Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4660
Peer-review started: July 29, 2020
First decision: August 7, 2020
Revised: August 12, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: October 6, 2020
Processing time: 58 Days and 10.5 Hours
The occurrence of a diaphragmatic hernia during the third trimester of pregnancy is rare; to our knowledge, there has only been a single case report related to congenital Bochdalek hernia complicated with mild acute pancreatitis during pregnancy. Nonspecific symptoms and lack of experience due to its rarity make the diagnosis of this condition very challenging. We report a case of diaphragmatic hernia accompanied by mild acute pancreatitis in the third trimester of pregnancy, which was misdiagnosed as severe acute pancreatitis.
A 19-year-old woman presented at gestation of 31+2 weeks with continuous distension pain for 3 d in the left lumbar region of no obvious cause. Ultrasonographic findings of left ureterectasis, with nonspecific lumbago and abdominal pain, led to the misdiagnosis of renal colic. Increased serum amylase and/or lipase levels indicated acute pancreatitis. Following the treatment of pancreatitis, her condition deteriorated. The patient was finally diagnosed with a diaphragmatic hernia complicated with mild acute pancreatitis on magnetic resonance imaging at our hospital. Caesarean section was performed at gestation of 31+6 weeks, followed by hernia repair, and the pancreatitis was treated sequentially. The patient was discharged in good condition 20 d after the surgery.
In this case, surgical treatment was not the same as that for non-pregnant diaphragmatic hernia repair. It is important to first perform a cesarean section before commencing the therapy.
Core Tip: Pregnancy with acute pancreatitis is rare, and pregnancy with diaphragmatic hernia is even rarer. To our knowledge, there has only been a single case report related to congenital Bochdalek hernia complicated with mild acute pancreatitis during pregnancy. We here report a case of a 19-year-old woman in the third trimester of pregnancy presenting with continuous distension pain for 3 d in the left lumbar region, who was misdiagnosed with severe pancreatitis. She was finally diagnosed with diaphragmatic hernia complicated with mild acute pancreatitis on magnetic resonance imaging at our hospital. The patient underwent caesarean section followed by hernia repair and was subsequently treated for pancreatitis. The patient's condition progressively improved 15 d after the surgery, and the patient was discharged in good condition 20 d after the surgery.
- Citation: Zou YZ, Yang JP, Zhou XJ, Li K, Li XM, Song CH. Bochdalek hernia masquerading as severe acute pancreatitis during the third trimester of pregnancy: A case report. World J Clin Cases 2020; 8(19): 4660-4666
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4660.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4660
Pregnancy with acute pancreatitis is rare, and pregnancy with diaphragmatic hernia is even rarer. The maternal and foetal mortality rate from acute pancreatitis during pregnancy was reported to be 20%-50%[1]. The mortality rate from diaphragmatic hernia during pregnancy was 40%[2]. There have been case reports of congenital Bochdalek hernia and mild acute pancreatitis during pregnancy; however, to our knowledge, there has been only a single case report of congenital Bochdalek hernia complicated with mild acute pancreatitis during pregnancy[3]. Diaphragmatic hernias are often misdiagnosed due to nonspecific symptoms or lack of experience, placing pregnant women at risk. Here, we report the case of a young woman with a diaphragmatic hernia and acute pancreatitis in the third trimester of pregnancy that was misdiagnosed as severe acute pancreatitis at a local tertiary referral centre and was treated for severe acute pancreatitis. The presentation of such case tended to be ignored and easily misdiagnosed as severe acute pancreatitis. We summarize the key points of accurate diagnosis and the experience of successful treatment for this condition.
A 19-year-old woman (gravida 1, para 0, at 31+2 wk of gestation) was admitted to hospital with continuous distension and pain for 3 d in her left lumbar region. Pain occurred with no obvious cause during sleep and radiated to the shoulder and back. Other serious clinical manifestations included nausea, vomiting, chest tightness, and shortness of breath. The pain was not relieved after vomiting. There were no similar symptoms (diaphragmatic hernia) before pregnancy.
The patient had natural pregnancy. She did not perform physical work during pregnancy, and there was no severe vomiting in the first and second trimesters. Routine prenatal physical examinations showed no abnormalities. Signs of constipation appeared in the last 2 mo. No history of trauma or surgery was recorded.
Physical examination at our hospital showed increases in the pulse rate (144 times/min) and respiratory rate (49 breaths/min). Normal blood pressure, shortness of breath, diminished breath sounds in the left lung, and no dry or moist rales were observed. The patient's breathing was mainly through abdominal respiration. No abnormal skin changes, subcutaneous bleeding, ecchymosis, abdominal varicose veins, peristaltic waves, or umbilicus protrusion were observed. Abdominal muscle strain, abdominal pain, and rebound tenderness were reported, and the bowel sounds were weak (3 times/min). Gynaecological examination revealed that the foetal heart rate was 148 times/min, and there was no sign of labour.
Laboratory tests showed elevated levels of serum amylase (639.00 U/L), serum lipase (2525.00 U/L), white blood cells (24.85 × 109/L), neutrophils (N% = 0.93), triglycerides (1.78 μmol/L), and total bilirubin (23.70 μmol/L).
Bedside ultrasound revealed left ureterectasis. The patient was diagnosed with left-sided renal colic developing into acute pancreatitis during pregnancy. After 3 d of treatment for acute pancreatitis, the signs of acute pancreatitis did not improve, but worsened with continuous abdominal pain and distension. Eventually, the patient was referred to our hospital with the diagnosis of severe acute pancreatitis.
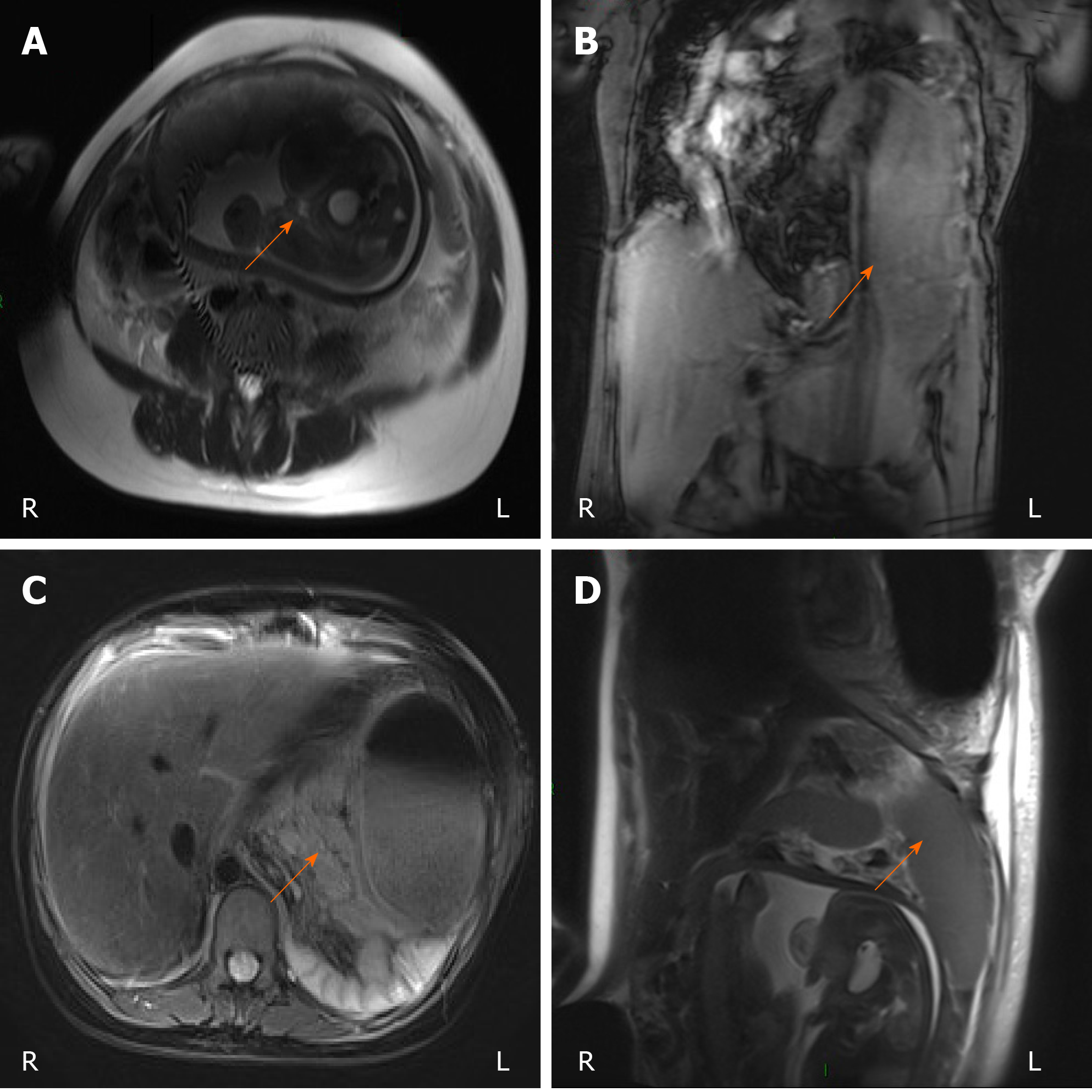
Emergency laboratory tests at our hospital indicated high levels of serum amylase (692.00 U/L), white blood cells (29.11 × 109/L), neutrophils (N% = 0.90), and total bilirubin (33.50 μmol/L). The coagulation function indices were all within the normal ranges. Abdominal bedside ultrasonography showed that the bile duct of the patient was slightly dilated inside and outside the liver, the pancreas was not clear, the left kidney collection system was separated, and ascites were identified. In the third trimester, the foetus survived. Chest and abdominal magnetic resonance imaging (MRI) revealed a possible diaphragmatic hernia in the left thoracic cavity (Figure 1).
This situation was a great threat to both the pregnant woman and the foetus. The uterine volume of pregnant women in the middle and third trimester of pregnancy increases, squeezing the viscera in the abdominal cavity upward, while the pancreas behind the peritoneum is indirectly compressed, which can cause poor discharge of pancreatic fluid, increased pancreatic duct pressure, and pancreatic microcirculation disorder, leading to the development of pancreatitis. Changes in the internal environment during pregnancy, especially changes in endocrine and metabolic levels, will cause physiological changes in the biliary system.
Changes in hormone and metabolic levels, combined with foetal growth, lead to increased intra-abdominal pressure, increasing the risk of diaphragmatic hernia. Because of concerns about radiation risk from X-rays and computed tomography (CT) scans, pregnant women often refuse these examinations. Therefore, an emergency MRI might be suitable. However, in some cases, MRI is not immediately available. According to the latest update, the side effects of radiography or CT scans are far less harmful to the foetus, and these tests should not be denied to pregnant women if necessary.
Diaphragmatic hernia is a common diaphragmatic disease. It refers to the phenomenon of intraperitoneal or retroperitoneal organs entering the thoracic cavity through a diaphragm defect or weak spot caused by trauma. The specific conditions of pregnancy, diaphragmatic hernia, and pancreatitis require further intraoperative exploration.
During pregnancy, constipation and breath holding are often associated with increased intra-abdominal pressure, in turn increasing the risk of diaphragmatic rupture. I agree with the gastroenterologist's opinion, but the relationship between the pancreatitis and the diaphragmatic hernia is not clear. Therefore, we require further analysis.
The patient was at < 37 wk of gestation but required emergency laparotomy and termination of pregnancy due to her condition. Therefore, short-term and long-term complications and developmental problems of premature infants should also be carefully considered. Furthermore, informed consent of the patient and her family should be obtained.
Acute pancreatitis complicated with diaphragmatic hernia in the third trimester of pregnancy with an acute physiology and chronic health evaluation II(APACHE II) score of 8, systemic inflammatory response syndrome (SIRS) score of 3, bedside index of severity in acute pancreatitis (BISAP) score of 2, and improved Marshall score of 1.
After multidisciplinary discussion in the emergency department, the final decision was made. Cesarean section was performed at gestation of 31+6 wk, followed by hernia repair, and the pancreatitis was treated sequentially.
Specifically, an emergency exploratory laparotomy was performed. During laparotomy, a massive amount of free pale-yellow abdominal fluid (ascites) was found in the abdominal cavity, and the left diaphragm muscle was injured. The obstetricians and gynecologists performed an emergency caesarean section and delivered a 1.4 kg boy baby in the head position. The foetal heart rate was 148 times/times (cardiotocogram). The Apgar score was 7. No abnormality was found in the cord blood gas. No abnormalities in postnatal neonatal development or brain damage were observed. Most of the stomach, as well as part of the duodenum, jejunum, transverse colon, mesangium, and omentum, were herniated into the thoracic cavity. Other abdominal organs were displaced to varying degrees, and the retroperitoneal pancreas was enlarged and distorted. Next, left posterolateral thoracotomy was performed through the 8th intercostal space. A small amount of fluid was found in the thoracic cavity, and a rupture of approximately 10 cm from the left posterior diaphragm and approximately 2 cm from the esophageal hiatus was observed. The hernia contents were manually returned to the abdominal cavity. An adequate patch size was used for repairing the hernia opening. An indwelling catheter was inserted into the chest cavity for drainage.
After surgery, the patient continued to receive treatment for acute pancreatitis in the specialized intensive care unit. The patient's condition progressively improved 15 dlater , and the patient was discharged in good condition 20 d after surgery. The whole process of diagnosis and treatment was successful with the active cooperation of the patient. After 5 years of outpatient and telephone follow-ups, the mother and child are in good health. The results of the routine laboratory tests upon admission and post-discharge follow-ups are presented in Table 1.
| Items | Disease duration (d) | Post-discharge (yr) | ||||||
| 1 | 3 | 5 | 7 | 14 | 35 | 0.5 | 1 | |
| ALT (mmol/L) | - | 38.00 | 34.00 | 27.00 | 24.00 | 19.00 | 16.00 | 23.00 |
| TC (mmol/L) | 1.78 | - | 1.81 | 2.10 | 2.41 | 2.17 | 2.23 | 2.11 |
| N % | 0.93 | 0.90 | 0.91 | 0.86 | 0.85 | 0.83 | 0.67 | 0.63 |
| Hb (g/L) | 108 | 105 | 92.10 | 81.00 | 92.00 | 94.00 | 116.00 | 121.00 |
| Ca2+ (mmol/L) | - | - | 1.92 | 1.85 | 2.02 | 2.18 | 2.05 | 2.20 |
| TBiL (μmol/L) | 23.70 | 33.50 | 12.80 | 6.00 | 4.20 | 4.60 | 4.40 | 5.80 |
| CRP | - | - | 81.70 | 64.40 | 15.30 | 7.90 | 5.30 | 3.70 |
| WBC (× 109/L) | 24.85 | 29.11 | 24.96 | 20.41 | 15.22 | 13.53 | 8.78 | 7.96 |
| AMY (U/L) | 639.00 | 692.00 | 232.00 | 38.00 | 57.00 | 80.00 | - | - |
| Lip (U/L) | 2525.00 | - | 769.00 | 21.00 | 85.00 | 63.00 | - | - |
This case suggests that, in a pregnant woman with such conditions, diagnosis and treatment should not be undertaken focusing only a single condition; complex independent conditions could be associated with each other, and identifying these associations is important for accurate diagnosis and optimal treatment. The general clinical symptoms of pregnancy, pancreatitis and diaphragmatic hernia tend to be similar and can have nonspecific clinical manifestations. Therefore, it is important to make a diagnosis based on abnormal manifestations, such as vomiting in the third trimester. If the patient has dyspnoea and an increased heart rate, a chest examination should be performed. In a diaphragmatic hernia, if the diaphragm is compressed, it can cause the apex to shift unreachably. Excitement of the sympathetic nerve can cause an increased heart rate. If the lungs are compressed, they cannot expand normally, resulting in reduced thoracic expansion and asymmetry of the vocal fremitus. The breath sound on the affected side will be weakened, and the trachea will shift to the healthy side. If gastrointestinal obstruction occurs in the hernia, gurgling can be heard in the chest. The above conditions should be carefully identified to avoid misdiagnosis and delayed diagnosis. In cases in which the diagnosis does not fully explain the condition, and specialist treatment is not effective, it is important to develop a better therapy through a multidisciplinary discussion.
Close observation is required after delivery, and long-term follow-up is required after discharge. In this case, the surgical treatment was not the same as that for non-pregnant diaphragmatic hernia repair. Hernia repair should be performed first during the surgery of non-gestational diaphragmatic hernia, and then the cause of diaphragmatic hernia was eliminated.. Diaphragmatic hernia during pregnancy should first involve decreasing the abdominal pressure (cesarean section). In principle, the treatment of acute pancreatitis during pregnancy should be the same as that of non-pregnant acute pancreatitis. If there were no complications or organ failure, conservative treatment would be very effective. Surgical treatment is appropriate only for severe pancreatitis.
The only a case published by Islah et al[3] is similar to ours. The diaphragmatic hernia was not initially considered in either case. After the treatment of pancreatitis, the condition worsened; thus, a proper diagnosis could only be made by further imaging examination and surgical exploration. However, the imaging examinations used differed between the two cases. The preferred imaging examination in our case was MRI, which is highly sensitive to soft tissues. In contrast, X-ray and CT were used in the case reported by Islah et al[3], and these imaging techniques not only have more radiation but also have lower sensitivity to soft tissues than MRI. Such low sensitivity might lead to an unclear diagnosis, and additional examinations could aggravate tissue damage. It has been confirmed that the contrast used in enhanced CT can induce allergic reactions and nephropathy[4]. The contrast used in X-rays can induce late-onset allergy-like reactions mediated by T cells[3,4]. However, there is no clear evidence that MRI induces severe side effects, except for contraindications[5,6]. The ACOG Committee Opinion, published in 2016, reported that ultrasonography and MRI have no risk for pregnant women[7]. The side effects of radiography, CT scans, or nuclear medical imaging are far less harmful to the foetus and should not be denied to pregnant women if it is necessary to combine it with ultrasound or MRI to establish the diagnosis [7].
The pathogenesis of this case remains largely unclear. Gestation is a unique period in women’s lives. In addition to hormonal and metabolic changes, the uterus will also expand several times in the third trimester of pregnancy, which can increase the pressure in the abdomen. As part of the diaphragm between the abdominal cavity and thoracic cavity disappears, there is more space for the abdominal viscera to move[8]. The gradually enlarging gravid uterus and higher abdominal pressure squeeze the body organs, such as the liver, stomach, and small intestine, into the thoracic cavity. Breathing movements can affect the pressure difference between the chest and abdominal cavity, resulting in "sucking" and "pushing" effects. In addition, the enlarged uterus, which increases the abdominal pressure, affects the blood flow to the diaphragm, thus making the diaphragm thinner. Under the influence of progestin, the diaphragm tension is reduced, and the surrounding ligaments are stretched and relaxed[9]. The diaphragm hiatus is gradually enlarged, the function of the esophageal lower sphincter is attenuated, and the tension in the gastric and intestinal smooth muscles is decreased, resulting in gastric retention or gastroesophageal reflux[10,11]. Gastric retention or gastroesophageal reflux triggers coughing and severe vomiting in the first trimester, as well as inappropriate or involuntary vigorous perinatal activities that can cause a sudden rise in abdominal pressure, leading to rupture of the diaphragm. Constipation and breath holding during pregnancy can both increase the abdominal pressure and increase the risk of diaphragmatic rupture.
In our case, we believe that the internal organs in the abdominal cavity entered the hernia, especially the stomach and part of the duodenum, leading to torsion of the stomach and duodenum, which increased the pressure in the intestinal lumen and impeded the outflow of the bile duct and pancreatic duct, leading to pancreatitis. In addition, poor blood circulation to the pancreas can also lead to pancreatitis. With expansion of the pancreas, the spleen can be compressed and damaged, in turn leading to acute pancreatitis. The increase in progesterone levels caused decreased bile duct tension, easily resulting in gallstones. Other studies have reported that gallstones fall off and block the pancreatic duct, in turn inducing pancreatitis. Hypertri-glyceridemia is a rare but well-known cause of acute pancreatitis[1,12]. Excessive ingestion of proteins and lipids can increase the levels of triglycerides. Lipids are hydrolysed by lipase during pancreatic microcirculation, thereby releasing a large number of free fatty acids, which can directly induce toxicity to the surrounding pancreatic acinar cells. In patients with triglyceridemia , ischaemic necrosis and impaired pancreatic microcirculation can occur in pancreatic cells due to abnormal fatty acid infiltration, eventually leading to hyperlipidaemic pancreatitis[1].
In this case report, we recorded the symptoms, diagnosis, treatment and outcomes in detail. We attempted to determine the pathogenesis and to suggest diagnostic and therapeutic approaches, and we compared similar published cases. A limitation of this case report was the missing data in the local hospital .
Diaphragmatic hernia complicated with acute pancreatitis during pregnancy is an extremely rare emergency condition that can lead to serious outcomes. Correct diagnosis and optimal treatment should be undertaken as soon as possible. The condition leads to increased intra-abdominal pressure in the third trimester of pregnancy, leading to a diaphragmatic hernia, which in turn induces mild acute pancreatitis. In this case, surgical treatment is not the same as that for non-pregnant diaphragmatic hernia repair. It is important to perform a cesarean section first.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mimura K S-Editor: Huang P L-Editor: MedE-Ma JY P-Editor: Xing YX
| 1. | Mali P. Pancreatitis in pregnancy: etiology, diagnosis, treatment, and outcomes. Hepatobiliary Pancreat Dis Int. 2016;15:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Lee SY, Tan KH. Antenatally diagnosed congenital diaphragmatic hernia in Singapore: a five-year series. Singapore Med J. 2013;54:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Islah MA, Jiffre D. A rare case of incarcerated bochdalek diaphragmatic hernia in a pregnant lady. Med J Malaysia. 2010;65:75-76. [PubMed] |
| 4. | Caraiani C, Petresc B, Dong Y, Dietrich CF. Contraindications and adverse effects in abdominal imaging. Med Ultrason. 2019;21:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Christiansen C, Pichler WJ, Skotland T. Delayed allergy-like reactions to X-ray contrast media: mechanistic considerations. Eur Radiol. 2000;10:1965-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Christiansen C. Late-onset allergy-like reactions to X-ray contrast media. Curr Opin Allergy Clin Immunol. 2002;2:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | American College of Obstetricians and Gynecologists' Committee on Obstetric Practice. Committee Opinion No. 656: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol. 2016;127:e75-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Reddy M, Kroushev A, Palmer K. Undiagnosed maternal diaphragmatic hernia - a management dilemma. BMC Pregnancy Childbirth. 2018;18:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 9. | Ménassa M, Bergeron AM, Drolet S, Bouchard A. Strangulated Congenital Diaphragmatic Hernia of Bochdalek Diagnosed in Late Pregnancy: A Case Report and Review of the Literature. J Obstet Gynaecol Can. 2019;41:1482-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Body C, Christie JA. Gastrointestinal Diseases in Pregnancy: Nausea, Vomiting, Hyperemesis Gravidarum, Gastroesophageal Reflux Disease, Constipation, and Diarrhea. Gastroenterol Clin North Am. 2016;45:267-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Close H, Mason JM, Wilson D, Hungin AP. Hormone replacement therapy is associated with gastro-oesophageal reflux disease: a retrospective cohort study. BMC Gastroenterol. 2012;12:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Luo L, Zen H, Xu H, Zhu Y, Liu P, Xia L, He W, Lv N. Clinical characteristics of acute pancreatitis in pregnancy: experience based on 121 cases. Arch Gynecol Obstet. 2018;297:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |