hybrid operations have provided new ideas for neurosurgeons in treating cerebrovascular diseases. Our hospital completed the building of a Hybrid-OR in 2011, which successfully treated 12 intracranial large and giant carotid-ophthalmic aneurysm cases between March 2012 and December 2015, and its effectiveness is summarized in the present study.
During the period from March 2013 to December 2019, our department of neurosurgery used the Hybrid-OR for the treatment of 12 cases of large and giant carotid-ophthalmic aneurysms: 5 males and 7 females. The age of these patients ranged from 53 to 68 years with a mean of 53.9 years. All patients showed unruptured intracranial aneurysms: 5 had chronic headaches, 4 had visual impairments, and 3 had visual field defects.
All patients were diagnosed with computed tomography angiography (CTA), preoperative digital subtraction angiography (DSA), MRI, and 3D-DSA examinations. The ICA balloon occlusion test (BOT), cross circulation test and Allcock’s test were performed to understand the condition of cerebral blood flow compensation. Twelve patients were diagnosed with large or giant aneurysms. The maximum diameter of the aneurysmal dome varied from 10.5 to 38.1 mm, the neck measured from 3.9 to 15.4 mm and the dome-neck (D/N) ratio varied from 1.4 to 9.8. A summary of the demographic and clinical characteristics of the 12 patients is shown in Table 1.
| No. | Age/sex | Side | Maximal diameter (mm) | Neck size (mm) | D/N ratio | Balloon location in operation | Residual aneurysm neck found by intraoperative DSA | Vessel stenosis found by intraoperative DSA | Times of intraoperative DSA | Thromboembolic event | mRS at discharge | Aneurysm recurrence during follow-up |
| 1 | 56/F | R | 25.3 | 4.5 | 5.6 | C2-C3 of ICA | + | Ophthalmic artery and ICA stenosis | 3 | - | 0 | No |
| 2 | 54/F | R | 21.4 | 13.4 | 1.6 | Aneurysm neck | + | ICA stenosis | 3 | - | 0 | No |
| 3 | 35/F | R | 13.7 | 9.5. | 1.4 | Aneurysm neck | - | - | 1 | - | 0 | No |
| 4 | 45/F | L | 17.1 | 6.5 | 2.6 | C2-C3 of ICA | - | - | 1 | - | 0 | No |
| 5 | 64/F | R | 23.8 | 9.3 | 3.9 | Aneurysm neck | - | - | 1 | - | 0 | No |
| 6 | 56/F | L | 25.9 | 7.1 | 2.6 | C2-C3 of ICA | - | - | 1 | Ischemic infarction in operation | 1-2 | No |
| 7 | 62/F | R | 26.8 | 15.4 | 1.7 | Aneurysm neck | + | - | 2 | - | 0 | No |
| 8 | 69/F | L | 13.2 | 4.7 | 2.8 | C2-C3 of ICA | - | - | 1 | - | 0 | No |
| 9 | 67/F | R | 10.5 | 5.9 | 1.8 | C2-C3 of ICA | - | - | 1 | - | 0 | No |
| 10 | 45/F | R | 38.1 | 3.9 | 9.8 | C2-C3 of ICA | - | - | 1 | - | 0 | No |
| 11 | 43/M | L | 14.1 | 4.8 | 2.9 | C2-C3 of ICA | - | - | 1 | - | 0 | No |
| 12 | 59/F | L | 33.0 | 8.3 | 3.5 | Aneurysm neck | - | - | 1 | - | 0 | No |
First, preoperative Hybrid-OR preparation was carried out as follows. All patients were placed in the supine position under general anaesthesia. Unilateral femoral puncture was performed using the Seldinger technique, and then a 6-F guiding catheter was placed in C1 of the ICA of the ipsilateral side of the aneurysm with continuous flushing with 5000 U heparin/L normal saline solution. MTI balloon (Hyperglide, EV3/4 mm x 20 mm) deployment: When the neck of the aneurysm was ≥ 8 mm, the balloon was deployed across the neck of the aneurysm; when the neck of the aneurysm was < 8 mm, the balloon was deployed in C2-C3 of the ICA. Based on this principle, the balloon was placed in the C2-C3 segment of the ICA in 7 cases (1, 4, 6, and 8-11) and across the aneurysm neck in 5 cases (2, 3, 5, 7 and 12), and the balloons remained deflated after initial deployment. Neither pre-procedural nor intra-procedural antiplatelet systemic heparinization agents were administered.
Second, the head was secured by a carbon fibre fixation system, and electrophysiological monitoring was used. Conventional pterional approach: The intradural anterior clitoridectomy (IAC) was performed to remove the anterior clinoid process (ACP). The DDR of the optic sheath was opened to sufficiently dissociate the nerve from the aneurysm neck.
Third, the balloon was inflated to block the ICA when clipping the aneurysm. After the aneurysms were clipped, the suction decompression was initiated to improve the surgical view and visualization of the aneurysm neck and its surrounding structures and to place the permanent clips safely and correctly. Then, intraoperative DSA was administered repeatedly to identify residues of the aneurysm neck and vessel stenosis because of incorrect clipping. If the clipping was not ideal, the clip would be readjusted to gain the best effect.
Residual aneurysm necks were discovered by intraoperative DSA in 3 cases (1, 2 and 7). For these 3 cases, the residual aneurysm necks were successfully clipped by adjusting the clips, and they were verified by second intraoperative DSA. With the assistance of the Hybrid-OR, the rates of residual aneurysm necks decreased from the original 25% (3/12) to 0%.
Vessel stenosis was found in 2 cases (1 and 2) by intraoperative DSA. For case 1, clipping was readjusted, and second intraoperative DSA displayed that the stenosis of the parent carotid artery and ophthalmic artery was relieved, while for case 2, clipping was readjusted, but mild stenosis of the parent ICA still remained as severe atherosclerosis in the aneurysm neck. The intraoperative electrophysiology monitoring showed no abnormalities. With the assistance of the Hybrid-OR, the rate of vessel stenosis decreased from 16.7% (2/12) to 8.35% (1/12).
In terms of thromboembolic events, one patient experienced ischemic infarction complications (case 6), and none of the patients experienced embolic infarction complications. Case 6 experienced contralateral limb weakness after the operation. Her pre-operative BOT demonstrated clinical intolerance (positive), and the pre-operative cross circulation test demonstrated inadequate compensation. The balloon was inflated to block the ICA in the C2-C3 segment of the ICA for 45 min, and hyper-intensity signals located in the watershed area of the hemisphere on the operating side were found in diffusion-weighted imaging post-operative MRI images. It was considered that ischemic infarction was caused by balloon block and inadequate compensation.
The postoperative conditions were evaluated with the modified Rankin Scale (mRS): 11 patients showed no symptoms (mRS = 0), 1 patient showed slight disability (mRS = 1-2), and none of the patients had severe disability (mRS = 5) or died (mRS = 6). All patients were followed for an average of 3 years. Postoperative CTA was performed to observe the effect of occlusion of the aneurysms, and no aneurysms recurred. More details of the patients are shown in Table 1.
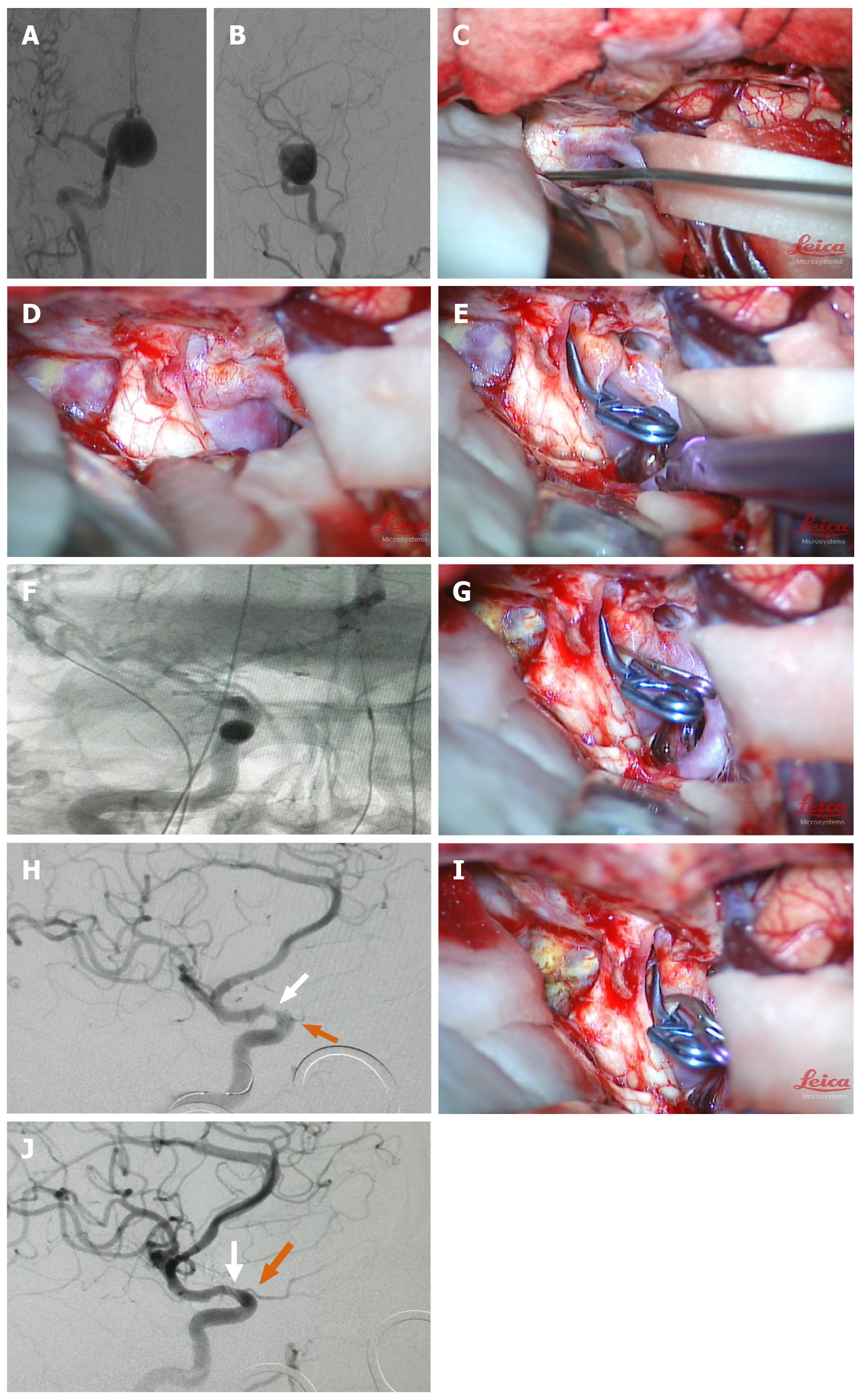
Case 1: A 56-year-old female presented to our institution with progressive vision loss. Her medical history was non-contributory. An ophthalmological evaluation showed a right central scotoma. Cranial MRI showed a mass suggestive of a carotid artery aneurysm. DSA revealed a 25.3 × 18.5 mm aneurysm with a 4.5 mm neck arising from the medial wall of the right ophthalmic segment (Figure 1A and B), and the BOT of the ICA and Allcock’s test were performed to understand the condition of cerebral blood flow compensation. To clip the aneurysm and reconstruct the right ICA, she was offered both surgical and endovascular treatment, considering the risks and benefits. The management plan was formulated to proceed with the hybrid operation as an elective procedure. During the operation, we placed the balloon in the C2-C3 segment of the ICA due to a relatively narrow-necked aneurysm (D/N ratio of 5.6). Then, we performed craniotomy via a pterional approach using the IAC technique to remove the ACP. We found that the right optic nerve was compressed by the giant aneurysm and that the optic nerve was crossed by the aneurysm neck; removal of the ACP and dissociation of the optic nerve from the aneurysm neck sufficiently revealed the aneurysm neck (Figure 1C and D). After the placement of the first clip, first intraoperative DSA showed that the aneurysm had been clipped and that there were small amounts of residue of the aneurysm neck (Figure 1E and F). A supplementary miniature clip was placed during the operation. Second intraoperative DSA showed that the aneurysm had been completely clipped. However, stenosis occurred in the ICA with poor visualization of the optic artery and poor visualization of the distal branch vessel of the ICA (Figure 1G and H). A miniature clip with angular tips was used to replace the original clip. Third intraoperative DSA showed that the aneurysm was completely clipped. The ICA with stenosis was relieved, and visualization of the optic artery and distal branch vessel of the MCA was improved (Figure 1I and J).
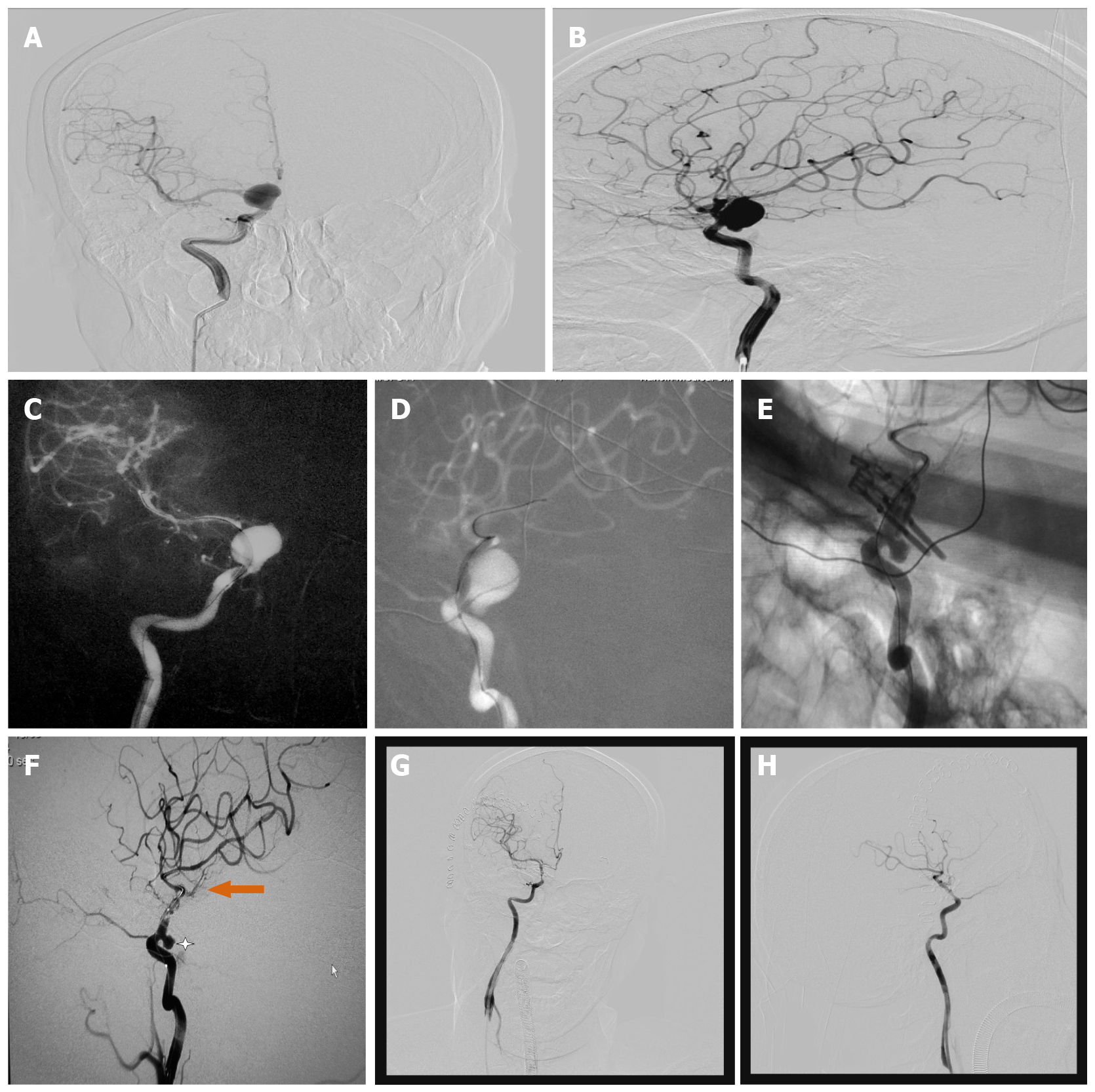
Case 2: A 54-year-old woman presented with a 6-mo history of chronic headache. MRI and pre-operative DSA established the diagnosis of a 21.4-mm large carotid-ophthalmic aneurysm with a 13.4-mm wide neck (Figure 2A and B). A combined approach was used in the Hybrid-OR. First, a 4 mm x 20 mm balloon (Hyperglide; Micro Therapeutics, Inc., Irvine, CA) was deployed across the ophthalmic aneurysm neck, and the balloon was inflated (Figure 2C and D). The aneurysm softened sufficiently to place two arrays of straight fenestrated and angled fenestrated clips (Aesculap AG, Tuttlingen) on the aneurysm. After that, second intraoperative DSA was performed and showed that the bulk of the aneurysm was clipped and not visualized, but a small portion of residual aneurysm was visualized (Figure 2E and F). After adjusting the clips, third intraoperative DSA showed that the aneurysm was completely clipped, but the parent artery was slightly narrowed due to the severe calcification of the aneurysmal neck (Figure 2G and H).
The size and shape of the aneurysms are the key issues for such operations. Previous studies have reported that the rates of fatality and morbidity are still as high as 20%-30% in neurosurgical centres that have well-developed technologies, although patients can undergo safer and relatively simpler operations such as aneurysm exclusion and extracranial-intracranial bypass. The critical difficulties in performing these operations are the proximal control of the ICA; the field of vision influenced by the aneurysm; the width of the aneurysm necks, complex branches and perforating blood vessels; the formation of endoluminal thrombosis; and atherosclerosis and dysplasia. For the clipping of giant carotid-ophthalmic aneurysms, control of the proximal end of the parent artery is the most crucial step. In treating patients with giant carotid-ophthalmic aneurysms, the removal of the ACP would provide sufficient space between the aneurysm neck and the DDR of the cavernous sinus for the placement of temporary blocking clips, including control of the proximal end of the ICA.
The vast majority of patients with giant carotid-ophthalmic aneurysms require an incision of the neck to be performed first to control the blood flow of proximal ICA. However, both ACP removal and neck incision would increase operational trauma and lengthen surgical time, especially for elderly patients aged over 65 years. Elderly patients are less tolerable to trauma. Research by the international subarachnoid aneurysm trial subgroup shows that patients over the age of 65 have higher rates of epilepsy, lung infection, cerebral vasospasm, infarction and neurological complications[4]. Therefore, intraoperative neck incision and blocking of the proximal part should be prevented in the treatment of patients with carotid-ophthalmic aneurysms. This should minimize surgical trauma and shorten the operation time.
Due to the size of carotid-ophthalmic aneurysms, the aneurysm necks located in the ICA lead to the skull around the ACP, and the optic nerve blocks the field of vision. As a result, finding the aneurysm neck and its branches through craniotomy would be difficult due to blind spots. In particular, for large and complex intracranial aneurysms, the aneurysm neck might be obstructed, which could lead to rupture before clipping[5]. As reported in the literature, after conventional aneurysm clipping with intraoperative indocyanine green (ICG) video angiography, post-operative DSA showed that the rate of incompletion of aneurysm clippings was 6.3%, while parent vessel stenosis was 5.7%. For giant aneurysms, the rates of incompletion of clippings and occurrence of stenosis were as high as 15% or more[6]. Therefore, compared to intraoperative DSA, intraoperative ICG could not clearly and accurately demonstrate images of parent vessel stenosis and aneurysm neck residues due to blocked vision and other reasons. In addition, intraoperative ICG was not able to directly observe the situation of distal blood vessels.
Recently, interventional treatments for intracranial aneurysms have been in rapid development, which reveal their advantages in treating intracranial aneurysms. Currently, the usable interventional technologies for treating complex wide-necked aneurysms include balloon-assisted coiling embolization, stent-assisted embolization and dual micro-catheter coiling embolization. However, a wide-necked aneurysm with a complex conformation is difficult to be completely removed with endovascular treatments. Although new interventional devices, tools, and technologies are evolving, a variety of interventional techniques are challenging for treating giant carotid-ophthalmic aneurysms. For the method of stent-assisted coiling embolization, it is possible that the stent guidewire does not overcome the technical difficulties of placing the stent through an aneurysm. The stent is used in stent-assisted coiling embolization to reconstruct the parent vessel and set as a block on the aneurysm neck and to prevent coil dislocation. However, within a few months of surgery, dual antiplatelet therapy must be strictly performed in patients until the internal membrane of the parent vessel has completely regenerated around the aneurysm neck[7]. Therefore, in most cases, stent-assisted coiling embolization technology cannot be safely used to treat ruptured aneurysms[8].
Carotid-ophthalmic aneurysms often show mild or none symptoms until they grow to a larger size due to their unique anatomic features. It was reported that 30%-50% of patients had carotid-ophthalmic aneurysms that had grown to a giant size (diameter > 25 mm) at their first visit to the hospital. The main symptoms are often loss of vision and visual field defects[9]. Interventional embolization was used to treat these patients; however, the aftermath of the aneurysm could not be eliminated, and the symptoms were not alleviated after the operation but continued to grow progressively, which required craniotomy to remove the emboli in order to save the patients’ vision and visual field[10,11]. Moreover, carotid-ophthalmic aneurysms often contain important vessels or artery branches that originate from the aneurysm dome or fundus parts, such as the ophthalmic artery, PComA, and even anterior choroidal artery.
Under these circumstances, the ideal treatments are to reconstruct the parent vessel based on retaining important perforator vessels and its branches. In this case, using the common interventional embolization technologies and flow-directed devices to solve the aforementioned problem is very difficult. Furthermore, difficulty in treating giant complex carotid-ophthalmic aneurysms is the high risk of relapse after surgery. As reported, the incidence of relapse after carotid-ophthalmic aneurysm operations ranged from 27.4% to 31.6%[12-14] however, the rates of relapse after interventional embolization reached 50% in treating giant aneurysms[15]. Therefore, it is extremely difficult to use the interventional embolization method to treat patients with wide-necked, giant and/or branch vessel-accumulated carotid-ophthalmic aneurysms.
In recent years, flow diverters (e.g., SILK embolization devices and pipeline embolization devices) have been widely used to treat complex anterior circulation aneurysms, especially giant types of carotid-ophthalmic segment and clinoid segment aneurysms. However, it has been reported that flow diverters used for managing giant anterior circulation aneurysms, dissecting aneurysms and microaneurysms are relatively rare. However, due to the rigidity and low compliance characterized by the hardness of the dense diverter, the excessive friction when the diverter passes through the tortuous parts of vessels, for instance, the ICA siphon, might cause stent translocation, insufficient expansion, stent fracture, in-stent restenosis or occlusion, and covered branch or perforator vessel stenosis or occlusion. In addition, after placing the flow-diverter stent, follow-up treatment would be extremely difficult if there were recurrent or residual aneurysms.
Current surgical limitations have been overcome due to this technological breakthrough while expanding the potential of surgery. The DSA system in the Hybrid-OR provides neurosurgeons with a brand new, intraoperative, image-guided environment that eliminates the limitation of traditional operating rooms and DSA systems when they function individually.
First, the use of balloon occlusion with the Hybrid-OR could quickly block the proximal end of the ICA. The control of the proximal end of the ICA had the following advantages: (1) Preventing neck incisions, shortening the operation time, and reducing surgical trauma; (2) Preventing operation of two surgical sites, which reduces the possibility of complications of intracranial infection; (3) Reducing operations on the ICA, which could decrease the rate of complications of intraoperative vasospasm and cerebral infarction; and (4) Treating ACP using the IAC rather than the extradural anterior clitoridectomy (EAC), with no need to completely remove the ACP but to remove a portion of the ACP in accordance with operational needs. The IAC technique was safer and more timesaving than the EAC technique.
Second, compared to direct observation under microscope or the intraperative ICG technique, the use of intraoperative DSA is clearer and more accurate for evaluating the effect of aneurysm clipping. It was extremely difficult under microscope at 360 degrees to detect and observe the relationship between the aneurysm and its surrounding branch vessels. Giant carotid-ophthalmic aneurysms were large in size, deep in location, obscured the ACP and optic nerve and had wide necks. Additionally, it is impossible to use ICG to observe residual aneurysms, ICA stenosis, preservation of important perforator vessels, or the status of the distal blood supply[16]. These were the reasons why the rates of incomplete clipping and the occurrence of stenosis were as high as 15% or more by the conventional aneurysm clipping method with intraoperative ICG verification. Nevertheless, the Hybrid-OR was applied to observe and evaluate aneurysm clipping at 360 degrees by intraoperative DSA. As shown in our cohort, 3 incomplete clipping events (cases 1, 2 and 7) and 2 vessel stenosis events (cases 1 and 2) were found via intraoperative DSA in the Hybrid-OR.
Furthermore, balloons can be directly deployed across the aneurysm neck and block the blood for wide-necked carotid-ophthalmic aneurysms. This kind of cross-neck deployment was effective in decreasing the escape of blood clots in the aneurysm sac and in the proper placement of the aneurysm clip and the good reconstruction of the ICA. In our study, the balloon was deployed across the aneurysm neck in 5 patients with a wide neck (≥ 8 mm). Four patients (80%) achieved good reconstruction of the parent ICA; however, good reconstruction of the parent ICA could not be obtained in one patient (case 2) because of severe atherosclerosis in the aneurysm neck. No balloon-deployment-related vasospasms occurred in the 12 patients.
The Hybrid-OR provides new ideas for the surgical clipping of large or giant intracranial carotid-ophthalmic aneurysms, and it effectively decreased the rate of intraoperative vessel stenosis and unsuccessful clipping.
A hybrid operating room (Hybrid-OR) is a surgical space that combines a conventional operating room with advanced medical imaging devices.
Currently, there is no established evidence on the application of hybrid operating rooms in the treatment of large or giant intracranial carotid-ophthalmic aneurysms.
The purpose of this study was to explore and summarize the technical features and effectiveness of the application of a Hybrid-OR in treating major intracranial carotid-ophthalmic aneurysms.
The Department of Neurosurgery treated 12 cases of large or giant intracranial carotid-ophthalmic aneurysms between March 2013 and December 2019 in a Hybrid-OR. All cases were treated with clipping and parent vessel reconstruction.
With the assistance of the Hybrid-OR, the rate of incomplete intraoperative aneurysm clipping decreased from 25% (3/12) to 0%, while the rate of vessel stenosis decreased from 16.7% (2/12) to 8.35% (1/12). In terms of thromboembolic events, ischemic infarction complication occurred in only one patient, and none of the patients experienced embolic infarction complications. All 12 patients were followed for an average of 3 years, and no aneurysms recurred. The postoperative recovery was evaluated with the modified Rankin Scale (mRS): 11 patients showed no symptoms (mRS = 0), 1 patient showed slight disability (mRS = 1-2), and none of the patients had severe disability (mRS = 5) or died (mRS = 6).
The Hybrid-OR provides new ideas for the surgical clipping of large or giant intracranial carotid-ophthalmic aneurysms and effectively decreases the rate of intraoperative vessel stenosis and unsuccessful clipping.
Although the Hybrid-OR has received widespread attention for treating large or giant intracranial carotid-ophthalmic aneurysms, there are still no definitive conclusions. This study shows that the safety and ease of use make Hybrid-OR combined with microsurgery and intraoperative digital subtraction angiography systems an attractive modality for managing large or giant intracranial carotid-ophthalmic aneurysms.
Manuscript source: Unsolicited manuscript
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Munakomi S S-Editor: Liu M L-Editor: MedE-Ma JY P-Editor: Wu YXJ
| 1. | Choudhri O, Mukerji N, Steinberg GK. Combined endovascular and microsurgical management of complex cerebral aneurysms. Front Neurol. 2013;4:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Kim SH, Song S, Kim SP, Lee J, Lee HC, Kim ES. Hybrid technique to correct cerebral malperfusion following repair of a type a aortic dissection. Korean J Thorac Cardiovasc Surg. 2014;47:163-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Angelini GD, Wilde P, Salerno TA, Bosco G, Calafiore AM. Integrated left small thoracotomy and angioplasty for multivessel coronary artery revascularisation. Lancet, 1996, 347: 757-8. . [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 175] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Ryttlefors M, Enblad P, Kerr RS, Molyneux AJ. International subarachnoid aneurysm trial of neurosurgical clipping vs endovascular coiling: subgroup analysis of 278 elderly patients. Stroke. 2008;39:2720-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Cockroft KM, Marks MP, Steinberg GK. Planned direct dual-modality treatment of complex broad-necked intracranial aneurysms: four technical case reports. Neurosurgery. 2000;46:226-30; discussion 230. [PubMed] |
| 6. | Katz JM, Gologorsky Y, Tsiouris AJ, Wells-Roth D, Mascitelli J, Gobin YP, Stieg PE, Riina HA. Is routine intraoperative angiography in the surgical treatment of cerebral aneurysms justified? Neurosurgery. 2006;58:719-27; discussion 719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Leung GK, Tsang AC, Lui WM. Pipeline embolization device for intracranial aneurysm: a systematic review. Clin Neuroradiol. 2012;22:295-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Shapiro M, Becske T, Sahlein D, Babb J, Nelson PK. Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol. 2012;33:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Day AL. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990;72:677-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 286] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Tawk RG, Villalobos HJ, Levy EI, Hopkins LN. Surgical decompression and coil removal for the recovery of vision after coiling and proximal occlusion of a clinoidal segment aneurysm: technical case report. Neurosurgery. 2006;58:E1217; discussion E1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Schmidt GW, Oster SF, Golnik KC, Tumialán LM, Biousse V, Turbin R, Prestigiacomo CJ, Miller NR. Isolated progressive visual loss after coiling of paraclinoid aneurysms. AJNR Am J Neuroradiol. 2007;28:1882-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Park HK, Horowitz M, Jungreis C, Kassam A, Koebbe C, Genevro J, Dutton K, Purdy P. Endovascular treatment of paraclinoid aneurysms: experience with 73 patients. Neurosurgery. 2003;53:14-23; discussion 24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Khan N, Yoshimura S, Roth P, Cesnulis E, Koenue-Leblebicioglu D, Curcic M, Imhof HG, Yonekawa Y. Conventional microsurgical treatment of paraclinoid aneurysms: state of the art with the use of the selective extradural anterior clinoidectomy SEAC. Acta Neurochir Suppl. 2005;94:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Sherif C, Gruber A, Dorfer C, Bavinzski G, Standhardt H, Knosp E. Ruptured carotid artery aneurysms of the ophthalmic (C6) segment: clinical and angiographic long term follow-up of a multidisciplinary management strategy. J Neurol Neurosurg Psychiatry. 2009;80:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Roy D, Raymond J, Bouthillier A, Bojanowski MW, Moumdjian R, L'Espérance G. Endovascular treatment of ophthalmic segment aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol. 1997;18:1207-1215. [PubMed] |
| 16. | Ng PY, Huddle D, Gunel M, Awad IA. Intraoperative endovascular treatment as an adjunct to microsurgical clipping of paraclinoid aneurysms. J Neurosurg. 2000;93:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |