Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4572
Peer-review started: May 22, 2020
First decision: July 29, 2020
Revised: August 11, 2020
Accepted: September 5, 2020
Article in press: September 5, 2020
Published online: October 6, 2020
Processing time: 128 Days and 1.9 Hours
Squamous cell carcinoma antigen (SCCA) is regarded as a specific indicator of epithelial malignancies and is widely used in the diagnosis of squamous cell carcinoma (SCC). However, the expression of SCCA in gastric adenocarcinoma has not been studied in detail.
A 52-year-old man was admitted to our hospital for a 2.5 cm × 2.5 cm ulcer at the antrum-body junction with dull pain and fullness in the upper abdomen for 2 mo. His pre-surgery serological testing results showed 0.51 ng/mL SCCA (reference interval, < 1.5 ng/mL) and 9.9 ng/mL carcinoembryonic antigen (reference range, < 4.7 ng/mL). He underwent radical distal gastrectomy and Roux-en Y anastomosis and was diagnosed with poorly differentiated mucinous adenocarcinoma (Lauren classification: Diffuse) by pathological examination of the resected lesion. Immunohistochemistry showed that SCCA was highly expressed in the cytoplasm of cancer cells. After surgery, the patient received an S-1 adjuvant chemotherapy regimen for six cycles containing tegafur, gimeracil, and oteracil potassium. He showed no sign of recurrence or metastasis within 24-mo follow-up.
This is a frontal report of SCCA overexpression in poorly differentiated adenocarcinoma of the stomach.
Core Tip: We report a typical case of poorly differentiated mucinous adenocarcinoma (pT3aN3aM0) with squamous cell carcinoma antigen (SCCA) overexpression. Since the patient had no sign of recurrence or metastasis up to 24 mo, it might contribute to improving the understanding of the nature of SCCA as a protease inhibitor in cancer. This report also emphasizes a subgroup of patients who have been diagnosed with adenocarcinoma and serves as a reminder to oncologists that a prospective cohort study should be carried out to evaluate SCCA-related prognosis.
- Citation: Wang L, Huang L, Xi L, Zhang SC, Zhang JX. High expression of squamous cell carcinoma antigen in poorly differentiated adenocarcinoma of the stomach: A case report. World J Clin Cases 2020; 8(19): 4572-4578
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4572.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4572
Gastric cancer (GC) is a common malignancy and the second leading cause of cancer related death[1]. Gastric adenocarcinoma, arising from the glands of the most superficial layer or the mucosa, accounts for more than 90% of all GC cases[2]. The survival rate of GC is dismal, and the 5-year survival rate is generally between 30%-40%, except in Japan and Korea[3]. Although the addition of trastuzumab to first line chemotherapy has improved the overall survival of some advanced patients, only 20% of patients benefit from this strategy[4,5]. Researchers are still devoting to identify new effective therapeutic targets.
Squamous cell carcinoma antigen (SCCA) is a tumor marker for squamous cell carcinoma (SCC). It is overexpressed in neoplastic tissues of epithelial origin, such as cervical[6], esophageal[7], and head and neck SCCs[8]. Elevated serum SCCA is often associated with therapeutic resistance and poor prognosis[9]. However, SCCA expression has not been studied in depth neither in serum nor in tissue of GC patients.
A 52-year-old man presented to our hospital complaining of dull pain and fullness in the upper abdomen for 2 mo, especially after eating. He reported no fever, diarrhea, hematemesis, black stool, or other discomfort.
The patient’s symptoms started 2 mo ago. He took domperidone but had no relief.
The patient had tuberculosis 20 years ago, and he had habits of drinking alcohol 150 mL per day and smoking 20 cigarettes per day for over 30 years.
Upon admission, the patient’s temperature was 37.0 °C, heart rate was 80 bpm, respiratory rate was 18 breaths per min, and blood pressure was 120/80 mmHg. Physical examination showed tenderness in the upper abdomen.
Routine blood examination showed mild erythrocytopenia (4.19 × 1012/L). Serum carcinoembryonic antigen (CEA) level was 9.9 ng/mL (reference range, < 4.7 ng/mL), and other serum tumor markers were normal, including CA19-9, CA724, SCCA, and α-fetoprotein.
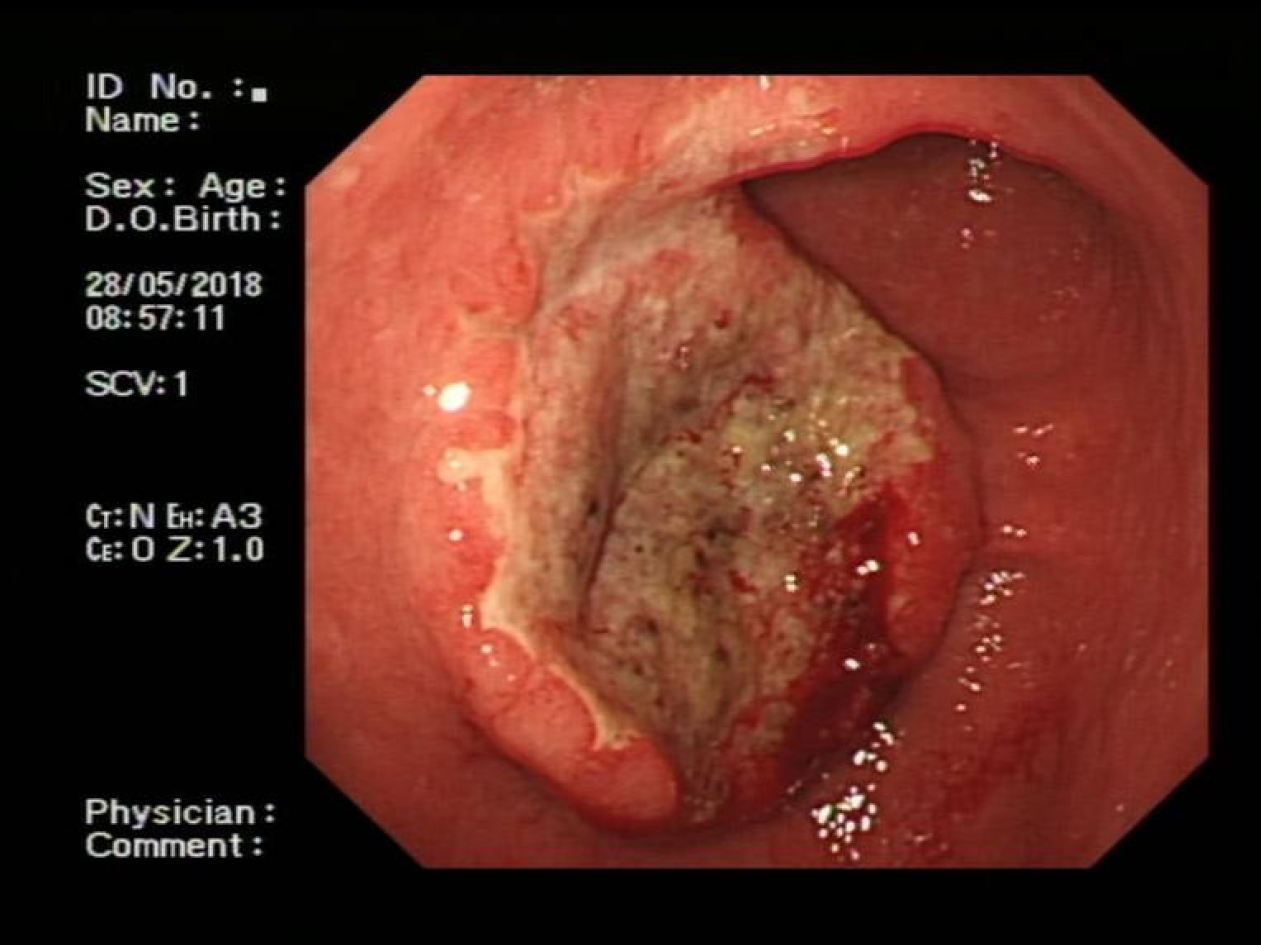
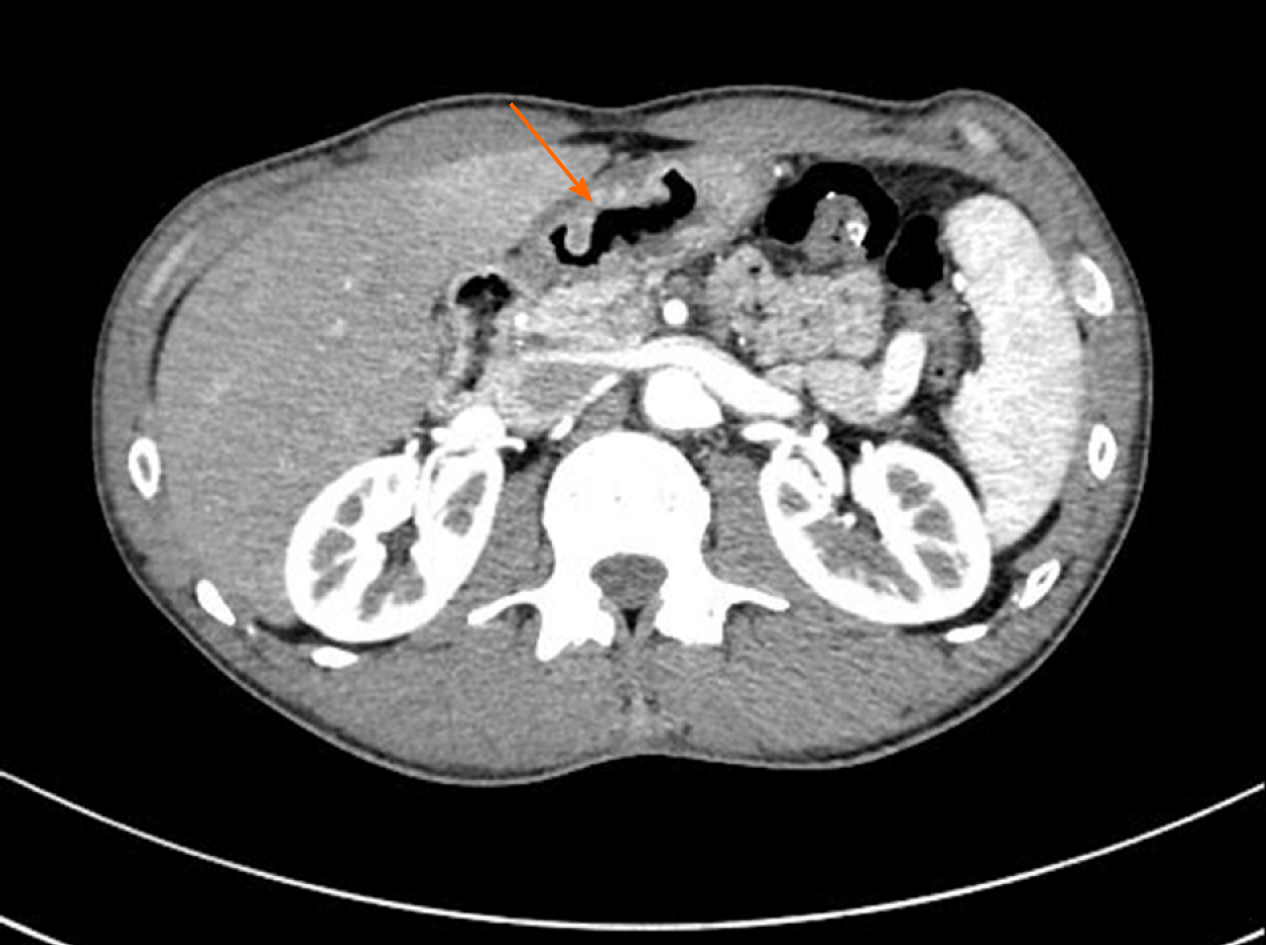
Endoscopy showed a congestive mucosa in the gastric fundus with edema and an irregular ulcer approximately 2.5 cm × 2.5 cm in dimension at the antrum-body junction (Figure 1). Abdominal contrast-enhanced computed tomography further revealed irregular thickening and enhancement of the wall of the gastric antrum near the pylorus (Figure 2). No abnormalities were found in the esophagus, duodenum, liver, pancreas, kidney, or bladder.
The patient should undergo radical distal gastrectomy.
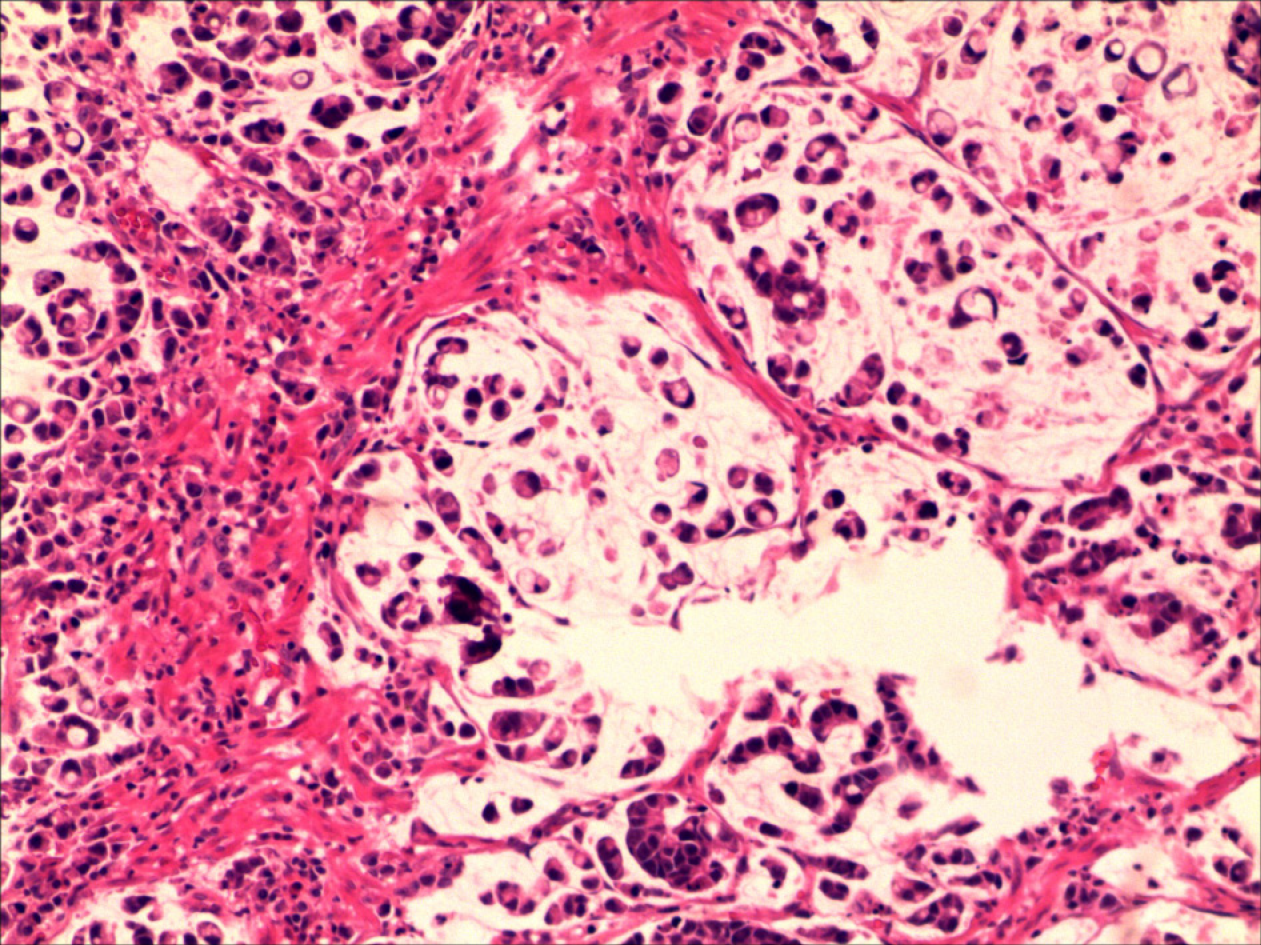
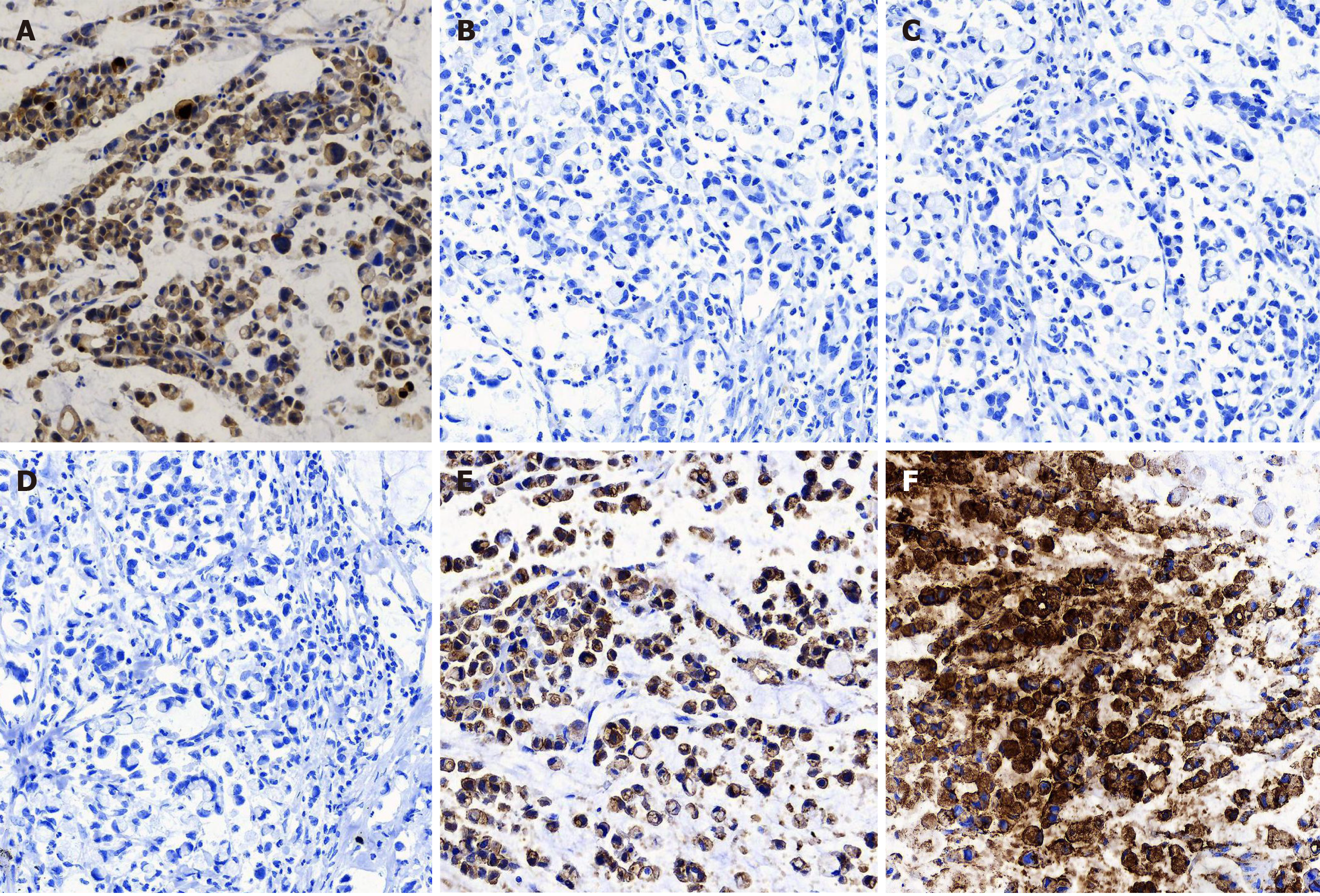
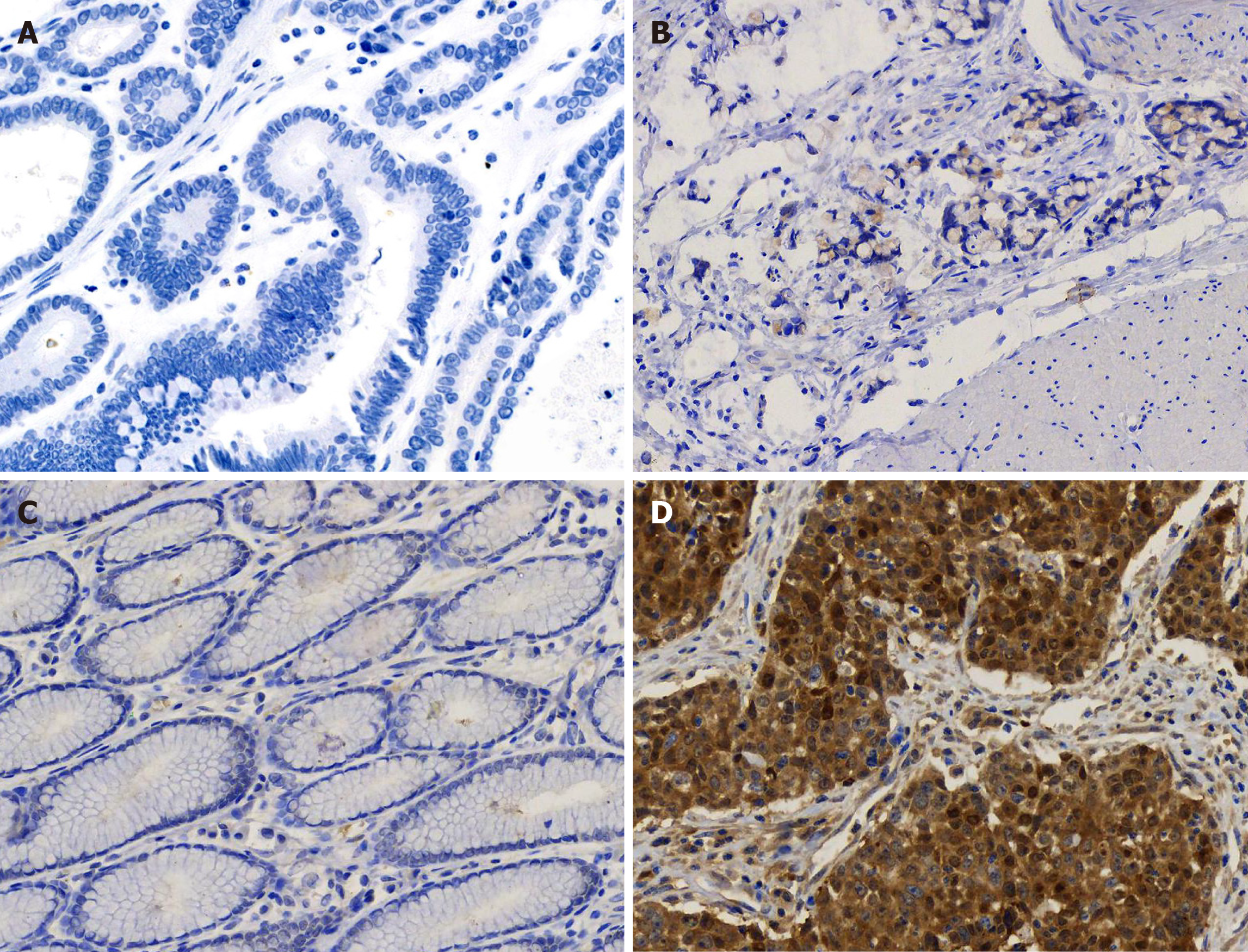
The pathological examination of the distal stomach showed poorly differentiated mucinous adenocarcinoma (Lauren classification: Diffuse), mostly signet-ring cells, and lymph node metastasis (pT3aN3aM0) (Figure 3). Immunohistochemical staining showed that the tumor cells were positive for CK-L (focal +++), CEA, SCCA (> 90%, +++), and E-cadherin but negative for CD68, Her-2, p40, p63, CK5/6, CK7, CAM5.2, CK20, and Ki-67 (Figure 4).
The patient underwent radical distal gastrectomy and Roux-en Y anastomosis. A 4 cm × 3 cm × 2 cm mass was found in the greater gastric curvature that had penetrated the serosa, and multiple enlarged lymph nodes were found in the lesser gastric curvature. After surgery, he orally took 50 mg BID of tegafur, gimeracil, and oteracil potassium capsules for six cycles.
During the follow-up period of 24 mo, the patient regularly came to our hospital for reexamination and he had no sign of recurrence or metastasis.
GC is one of the most common gastrointestinal malignancies worldwide, most of which are adenocarcinoma. Due to the high incidence of recurrence after resection and chemotherapy resistance, the 5-year overall survival rate of GC is less than 50%. Treatment of GC patients is extremely challenging due to patients being commonly treated in a uniform fashion irrespective of disease subtype. Existing traditional clinicopathologic criteria are inadequate for guiding individualized therapy[10]. With the deepening of studies at the molecular level, some new GC subtypes based on molecular characteristics have been proposed, providing a roadmap for patient stratification, treatment options, and drug selection[11]. Understanding the pathogenesis of GC and finding potential therapeutic targets to improve medical management and survival from this deadly disease are urgent tasks.
Here, we report a case of SCCA overexpression in gastric poorly differentiated adenocarcinoma. The specificity of this overexpression was further confirmed by its absence in gastritis tissue and well differentiated adenocarcinoma (Figure 5). SCCA, a member of the ovalbumin serpin (ov-serpin)/clade B serpin family, was originally isolated from SCC tissues of the uterine cervix by Kato et al[12] in the 1970s and used as the earliest marker to diagnose SCC. It has two subtypes with an identical 45-kDa molecular weight: SCCA1 (Serpin B3) and SCCA2 (Serpin B3); Both contain a key reactive center loop for interaction with the target protease and function as irreversible 'suicide' inhibitors for cellular proteases through reactive center loop cleavage[13]. This patient has been shown to have no sign of recurrence up to 24 mo. It seems that he has a better prognosis compared with other patients in the same TNM stage[14,15]. Petty et al[16] found that SCCA leads to entirely opposite outcomes in two non-small cell lung cancer subtypes-squamous carcinoma and adenocarcinoma.
In this case, the patient’s serum SCCA was within the reference range by flow immunofluorescence assay. In fact, the current sensitivity of serum SCCA is unsatisfactory (44%-69% in cervical squamous carcinoma). More importantly, whether extracellular SCCA is a result of passive release from dead cells or active secretion from live cells is still debatable. Uemura et al[17] confirmed that SCCA (1 or 2) synthesized by squamous carcinoma cells is mainly retained in the cytoplasm, and only a small amount is secreted.
SCCA upregulation is also observed in adenocarcinoma of the lung[18], Barrett’s esophagus[19], breast[20], pancreas[21], and chronic inflammatory diseases of the skin (psoriasis, specific dermatitis, and eczema) as well as in respiratory inflammatory diseases (pulmonary tuberculosis, asthma, and chronic obstructive pulmonary disease). A large retrospective study found upregulated SCCA in patients with uremia, azotemia, diabetic nephropathy, and nephrotic syndrome[22]. SCCA regulates the differentiation of the normal squamous epithelium. It enhances tumor growth by promoting cell resistance to apoptosis and amplifies invasive potential by triggering epithelial-mesenchymal transition[23]. SCCA also inhibits chemotaxis and cytotoxicity of natural killer cells to suppress the immune system[24]. Turato et al[25] demonstrated that SerpinB3 contributes to hepatocellular carcinoma stem cell phenotype via miR-122.
Immunohistochemical markers of gastric SCC commonly used in pathology department include keratin CK, p63, and p40 (ΔNp63). Human p63 gene is composed of 15 exons and 2 independent promoters. Two proteins are encoded by this gene: (1) Full-length protein p63 with trans-activation domain transcribed from exon 1; and (2) P40 without trans-activation domain starting from exon 3. P63 is often expressed in the basal layer of epithelial tissue and plays an important role in the formation of the normal epithelium. Both p63 and p40 are expressed in various benign and malignant tumors of squamous cell origin, which often leads to a pathological diagnosis of SCC. Our data showed that the tumor cells in this patient were negative for CK5, CK6, CK7, CAM5.2, CK20, p63, and p40 but positive for SCCA, CEA, and E-cadherin, indicating that SCCA is an independent marker in gastric poorly differentiated adenocarcinoma cells. Such evidence underlines the “non-squamous” property of SCCA and clearly highlights the diverse biological functions of SCCA both inside and outside the cell.
We herein report a case of poorly differentiated adenocarcinoma of the stomach with abundant SCCA protein. Our data further enriches the knowledge that SCCA mRNA was detected in GC cell lines[26]. This patient was still alive 24 mo after surgery in a stable condition. To our knowledge, no data are currently available on the correlation between SCCA and the prognosis of GC, and SCCA has not yet been considered as a subtype marker for gastric tumor. Further in-depth study will focus on the correlation of serum level and tissue abundance of SCCA with clinical evaluation of gastric tumor (including grade, stage, recovery, metastasis, and recurrence) to evaluate the significance of SCCA in clinical laboratory.
We would like to thank Professor Xu ZK and Professor Wang J for expert advice on the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Link A, Mizuguchi T, Sirin G, Tandon RK S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55686] [Article Influence: 7955.1] [Reference Citation Analysis (132)] |
| 2. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1322] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 3. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3391] [Article Influence: 484.4] [Reference Citation Analysis (1)] |
| 4. | Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 678] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 5. | Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 423] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 6. | Yuan SH, Liang XF, Jia WH, Huang JL, Wei M, Deng L, Liang LZ, Wang XY, Zeng YX. Molecular diagnosis of sentinel lymph node metastases in cervical cancer using squamous cell carcinoma antigen. Clin Cancer Res. 2008;14:5571-5578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Tong YS, Wang XW, Zhou XL, Liu ZH, Yang TX, Shi WH, Xie HW, Lv J, Wu QQ, Cao XF. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol Cancer. 2015;14:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 270] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 8. | Guerra EN, Rêgo DF, Elias ST, Coletta RD, Mezzomo LA, Gozal D, De Luca Canto G. Diagnostic accuracy of serum biomarkers for head and neck cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;101:93-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Markovina S, Wang S, Henke LE, Luke CJ, Pak SC, DeWees T, Pfeifer JD, Schwarz JK, Liu W, Chen S, Mutch D, Wang X, Powell MA, Siegel BA, Dehdashti F, Silverman GA, Grigsby PW. Serum squamous cell carcinoma antigen as an early indicator of response during therapy of cervical cancer. Br J Cancer. 2018;118:72-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Chia NY, Tan P. Molecular classification of gastric cancer. Ann Oncol. 2016;27:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 11. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4828] [Article Influence: 438.9] [Reference Citation Analysis (2)] |
| 12. | Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI, Whisstock JC. An overview of the serpin superfamily. Genome Biol. 2006;7:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 522] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 14. | Warschkow R, Baechtold M, Leung K, Schmied BM, Nussbaum DP, Gloor B, Blazer Iii DG, Worni M. Selective survival advantage associated with primary tumor resection for metastatic gastric cancer in a Western population. Gastric Cancer. 2018;21:324-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Du CY, Chen JG, Zhou Y, Zhao GF, Fu H, Zhou XK, Shi YQ. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol. 2012;18:3610-3616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Petty RD, Kerr KM, Murray GI, Nicolson MC, Rooney PH, Bissett D, Collie-Duguid ES. Tumor transcriptome reveals the predictive and prognostic impact of lysosomal protease inhibitors in non-small-cell lung cancer. J Clin Oncol. 2006;24:1729-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Uemura Y, Pak SC, Luke C, Cataltepe S, Tsu C, Schick C, Kamachi Y, Pomeroy SL, Perlmutter DH, Silverman GA. Circulating serpin tumor markers SCCA1 and SCCA2 are not actively secreted but reside in the cytosol of squamous carcinoma cells. Int J Cancer. 2000;89:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Wang R, Wang G, Zhang N, Li X, Liu Y. Clinical evaluation and cost-effectiveness analysis of serum tumor markers in lung cancer. Biomed Res Int. 2013;2013:195692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Fassan M, Realdon S, Vianello L, Quarta S, Ruol A, Castoro C, Scarpa M, Zaninotto G, Guzzardo V, Chiarion Sileni V, Pontisso P, Rugge M. Squamous cell carcinoma antigen (SCCA) is up-regulated during Barrett's carcinogenesis and predicts esophageal adenocarcinoma resistance to neoadjuvant chemotherapy. Oncotarget. 2017;8:24372-24379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Catanzaro JM, Guerriero JL, Liu J, Ullman E, Sheshadri N, Chen JJ, Zong WX. Elevated expression of squamous cell carcinoma antigen (SCCA) is associated with human breast carcinoma. PLoS One. 2011;6:e19096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Catanzaro JM, Sheshadri N, Pan JA, Sun Y, Shi C, Li J, Powers RS, Crawford HC, Zong WX. Oncogenic Ras induces inflammatory cytokine production by upregulating the squamous cell carcinoma antigens SerpinB3/B4. Nat Commun. 2014;5:3729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Yang D, Wang J, Zhang L. Serum SCCA levels in patients suffering cancers or other diseases. Prog Mol Biol Transl Sci. 2019;162:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Quarta S, Vidalino L, Turato C, Ruvoletto M, Calabrese F, Valente M, Cannito S, Fassina G, Parola M, Gatta A, Pontisso P. SERPINB3 induces epithelial-mesenchymal transition. J Pathol. 2010;221:343-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Suminami Y, Nagashima S, Murakami A, Nawata S, Gondo T, Hirakawa H, Numa F, Silverman GA, Kato H. Suppression of a squamous cell carcinoma (SCC)-related serpin, SCC antigen, inhibits tumor growth with increased intratumor infiltration of natural killer cells. Cancer Res. 2001;61:1776-1780. [PubMed] |
| 25. | Turato C, Fornari F, Pollutri D, Fassan M, Quarta S, Villano G, Ruvoletto M, Bolondi L, Gramantieri L, Pontisso P. MiR-122 Targets SerpinB3 and Is Involved in Sorafenib Resistance in Hepatocellular Carcinoma. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Kyogo I, Mamor H. Squamous cell carcinoma antigen-derived peptide binding to HLA-A24 molecule. EP1930427A1. Available from: https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=EP&NR=1930427A1&KC=A1&FT=D&ND=4&date=20080611&DB=&locale=en_EP. |