Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4644
Peer-review started: March 31, 2020
First decision: April 28, 2020
Revised: May 11, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: October 6, 2020
Processing time: 180 Days and 7.7 Hours
Hemophilic pseudotumor (HP) is a rare complication in patients with hemophilia. The lesion most frequently occurs in the long bones, pelvis, small bones of the hands and feet, or rarely in the maxillofacial region. Postoperative changes in HP are seldom arrested, whereas angiogenesis characterized by disturbed wound healing in HP may cause vascular malformations.
We report the case of an 11-year-old boy who was affected by maxillary intraosseous venous malformation. Enucleation of an HP without factor replacement was performed initially on the right side of the maxilla 3 years ago. The patient was referred to us because of painless swelling in the same location. Factor replacement and subtotal maxillectomy were performed. Pathological examinations revealed intraosseous venous malformation.
This study is the first to document the development of intraosseous venous malformation after enucleation of an HP in the maxillofacial region. Angiogenesis characterized by disturbed wound healing in patients with hemophilia may be pivotal in the pathogenesis of this condition.
Core tip: Hemophilic pseudotumor (HP) is a rare complication in patients with hemophilia. We present herein the case of an 11-year-old boy who was affected by maxillary intraosseous venous malformation. Enucleation of an HP without factor replacement was performed initially on the right side of the maxilla 3 years ago. This case highlights that the potential angiogenesis characterized by disturbed wound healing may lead to vascular malformation.
- Citation: Cai X, Yu JJ, Tian H, Shan ZF, Liu XY, Jia J. Intraosseous venous malformation of the maxilla after enucleation of a hemophilic pseudotumor: A case report. World J Clin Cases 2020; 8(19): 4644-4651
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4644.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4644
Hemophilia A and B are uncommon X-linked recessive disorders that almost exclusively affect male individuals. A common complication, often following trauma, is bleeding in joints, muscles, and bones. Repeated intramuscular or intraosseous hemorrhage rarely results in an expanding lytic lesion termed “hemophilic pseudotumor (HP)”. In osseous tissues, HP commonly occurs in long bones, pelvis, and small bones of the hands and feet. Starker[1] first described the morbid anatomy of two subperiosteal hematomatas in a patient with hemophilia. HP usually manifests as chronic, encapsulated slow-growing hematomas resulting from recurrent hemorrhages. In the jaws, occlusal trauma caused by unilateral chewing habits in patients with hemophilia increases the prevalence of HP in severe and mild conditions.
Postoperative changes in HP are rarely arrested, whereas angiogenesis characterized by disturbed wound healing may cause vascular malformations. Coagulation abnormalities affect vasculogenesis and angiogenesis. A population-based case-control study has revealed that coagulation abnormalities are biological risk factors for severe cardiovascular malformations, and this finding is supported by clinical evidence and recent advances in vascular biology[2]. In hemophilic arthropathy, Bhat et al[3] found soft-tissue hyperproliferation with marked induction of neoangiogenesis and evolving abnormal vascular architecture in FVIII-deficient mice. This case report describes an 11-year-old male patient who suffered from venous malformation (VM) of the maxilla after he underwent enucleation of an HP.
An 11-year-old boy was admitted to Head and Neck Surgery Department with a major complaint of painless swelling of the right facial region.
The patient’s symptoms started 6 mo ago, and he was referred to us for a painless swelling in the right maxillary region that gradually enlarged for 6 mo.
In 2011 in Hunan province, China, the patient was subjected to enucleation of the same region without factor replacement. The lesion was first diagnosed as a simple bone cyst, and the pathology consultation finally diagnosed it as a hemophilic pseudotumor of the right maxilla.
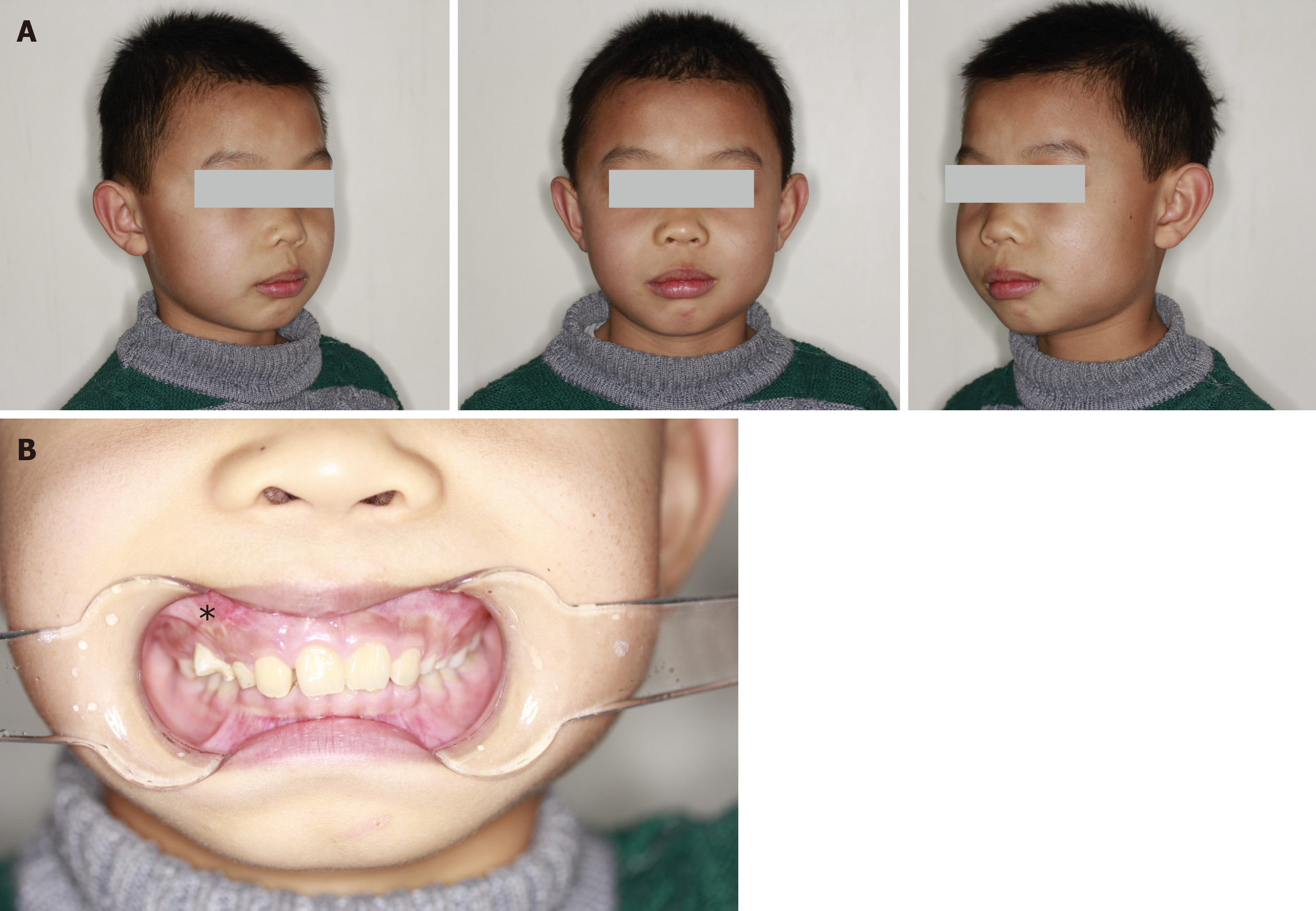
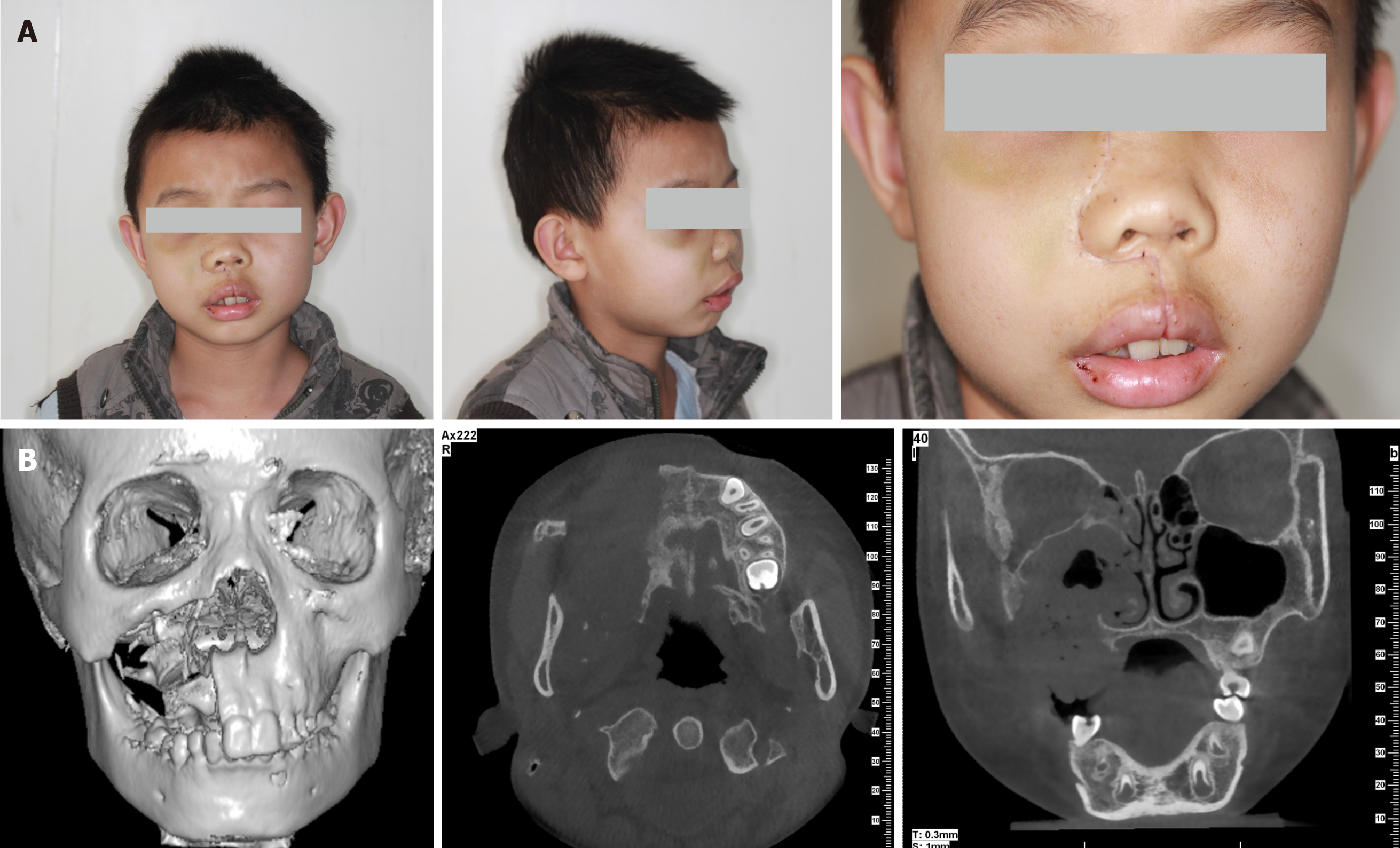
The face of the patient was symmetrical (Figure 1A). The partially enlarged right maxilla was palpated in intraoral physical examination. The upper right side of the vestibular groove was shoaled (Figure 1B).
Preoperative examination revealed a coagulation disorder diagnosed as hemophilia A (factor VIII, 23% of normal activity).
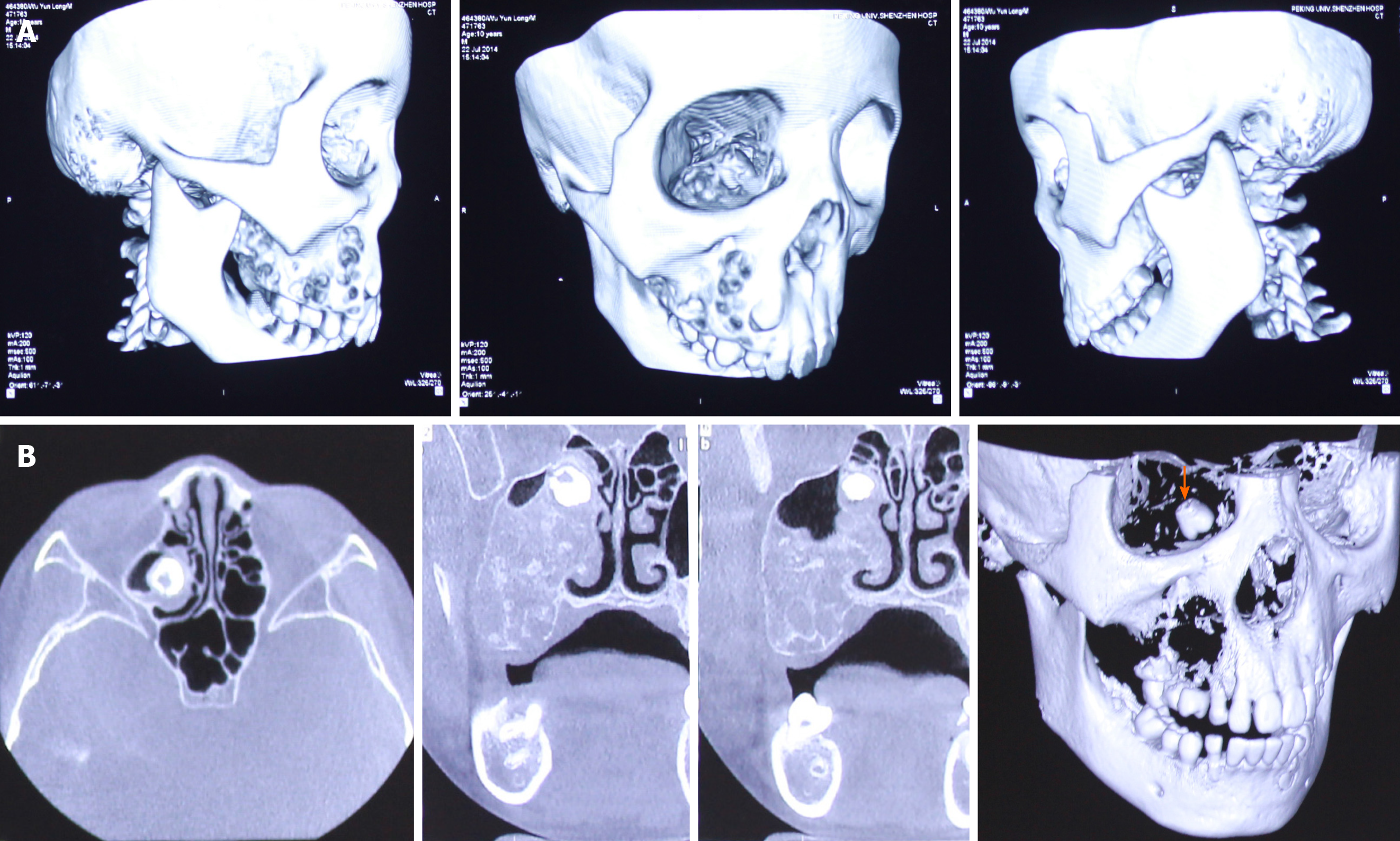
CT displayed a multicystic low-density mass measuring 3 cm × 2.5 cm × 2.5 cm in the right maxilla. The body of the right maxilla manifested irregular swelling (Figure 2A). The lesion occupied the right maxillary sinus. A molar was squeezed into the right maxillary sinus and embedded in the superior part of the lesion close to the canalis opticus (Figure 2B).
Odontogenic tumor of the maxilla
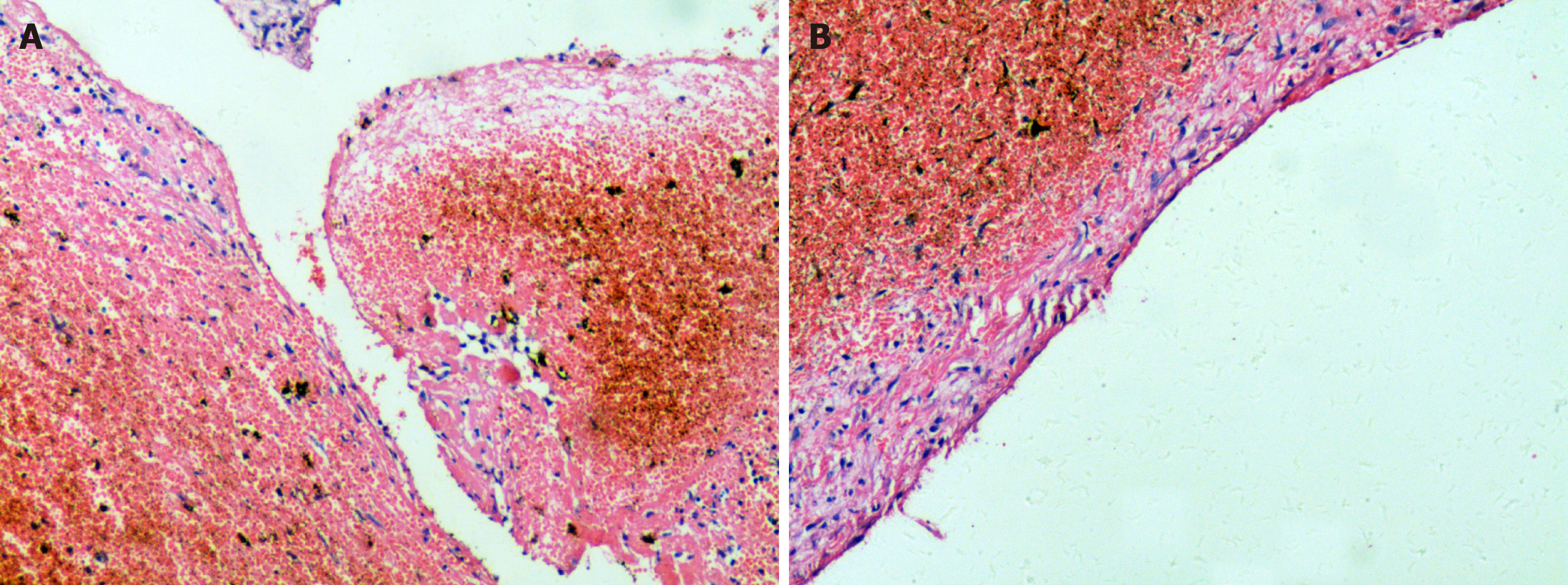
The specimens were retrieved from the Department of Pathology, The Second Xiangya Hospital of Central South University. In the lesion of the first surgery, no signs of epithelium were found, although a fibrous tissue lining was observed. In the wall of the lesion of the first surgery, remote hemorrhage and clot organization were obvious, which is different from simple bone cyst (Figure 3A). Abundant intracellular and extracellular hemosiderin deposition was noted (Figure 3B). Histological characteristics are necessary to rule out other entities that cause unilocular radiolucent jaw lesions. Combined with coagulation function results in this case, the multicystic radiolucent lesion in the maxilla was confirmed to be an HP.
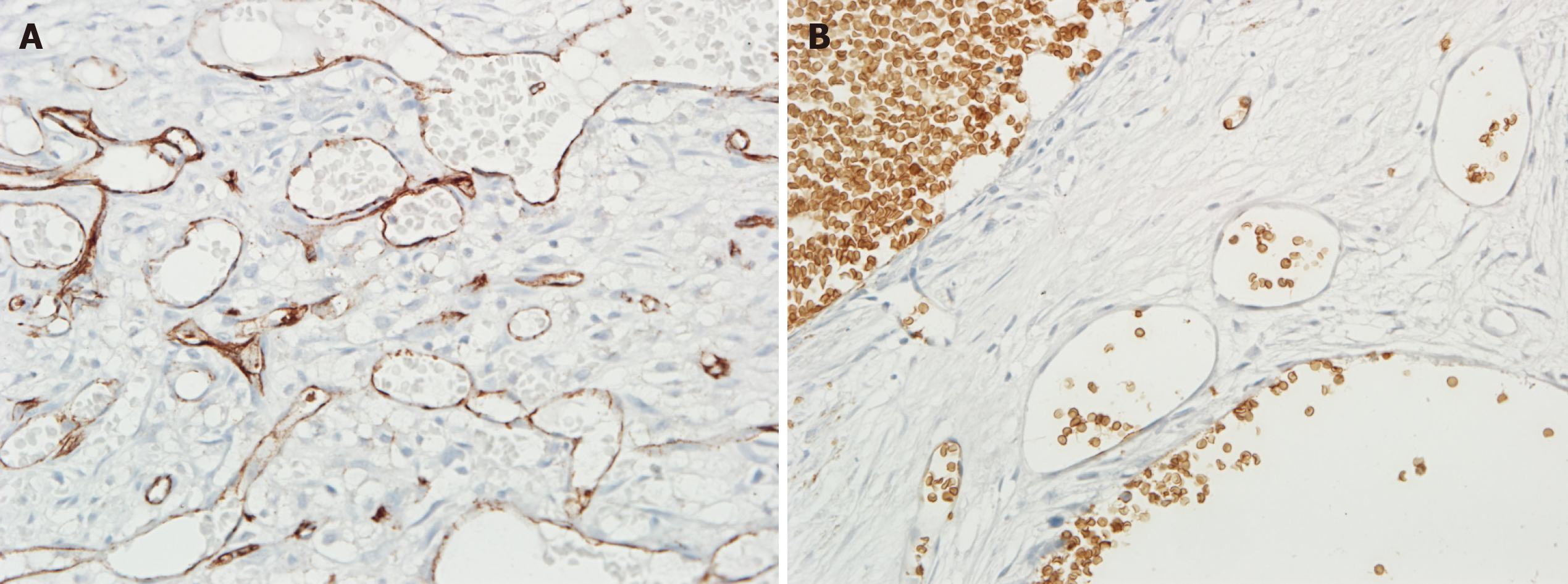
The postoperative pathological results of the second surgery verified the diagnosis of intraosseous VM of the right maxilla. Histomorphology and immunohistochemistry results revealed that the lesions were abundant in vessels positive for CD31, a marker of endothelial components, and negative for GLUT-1, which is strongly expressed by endothelial cells of infantile hemangiomas at all stages of their evolution and is not expressed by other benign vascular anomalies and reactive proliferations (Figure 4A and B). The lesions were diagnosed as intraosseous venous malformation according to the ISSVA classification. Venous malformations are most common in slow-flow vascular malformations that commonly form in the oral maxillofacial region. Angiogenesis characterized by normal pericyte coverage in patients with hemophilia has been reported in hemophilic arthropathy[4]. Moreover, the increased vascular density likely caused the formation of vascular anomalies.
The final diagnosis of the presented case was intraosseous venous malformation of the maxilla.
Cryoprecipitate was given preoperatively to correct coagulation disorders. Then, the patient underwent right subtotal maxillectomy. A nasal lateral and nasolabial sulcus incision was made. The complete resection of the lesion of the maxilla and removal of contents of the maxillary sinus and ethmoidal cellules were then performed, and the pavimentum orbitae and zygomatic process of the maxilla were preserved (Figure 5B).
Iodoform gauze was placed in the right maxillary sinus and wound, and drainage was performed through the inferior nasal channel in the first week. The patient had an uneventful postoperative clinical course. Factor VIII was given per 8 h after surgery for 3 d. The faciomaxillary region was supported by the residual bone and the face was almost symmetrical (Figure 5A). The pathological result indicated intra-maxillary venous malformation. No evidence of recurrence was found during the routine 5-year follow-up.
The pathogenesis of HP is poorly understood. In this case, the critical factors probably included hemorrhagic tendency, occlusal trauma, oppressive bone resorption, tooth germ development, and angiogenesis. Intraosseous hemorrhage, caused by unilateral chewing and following unconscious occlusal trauma, might be implicated in the origin and progression of this condition into remote hemorrhage and clot organization. Pathological results revealed that the multicystic bone cyst was covered with a thin layer of connective tissue without epithelial components. Remote hemorrhage and organization were found in the wall. Abundant intracellular and extracellular hemosiderin deposition was noted, which is similar to electron micrograph showing a deposit of electron-dense hemosiderin granules surrounded by bands of fibrous collagen in the study by Fernandez de Valderrama et al[5]. Similar to a simple bone cyst, the wall without epithelial components was pathologically interesting. Thus, this condition was easily misdiagnosed as simple bone cyst, with the consideration of hemosiderin deposition caused by enucleation. In simple bone cysts, materials for histological examination may be difficult to obtain because the soft tissue lining of the bony cavity may be entirely absent or thin. These materials usually consist of loose fibrovascular tissues with signs of previous hemorrhage, such as cholesterol clefts and macrophages loaded with iron pigment. Incidentally, threadlike calcification can be observed. In some instances, simple bone cysts may occur simultaneously with various fibro-osseous lesions, but the possibility of pseudocystic stromal degeneration in an ossifying fibroma cannot be ruled out for certain[6]. After the mass was subjected to enucleation, replacement therapy, which is important in sequential therapy for bony remodeling, was not performed. Hoffman et al[7] found that cutaneous wounds heal more slowly in hemophilic mice than in wild-type mice. Hemophilic mice also exhibit histological abnormalities even after their skin defect is closed. Hemophilic wounds are characterized by reduced inflammatory cell influx and increased angiogenesis. The radiological appearance of multicystic bone cysts may be related to occlusal trauma in the periapical region and tooth germ development, which are common in odontogenic cysts. In the patient’s dentition developmental period, tooth displacement was associated with the compression of the lesion between deciduous dentition and permanent dentition. Consequently, the second molar was pushed into the maxillary sinus. After 3 years, the second molar was pushed into the anterior superior part of the right maxillary sinus close to the optic canal. The loose maxillary structure may also participate in lesion progression.
Increased angiogenesis characterized by normal pericyte coverage in patients with hemophilia has been observed in hemophilic arthropathy. Zetterberg et al[4] investigated whether angiogenesis is disturbed in hemophilic arthropathies and found that microvascular density and VEGF expression are increased. Acharya et al[8] examined the potential role of neoangiogenesis in the pathogenesis of hemophilic arthropathies. The sera from patients with hemophilia elicit an angiogenic response in endothelial cells, but this response is abolished by blocking VEGF. In our patient, venous malformation occurred after the enucleation was completed. Therefore, neoangiogenesis may play a role in its pathogenesis. For further evidence, several resected specimens from the second surgery were retrieved from the Department of Oral Pathology, Stomatology School of Wuhan University, and the components of the vascular structure were confirmed by detecting the CD31 and Glut-1 expression levels. The representative immunohistochemical results are shown in Figure 4A and B. Compared with the specimens from the first surgery, the lesions from the second surgery contained numerous CD-31-positive vessels, which were negatively stained for Glut-1.
Described by Lazarovits et al[9] in 1968, the first report of HP in the maxillofacial region involved an 11-year-old patient with mild hemophilia and with a major complaint of gum bleeding and mass swelling in the left mandible. The present study reports the case of an 11-year-old male child who suffered from intraosseous VM of the maxilla after he underwent enucleation of an HP. This study is the first to document the development of intraosseous VM of the maxilla after HP surgery. Nevertheless, several sporadic cases of HP in the maxilla have been reported. We review the treatments for HP in the maxilla in the literature (Table 1)[10-14]. All of the patients are male and young. Five cases of HP in the maxilla have been published. In 2004, Steele et al[15] presented a case of HP in the paranasal sinus. We present an unusual case involving an 11-year-old male patient who suffered from intraosseous VM of the maxilla after he received enucleation of an HP. We provided insights into the possible link between hemophilia and vascular anomalies. Thus, further studies should be performed to determine whether hemophilia-related wound healing can cause vascular anomalies.
| No. | Ref. | Year | Age (yr) | Type | Severity | Treatment | Outcome |
| 1 | Marquez et al[10] | 1982 | 13 | A | Moderate | Hemimaxillary resection, conservative treatment | 5 yr: No recurrence |
| 2 | De Sousa et al[11] | 1995 | 11 | A | Severe | Curettage, FR | 1 yr: No recurrence |
| 3 | Zheng et al[12] | 1997 | 13 | A | Mild | Curettage | Not discussed |
| 4 | Lima et al[13] | 2008 | 12 | A | Mild | Enucleation/EACA, FR | 9 mo: No recurrence |
| 5 | Hu et al[14] | 2010 | 11 | A | Mild | Radiotherapy, enucleation, FR | 10 yr: No recurrence |
| 6 | The present case | 2011 | 9 | A | Mild | Enucleation | 2 yr: Development of venous malformation |
We herein describe the unusual case of 11-year-old male patient who suffered VM of the maxilla after enucleation of an HP, and meanwhile provide some underlying clues for the possible link between HP and VM development. Therefore, we emphasize that long-term routine follow-up after HP enucleation is necessary, and the up-regulated angiogenesis in patients with hemophilia should not be overlooked, especially in oral and facial regions.
We thank Professor Zhao YF at Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Wuhan University for pathological diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ogawa T S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Starker L. Knochenusur durch ein hamophiles, subperiostales Hamatom. Mitt Grenzgeb Med Chir. 1918;31:381-415. |
| 2. | Ferencz C. Coagulation affects vasculogenesis and angiogenesis: does it affect the development of the heart? J Hematother Stem Cell Res. 2002;11:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Bhat V, Olmer M, Joshi S, Durden DL, Cramer TJ, Barnes RF, Ball ST, Hughes TH, Silva M, Luck JV, Moore RE, Mosnier LO, von Drygalski A. Vascular remodeling underlies rebleeding in hemophilic arthropathy. Am J Hematol. 2015;90:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Zetterberg E, Palmblad J, Wallensten R, Morfini M, Melchiorre D, Holmström M. Angiogenesis is increased in advanced haemophilic joint disease and characterised by normal pericyte coverage. Eur J Haematol. 2014;92:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Fernandez de Valderrama JA, Spain M, Matthews JM. The haemophilic pseudotumour or haemophilic subperiosteal haematoma. J Bone Joint Surg Br. 1965;47:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Pieter S. Pathology of the Maxillofacial Bones. Switzerland: Springer; 2015: 29-30. [DOI] [Full Text] |
| 7. | Hoffman M, Monroe DM. Wound healing in haemophilia--breaking the vicious cycle. Haemophilia. 2010;16 Suppl 3:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Acharya SS, Kaplan RN, Macdonald D, Fabiyi OT, DiMichele D, Lyden D. Neoangiogenesis contributes to the development of hemophilic synovitis. Blood. 2011;117:2484-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Lazarovits P, Griem ML. Radiotherapy of hemophilia pseudotumors. Radiology. 1968;91:1026-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Marquez JL, Vinageras E, Dorantes S, Nussbaumer C, Flores A, Perez Rolon G. Hemophilic pseudotumor of the inferior maxilla. Report of a case. Oral Surg Oral Med Oral Pathol. 1982;53:347-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | de Sousa SO, de Piratininga J, Pinto Júnior DS, de Araújo N. Hemophilic pseudotumor of the jaws: report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Zheng K, Zheng P. Hemophilic pseudotumor involving maxilla and tibia. Chin Med J (Engl). 1997;110:233-235. [PubMed] |
| 13. | Lima GS, Robaina TF, de Queiroz Chaves Lourenço S, Dias EP. Maxillary hemophilic pseudotumor in a patient with mild hemophilia A. J Pediatr Hematol Oncol. 2008;30:605-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Hu Y, Zhu LF, Xu CS. [Successful treatment of hemophilic pseudotumor of maxilla by radiotherapy: a case with 10 years follow-up]. Zhonghua Kou Qiang Yi Xue Za Zhi. 2020;55:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Steele NP, Myssiorek D, Zahtz GD, Diamond A. Pediatric hemophilic pseudotumor of the paranasal sinus. Laryngoscope. 2004;114:1761-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |