Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4558
Peer-review started: June 1, 2020
First decision: July 25, 2020
Revised: July 29, 2020
Accepted: August 29, 2020
Article in press: August 29, 2020
Published online: October 6, 2020
Processing time: 118 Days and 13.7 Hours
The early diagnosis of basal ganglia and thalamus germinomas is often difficult due to the absence of elevated tumor markers, and atypical clinical symptoms and neuroimaging features.
Four male children aged 8 to 15 years were diagnosed with germinomas in the basal ganglia and thalamus by stereotactic biopsy from 2017 to 2019. All patients developed hemiplegia except patient 4 who also had cognitive decline, speech disturbance, nocturnal enuresis, polydipsia, polyuria, precocious puberty and abnormalities of thermoregulation. All four cases were alpha-fetoprotein and beta-human chorionic gonadotrophin (β-HCG) negative except patient 3 who had slightly elevated β-HCG in cerebrospinal fluid (CSF). No malignant cells were detected in the patients’ CSF. Brain magnetic resonance imaging findings were diverse in these patients with the exception of the unique and common characteristics of ipsilateral hemisphere atrophy, especially in the cerebral peduncle. All patients were diagnosed with germinomas of the basal ganglia and thalamus by stereotactic brain biopsy.
Stereotactic brain biopsy is necessary to confirm the diagnosis of ectopic germinomas. Serial neuroimaging studies can not only differentiate disease but also determine the biopsy site.
Core Tip: Basal ganglia and thalamus germinomas are rare and early diagnosis of these tumors is usually difficult due to insidious onset, absence of elevated tumor markers, and subtle and atypical neuroimaging features. The definite diagnosis of these ectopic germinomas depends on histopathological examination. In this report, we describe four intractable cases whose histopathological diagnoses were germinomas in the basal ganglia and thalamus. Ipsilateral hemiatrophy, which was a common characteristic on neuroimaging of germinomas in the basal ganglia and thalamus, may be an important feature in differentiating these lesions from other intracranial tumors.
- Citation: Huang ZC, Dong Q, Song EP, Chen ZJ, Zhang JH, Hou B, Lu ZQ, Qin F. Germinomas of the basal ganglia and thalamus: Four case reports. World J Clin Cases 2020; 8(19): 4558-4564
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4558.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4558
Intracranial germinomas account for approximately 50% of all central nervous system germ cell tumors and constitute 0.3%-3.4% of all brain cancers[1,2]. They are usually located at the midline structures including the pineal and suprasellar regions. The off-midline germinomas also called ectopic germinomas are rare including those in the basal ganglia and thalamus. Germinomas in the basal ganglia and thalamus are more frequently seen in the Asian population. Intracranial germinomas have a male predominance, especially those that originate in the basal ganglia and thalamus[3]. Pure intracranial germinomas are negative for alpha-fetoprotein (AFP) and beta-human chorionic gonadotrophin (β-HCG) in both body fluid and histological staining[3-6]. Slightly elevated β-HCG levels in the body fluid predict syncytiotrophoblastic giant cells in germinomas[7,8]. Early clinical diagnosis of a basal ganglia germinoma is more difficult than in the midline region due to unusual localization, slow clinical course and subtle or atypical neuroimaging findings. Definite diagnosis of germinoma depends on histopathological findings. The prognosis of an intracranial germinoma is usually favorable following chemotherapy and radiotherapy. However, treatment outcome of ectopic germinoma is worse if diagnosis is delayed. Here, we describe four atypical and intractable cases which were ultimately diagnosed as germinomas by stereotactic brain biopsy and histological staining.
All four patients were male and the age of onset ranged from 8-15 years. Patient 1 suffered from three episodes of transient numbness of his right extremities. Patient 2 and patient 3 developed slow progressive weakness of their hemilateral legs and arms. Patient 4 gradually developed walking and writing disorders, cognition decline, speech disturbance, nocturnal enuresis, polydipsia, polyuria, precocious puberty and abnormalities of thermoregulation.
All four patients had no particular medical history or family history.
Patients 1, 2 and 3 had mild to moderate spastic paresis of unilateral limbs, brisk deep reflexes and the Babinski sign. Cranial nerve palsy was not observed. Sensory examinations were almost bilaterally symmetric. Patient 4 presented with mild cognitive impairment, involuntary movement of his right arm, increased muscle tone of bilateral extremities without muscle weakness and precocious puberty signs including enlarged testicles and penis, and the appearance of pubic and underarm hair.
All four cases were AFP and β-HCG negative except patient 3 who had a slightly elevated β-HCG in cerebrospinal fluid (CSF, 22.9 mIU/mL, reference value 0-5 mIU/mL) (Table 1). Serum carcinoembryonic antigen and other tumor marker levels were also within the reference range. No malignant cells were detected in CSF. No other significant abnormalities in laboratory examinations were observed.
| Case | Onset age (yr) | Sex | Duration(mo) | AFP (S/C) | HCG (S/C) | Histological diagnosis |
| 1 | 12 | M | 2 | -/- | -/- | Germinoma |
| 2 | 10 | M | 3 | -/- | -/- | Germinoma |
| 3 | 15 | M | 18 | -/- | -/+ | Germinoma |
| 4 | 8 | M | 24 | -/- | -/- | Germinoma |
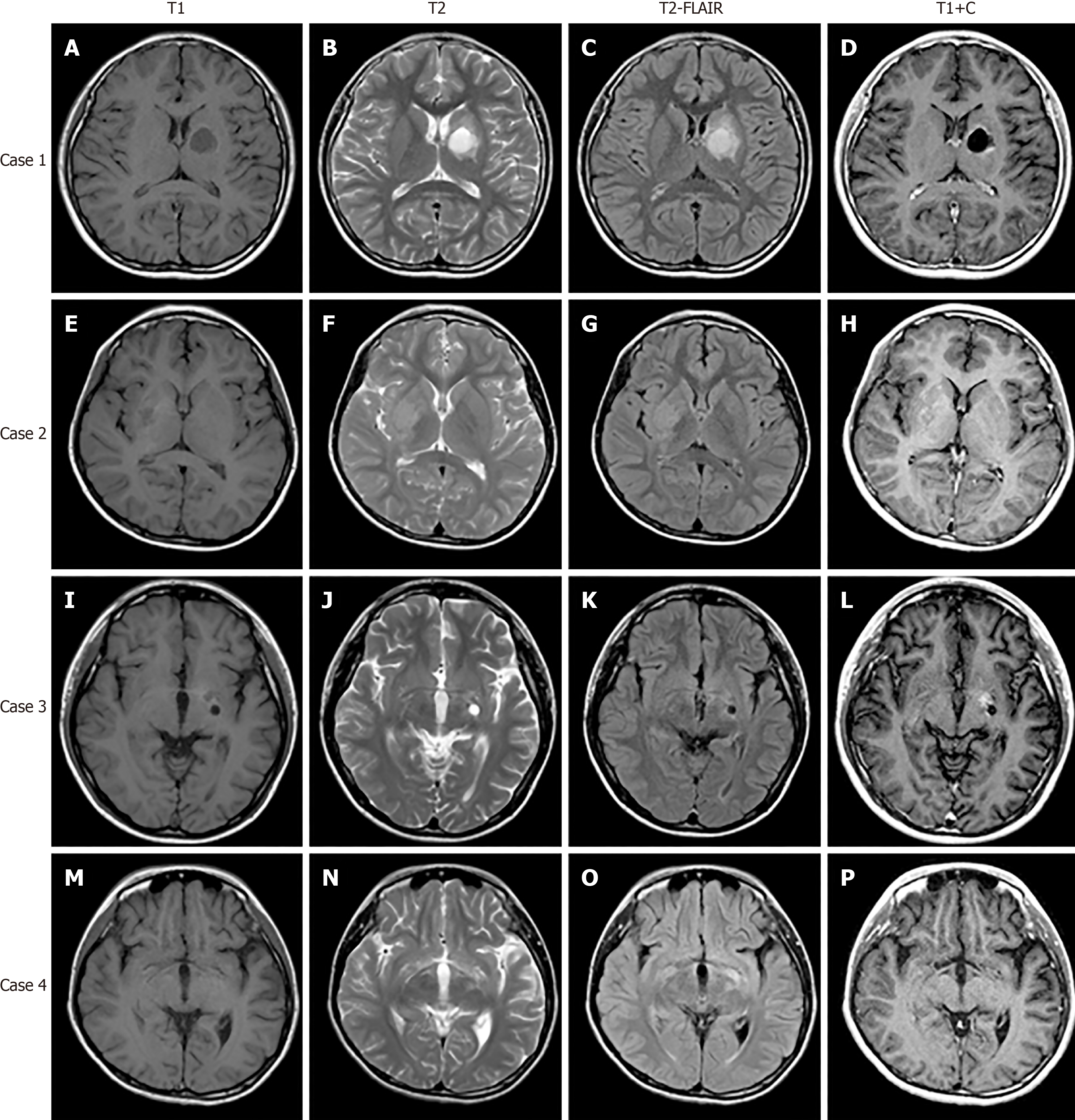
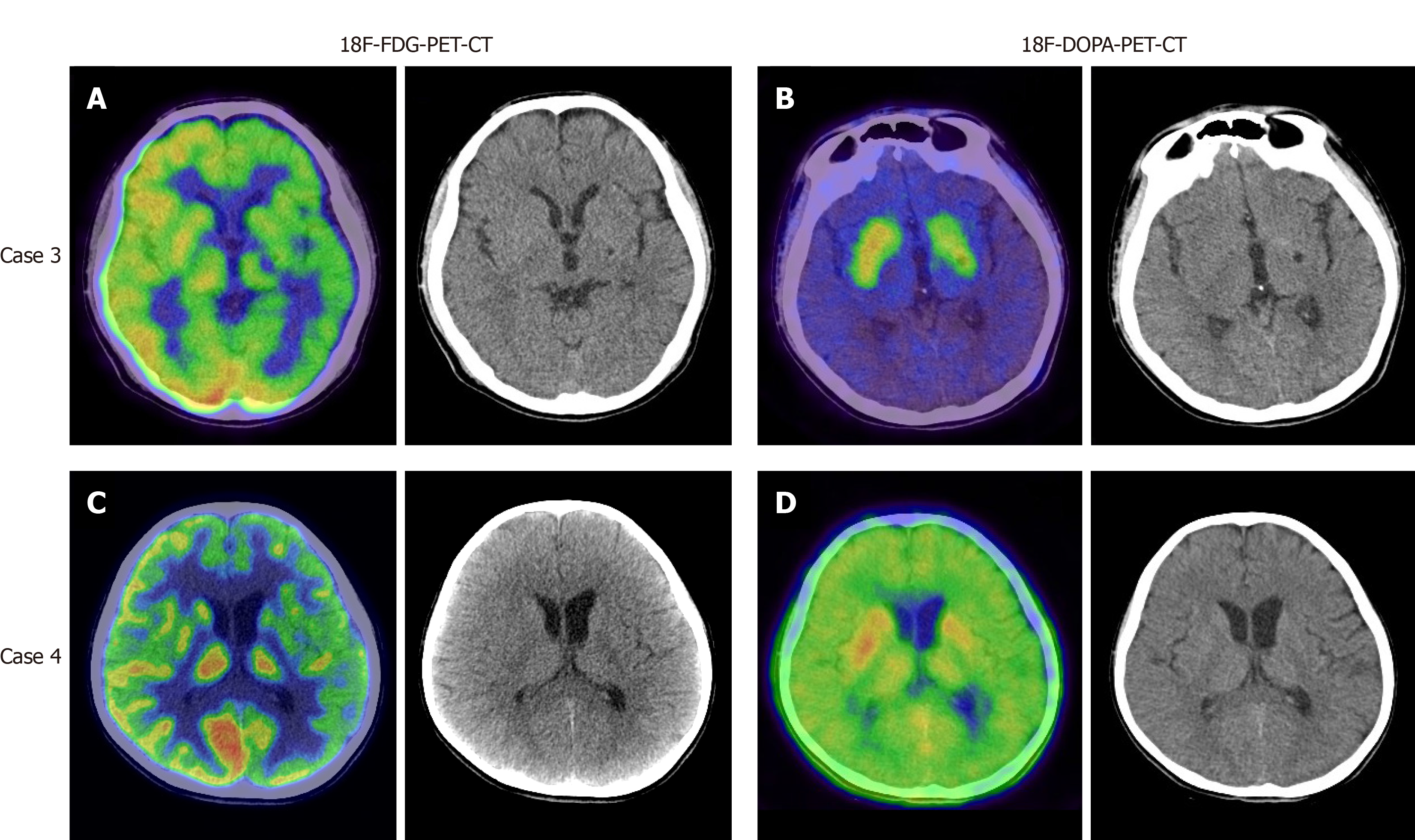
Magnetic resonance imaging (MRI) of the brain revealed local lesions in unilateral basal ganglia region in patients 1, 2, and 3. Brain MRI revealed subtle and ill-defined lesions in bilateral basal ganglia and thalamus in patient 4. The characteristics of these brain lesions are shown in Table 2 and Figure 1. In addition, patient 3 and 4 underwent both 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG-PET) and 18F-fluorodopa-positron emission tomography (18F-DOPA-PET). In patient 3, 18F-FDG-PET revealed diffuse low metabolism in the left cerebral cortex, basal ganglia and thalamus (Figure 2A). 18F-DOPA-PET showed slightly low metabolism in the left basal ganglia (Figure 2B). In patient 4, 18F-FDG-PET demonstrated low metabolism in the left hemisphere and left cerebral peduncle (Figure 2C). 18F-DOPA-PET showed normal metabolism (Figure 2D).
| Case | MRI findings | 18F-FDG-PET | 18F-DOPA-PET | ||||||||
| Contrast enhancement | Mass effect | Hemorrhage | Calcification | Cyst formation | Ipsilateral hemiatrophy | SWI | MRS | DTI | |||
| 1 | Y | Y | N | Y | Y | Y | Hypo | Low NAA | Nor | Low | Low |
| 2 | Y | N | N | N | N | Y | N/A | N/A | N/A | N/A | N/A |
| 3 | Y | N | N | N | Y | Y | N/A | N/A | Interrupted | N/A | N/A |
| 4 | N | N | N | N | N | Y (Bi) | Nor | Nor | N/A | Low | Nor |
All patients were diagnosed with germinomas of the basal ganglia and thalamus by stereotactic brain biopsy. Histopathological diagnoses were further confirmed by another hospital.
All four patients received whole brain radiotherapy and chemotherapy at another hospital.
The brain lesions on MRI were reduced or disappeared and their symptoms remained stable without aggravation.
Germinomas in the basal ganglia and thalamus show a male predominance[3,5,6,9-11]. The reason for this is unclear. They usually occur in young adolescents aged from 10 to 19 years. This may be correlated to gonad development in this age group[3]. Basal ganglia germinomas usually have an insidious onset and slow progression. The clinical presentation of these tumors depends on their localization. The most common symptoms are progressive hemiparesis, mental status change and cognitive decline. In this report, patient 1 developed paroxysmal paresthesia which is very rare. The other 3 patients had hemiplegia. Patient 4 developed cognitive decline, diabetes insipidus, precocious puberty in addition to hemiplegia. Although patients 3 and 4 had longer duration than patients 1 and 2, the brain lesions shown by MRI were much smaller and more ill-defined. Hence, the size of the lesion did not correspond to the duration and severity of the clinical presentation. Symptoms and signs are valuable for localization and contribute to the identification of subtle lesions on brain MRI.
Tumor markers of pure germinomas including AFP and β-HCG were negative in these patients[3-6]. β-HCG levels were slightly elevated in the body fluid of some patients which indicated syncytiotrophoblastic giant cells in the germinoma. Germinomas with elevated β-HCG in serum but not in CSF, might be associated with a poor outcome[7,8]. It was reported that intracranial germinomas with serum β-HCG levels higher than 15 mIU/mL had a high recurrence rate[7]. However, all the cases in that study were midline germinomas including those in the pineal region and suprasellar region or both sites. Further studies are needed to evaluate the prognosis of basal ganglia germinomas with elevated β-HCG. All our cases were AFP and β-HCG negative except patient 3 who had a slightly elevated level of β-HCG in CSF. Although these tumor markers are usually negative in germinomas, they serve to differentiate germinomas from other germ cell tumors.
According to the literature, typical brain MRI signs of basal ganglia germinomas are usually cystic formation, amorphous calcification, focal hemorrhage, peritumoral edema, contrast enhancement and ipsilateral cerebral and brain stem hemiatrophy[12-14]. None of our four cases presented all of the above typical neuroimaging characteristics. One patient had calcification, two patients exhibited cystic formation, and three patients had contrast enhancement. All these patients developed ipsilateral hemisphere atrophy especially in the cerebral peduncle. None had intratumoral hemorrhage. Although the MR images of case 1 shared overlapping features with craniopharyngioma, the location of the tumor was useful in differentiating it from craniopharyngioma. In cases 2-4, it was easy to miss the lesions on brain MRI. The most atypical case was patient 4 who showed bilateral involvement and did not present with the above signs except bilateral hemisphere atrophy. As shown in the literature, ipsilateral hemiatrophy is the predominant feature of basal ganglia germinoma[3,15]. Wallerian degeneration of the conduction tract is hypothesized to be the etiology of hemiatrophy[15]. In our report, patients 1 and 3 underwent diffusion tensor imaging examination which further supported this hypothesis. Why only germinomas rather than other brain tumors lead to ipsilateral hemiatrophy requires further investigation. Previous reports revealed that susceptibility weighted imaging (SWI) might be more sensitive in detecting early basal ganglia germinoma than conventional MRI, and MR spectroscopy (MRS) was helpful for monitoring the effects of treatment[6,16]. Patient 1 had similar SWI and MRS findings to those in the literature. A preoperative computed tomography scan was helpful in evaluating hemorrhage and calcification. Patients 3 and 4 underwent both 18F-FDG-PET and 18F-DOPA-PET. It seems that both these techniques had limited use for germinoma. Further studies are needed to confirm the value of PET examination in the diagnosis of germinoma. The reason why patient 4 had the longest clinical course before definite diagnosis was the limited findings on radiological examination.
According to the above findings, germinomas originating from atypical regions are not easy to diagnosis, especially in patients with small and ill-defined brain lesions. Stereotactic biopsy was valuable for early diagnosis. Serial neuroimaging studies are needed not only for disease differentiation but also for determining the biopsy site.
The diagnosis of germinomas in the basal ganglia and thalamus is often delayed due to the absence of elevated tumor markers, and atypical clinical symptoms and neuroimaging features. The association of a focal lesion in the basal ganglia or thalamus of children with progressive hemiparesis, neuroendocrine and neuro-psychiatric symptoms and ipsilateral hemiatrophy could prompt the diagnosis of ectopic germinoma. Histological examinations are necessary to confirm the diagnosis of an atypical lesion. Serial neuroimaging studies not only differentiate diseases but also determine the biopsy site.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zavras N S-Editor: Huang P L-Editor: MedE-Ma JY P-Editor: Xing YX
| 1. | Cuccia V, Galarza M. Pure pineal germinomas: analysis of gender incidence. Acta Neurochir (Wien). 2006;148:865-871; discussion 871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Finlay J, da Silva NS, Lavey R, Bouffet E, Kellie SJ, Shaw E, Saran F, Matsutani M. The management of patients with primary central nervous system (CNS) germinoma: current controversies requiring resolution. Pediatr Blood Cancer. 2008;51:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Hao S, Liu B, Tang J, Jia G, Zhang Y, Ma Z, Wang Z. Germinoma of basal ganglia in female: case report and review of the literature. Childs Nerv Syst. 2009;25:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Rasalkar DD, Chu WC, Cheng FW, Paunipagar BK, Shing MK, Li CK. Atypical location of germinoma in basal ganglia in adolescents: radiological features and treatment outcomes. Br J Radiol. 2010;83:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Konovalov AN, Kadyrov SU, Tarasova EM, Mazerkina NA, Gorelyshev SK, Khukhlaeva EA, Kobyakov GL, Trunin YY, Sanakoeva AV, Kholodov BV, Shishkina LV, Panina TN, Ryzhova MV. [Basal ganglia germinomas in children. Four clinical cases and a literature review]. Zh Vopr Neirokhir Im N N Burdenko. 2016;80:71-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Lou X, Ma L, Wang FL, Tang ZP, Huang H, Cai YQ, Wong EH. Susceptibility-weighted imaging in the diagnosis of early basal ganglia germinoma. AJNR Am J Neuroradiol. 2009;30:1694-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Utsuki S, Kawano N, Oka H, Tanaka T, Suwa T, Fujii K. Cerebral germinoma with syncytiotrophoblastic giant cells: feasibility of predicting prognosis using the serum hCG level. Acta Neurochir (Wien). 1999;141:975-977; discussion 977-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Utsuki S, Oka H, Tanaka S, Tanizaki Y, Fujii K. Long-term outcome of intracranial germinoma with hCG elevation in cerebrospinal fluid but not in serum. Acta Neurochir (Wien). 2002;144:1151-1154; discussion 1154-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Yasue M, Tanaka H, Nakajima M, Kamio M, Nakamura N, Numoto T, Tanaka J. Germ cell tumors of the basal ganglia and thalamus. Pediatr Neurosurg. 1993;19:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Tang J, Ma Z, Luo S, Zhang Y, Jia G, Zhang J. The germinomas arising from the basal ganglia and thalamus. Childs Nerv Syst. 2008;24:303-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Zou L, Gao B. Intracranial germinoma: clinical and MRI findings in 56 patients. Childs Nerv Syst. 2010;26:1773-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Moon WK, Chang KH, Kim IO, Han MH, Choi CG, Suh DC, Yoo SJ, Han MC. Germinomas of the basal ganglia and thalamus: MR findings and a comparison between MR and CT. AJR Am J Roentgenol. 1994;162:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Okamoto K, Ito J, Ishikawa K, Morii K, Yamada M, Takahashi N, Tokiguchi S, Furusawa T, Sakai K. Atrophy of the basal ganglia as the initial diagnostic sign of germinoma in the basal ganglia. Neuroradiology. 2002;44:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Kim DI, Yoon PH, Ryu YH, Jeon P, Hwang GJ. MRI of germinomas arising from the basal ganglia and thalamus. Neuroradiology. 1998;40:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Nagata K, Nikaido Y, Yuasa T, Fujimoto K, Kim YJ, Inoue M. Germinoma causing wallerian degeneration. Case report and review of the literature. J Neurosurg. 1998;88:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Li J, Zhang XY, Wang B, Geng JZ. MRI and MR spectroscopy findings of the evolution of an intracranial germinoma: A case report. Oncol Lett. 2015;10:1194-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |