Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4505
Peer-review started: May 10, 2020
First decision: June 7, 2020
Revised: June 18, 2020
Accepted: September 2, 2020
Article in press: September 2, 2020
Published online: October 6, 2020
Processing time: 140 Days and 11.3 Hours
Urinary tract lymphoepithelioma-like carcinoma is rarely seen. Although it is termed after lymphoepithelioma at the nasopharynx, it behaves more like high grade urothelial carcinoma by immunohistochemical features. Most published literatures focused on its rarity but few discussed results of long-term follow-ups. As no available guidelines are applicable, we postulated that principles should be similar to that of urothelial carcinoma at urinary tract. As of now, this work features the longest follow-up of this cancer at the upper urinary tract.
A 63-year-old female had a chief complaint of intermittent left flank pain for 2 mo, along with accompanying symptoms including vomiting and body weight loss, about 7 kg over 2 mo. Laboratory data showed normocytic anemia, mildly poor renal function, and hyperparathyroidism. Urine analysis showed mild hematuria. Computed tomography showed a 4.2-cm-width irregular mass over left renal pelvic and enlarged lymph node at the left renal hilum. Whole-body bone scan was negative of active bone lesions. Biopsy from ureteroscopy showed urothelial carcinoma. Specimen from laparoscopic nephroureterectomy with bladder cuff resection showed lymphoepithelioma-like carcinoma with muscular invasion (pT3). She took adjuvant chemotherapies of 2 cycles and full courses of radiation therapy. No recurrence was observed with designed investigative programs.
Locally advanced urinary tract lymphoepithelioma-like carcinoma could benefit from nephroureterectomy and bladder cuff excision in terms of recurrence-free survival.
Core Tip: Although urinary tract lymphoepithelioma-like carcinoma is rarely seen and no published guideline can be offered, we could still outline feasible principles from immunohistochemical evidence. In our previous experience, guideline of urothelial carcinoma at urinary tract can provide us a promising result. The case in this work comprises of most of the worst scenarios, including mixed histological type, muscular invasion, and involvement of lymph node. However, with left nephroureterectomy with bladder cuff resection and postoperative radiation therapy, our result stands superior to those other published literatures. Thus, in our experiences, the operative methods play the definite roles on patients’ prognosis.
- Citation: Yang CH, Weng WC, Lin YS, Huang LH, Lu CH, Hsu CY, Ou YC, Tung MC. Eight-year follow-up of locally advanced lymphoepithelioma-like carcinoma at upper urinary tract: A case report. World J Clin Cases 2020; 8(19): 4505-4511
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4505.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4505
Urinary tract lymphoepithelioma-like carcinoma (LELC) is termed after lymphoepithelioma (LE) at the nasopharynx but is actually a variant of urothelial carcinoma (UC). LELC is infrequently, 2% occurrence, identified at both upper and lower urinary tract. Malignancy is constituent of epithelial parts and inflammatory cells, such as myeloid cells or lymphoid cells under hematoxylin and eosin stain, making chemotherapy feasible. Immunohistochemical analysis differentiates urinary tract LELC from LE at the nasopharynx. No hybridization to Epstein–Barr virus, which is thought to be essential in cell expression and maintenance in LE of the nasopharynx, could be found in urinary tract LELC[1]. On the other hand, other characteristics such as presence of p53 make urinary tract LELC more resembling to high grade UC. In our previous published literature[2], we deliberated our postulation on urinary tract LELC, applying the same therapeutic principle as UC. From our experiences[2], nephroure-terectomy with bladder cuff excision without adjuvant therapies on urinary tract LELC showed no recurrence within one year after surgery. However, most published literatures tend to have more discussion on the lower and few on the upper urinary tract. Among case studies of upper urinary tract LELC, few had discussions on long-term follow-ups. Furthermore, compared to stages T1 and T2 upper urinary tract LELC, stage T3 is proved to be worse in terms of overall survival[3]. To date, this documentation has the longest investigations on the locally advanced upper urinary tract LELC.
On July 2012, a 63-year-old female came to the urology office with symptoms of intermittent left flank pain for 2 mo.
The pain was described to be localized at left fank. Neither precipitating factors nor exaggerating factors were mentioned. It occurred randomly without any special time or occasions, and can be suppressed by painkillers. Accompanying symptoms include vomiting and body weight loss, about 7 kg over 2 mo. She denied having visible red color urine, neither fever. Appetite was not changed. Neither specific travel histories nor cluster histories were mentioned.
She had uterine prolapse diagnosed 2 years before this episode, but did not have any interventions about it.
Her temperature was 35.9 °C, and heart rate was 74 bpm. Respiratory rate was 17 breaths per minute, and blood pressure was 127/81 mmHg. Oxygen saturation in room air was 98%. She ranked the pain subjectively 4 out of 10 on visual analog scale pain score. No knocking pain was examined at costovertebral angle, neither the tenderness on abdomen.
Normocytic anemia was seen on complete blood cell (White blood cell: 4 500/UL, Red blood cell: 3370000/UL, Hemoglobulin: 10.4 g/DL, Mean corpuscular volume: 93.5 FL). Mildly insufficiency was seen on renal functions tests (Creatinine: 1.4 mg/DL, Blood urea nitrogen: 16.2 mg/DL). Elevated Intact parathyrin (EIA/LIA) was 245 pg/ML. Free T4 (EIA/LIA) was 1.070 ng/DL, and TSH (EIA/LIA) was 1.5 UIU/ML. Tumor markers of carcino-embryonic antigen, CA-199, and alpha-fetoprotein were all within normal limits. Urine examination showed hematuria (red blood cell 5-10/HPF) and pyuria (white blood cell: 10-20/HPF). Urine cytology was gained, but no atypical cells were examined.
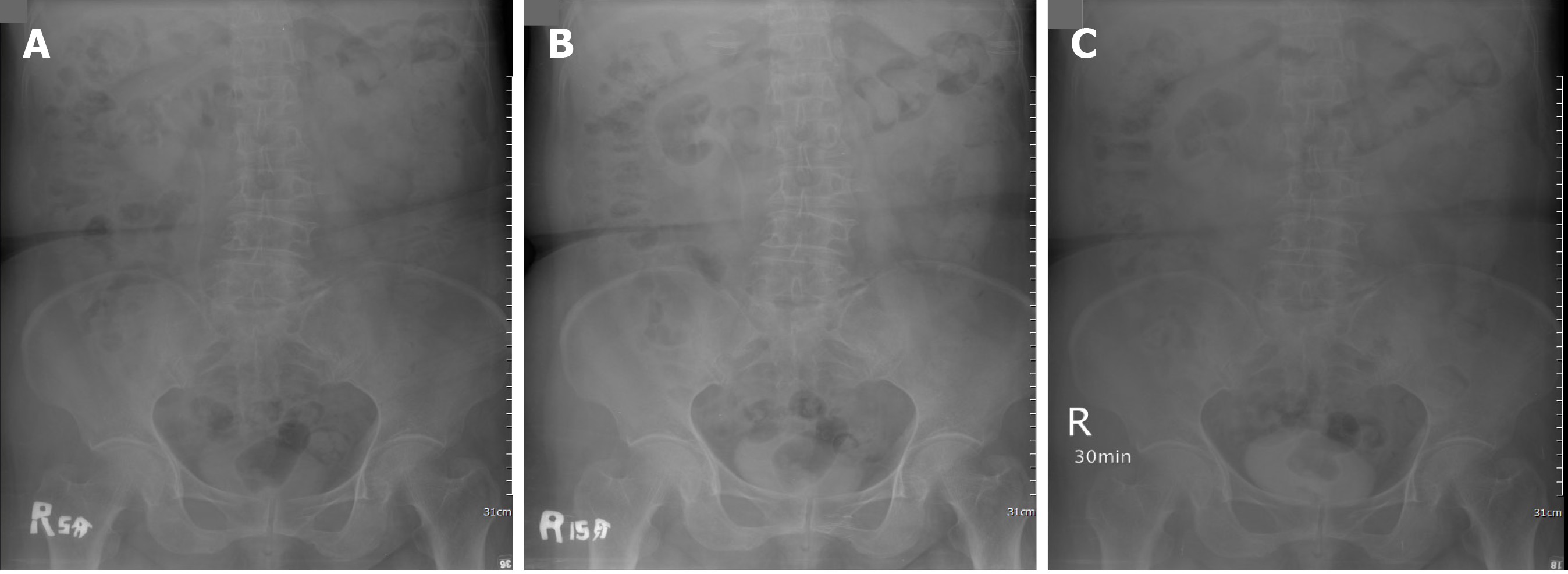
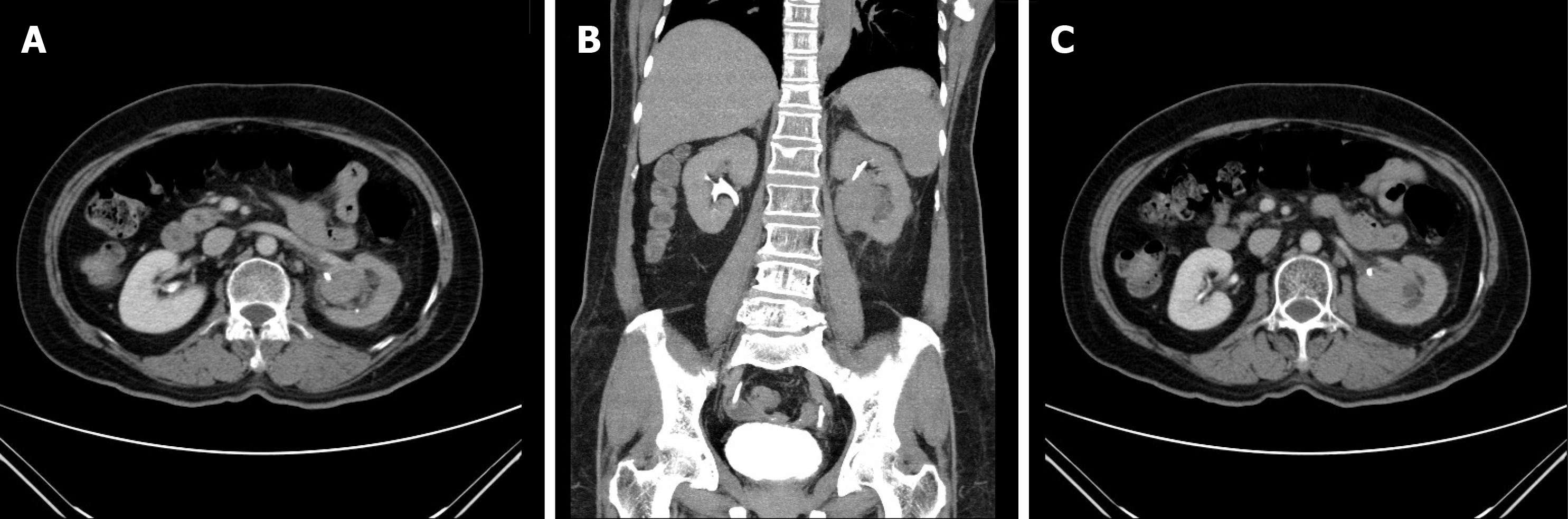
Left side hydronephrosis was evident on ultrasound. Intravenous pyelography showed a non-enhanced left kidney (Figure 1) and then computed tomography urogram was arranged (Figure 2) for comprehensive studies, which showed an enlarged lymph node close to the left renal pedicle and one irregularly ill-defined 4.2-cm width mass at the left renal pelvic.
Ureteroscopy was arranged and biopsy was done to this 4.2-cm mass at left renal pelvis. Pathology from the biopsy was proved to be high-grade (grade III) UC, which is invasive to lamina propria.
The final diagnosis of this case is upper urinary tract UC with clinical stage of T3N1Mx at left renal pelvis.
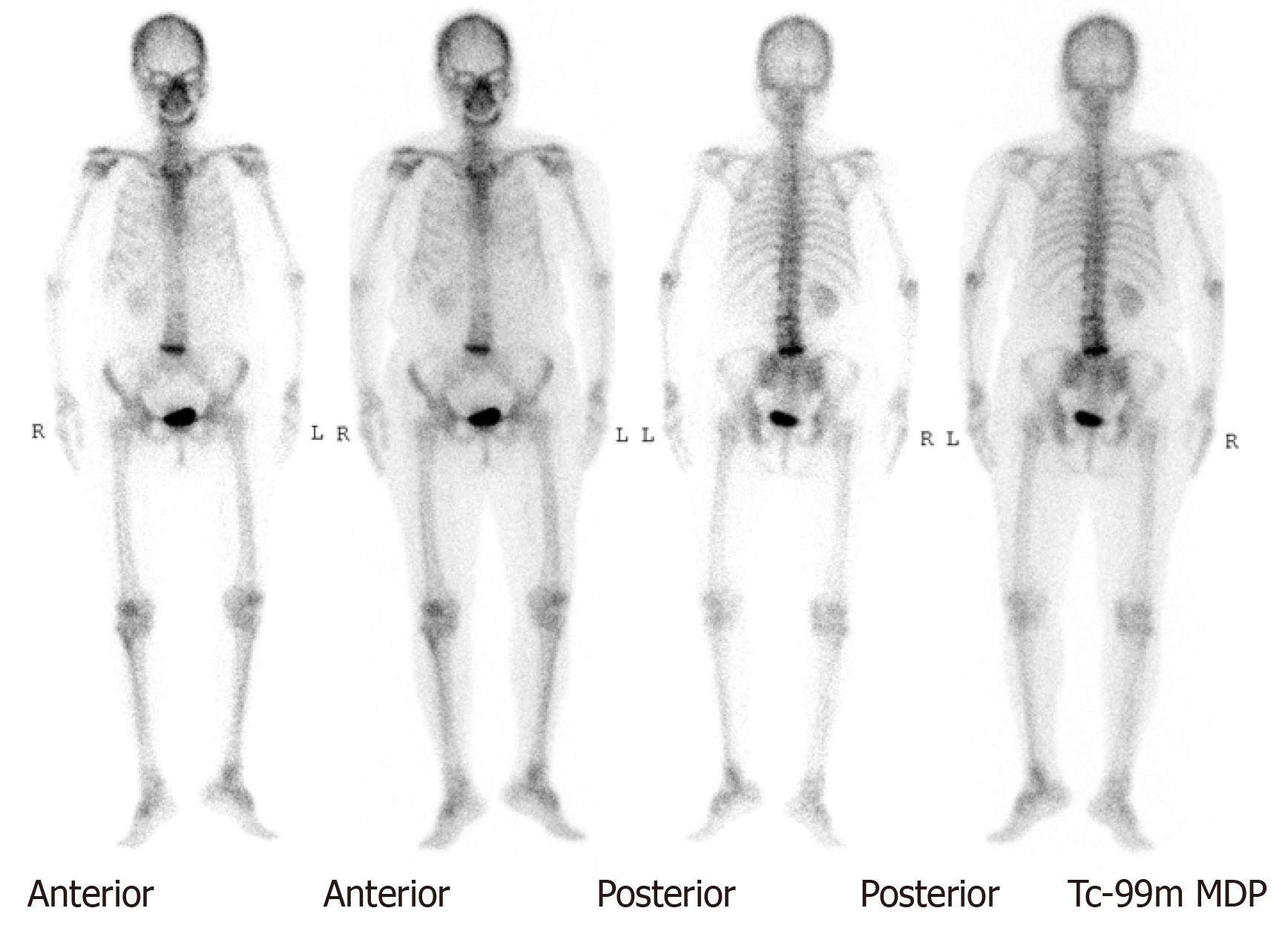
A whole-body bone scan showed a negative finding (Figure 3). After securing informed consent, the patient underwent laparoscopic left nephroureterectomy with bladder cuff excision.
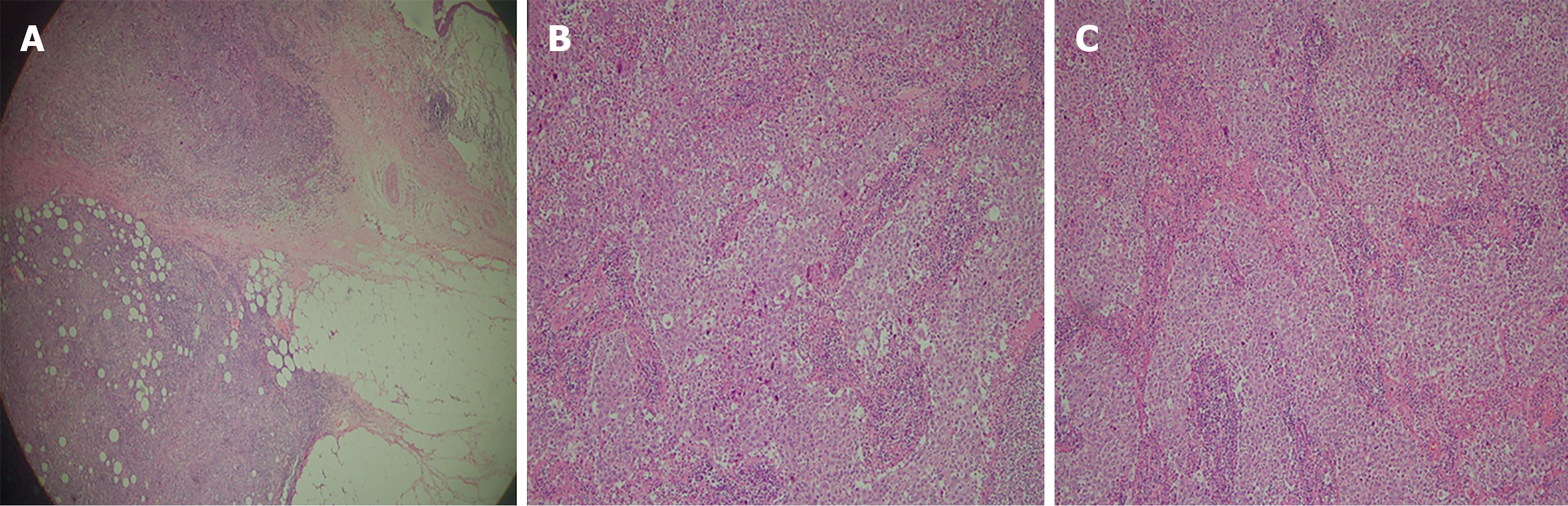
A tumor size of 3 cm × 2.5 cm× 2.5 cm from left nephroureterectomy with bladder cuff resection was examined as undifferentiated LELC (Figure 4), high grade (undifferentiated type, grade IV), and invasive into muscle at renal pelvis and peripelvic fat (pT3). Surgical margin, Gerota’s fascia, ureter, and resected bladder cuff were all free of tumor. No lymph node was identified from the specimen. Tissues from the other non-tumor kidney displayed chronic pyelonephritis with focal global sclerosis.
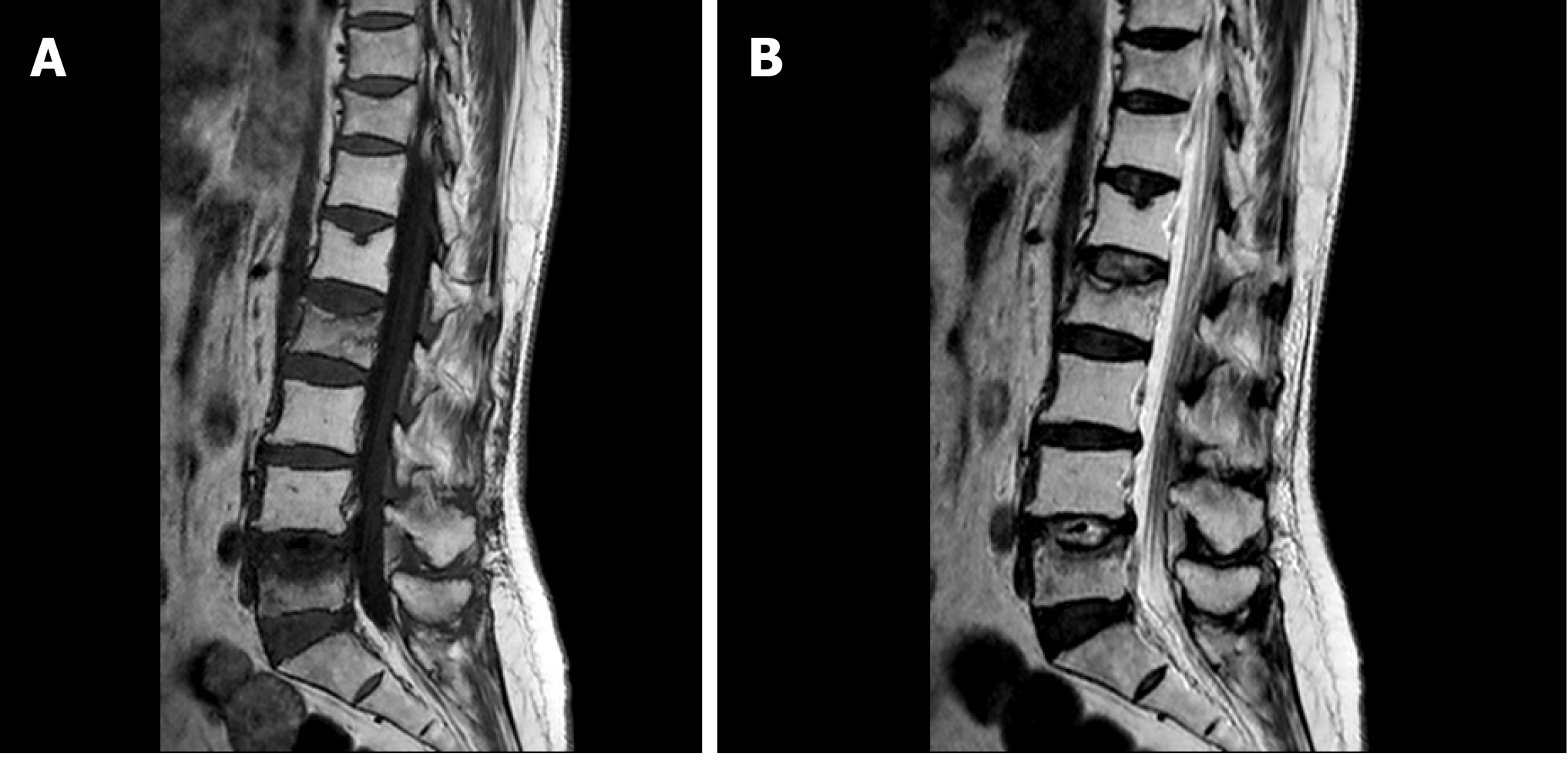
She received two cycles of gemcitabine (1000 mg/m2) and cisplatin (35 mg/m2) chemotherapy after the operation but stopped due to unbearable adverse effects, which was assessed to be grade I nausea and vomiting on World Health Organization criteria. Palliative radiation therapy (RT) was targeted to the enlarged lymph node at the left renal pedicle which had no proven pathologies. A total dosage of 5580 cGy was divided into 31 doses. However, 2 mo later, she suffered from low back pain and osteoporotic compression fractures were shown on X-ray and magnetic resonance imaging (Figure 5) of the L-spine (L2 and L5), which was a suspicious pathological change secondary to LELC. A second whole-body bone scan revealed no new onset changes compared to the preoperative one. The orthopedist performed palliative resection of the bone lesions and fixation with cages for symptomatic treatment. Fortunately, the specimen taken from bones showed null of metastatic cancer. Active follow-ups with urine cytology and cystoscopy every three months were planned. Computed tomography scan was done every six months postoperatively in the succeeding five years. After five years, biannual cystoscopy and annual computed tomography scan were done. She has no evidence of tumor recurrence postoperatively for 8 years till now. In conclusion, this pathological T3N1Mx mixed LELC with two cycles’ adjuvant chemotherapy and a full dose of palliative RT can lead to a recurrence-free survival up to 8 years after left nephroureterectomy with bladder cuff excision.
Generally speaking, urinary tract LELC can be categorized into pure (100% LELC) or mixed type (predominant: > 50% LELC), and pure type is reported to have more favorable prognosis. The case, described in this article, comprises of some of the worst scenarios of LELC at the upper urinary tract, with muscular invasion, lymph node involvement, and undifferentiated mixed type histology. Lopez-Beltran et al[3] issued the largest series of follow-ups on upper urinary tract LELC[3] with a maximal 4.8-year span. Their data suggests that the overall survival rate is comparable to conventional UC at the upper urinary tract (about 60% survival rate and 40% cancer-related death) across all stages. However, in their statistics, all cancer-related deaths are at pT3 stage. They also find that prognosis will be influenced by T stage, and T3 stage (time before cancer-related death: 15 ± 16.2 mo; median: 8.5 mo; range: 4-39 mo) will significantly be worse than T1 and T2. The other related reports about LELC at the upper urinary tract in the past 20 years are listed in Table 1[3-11].
| Ref. | Case amount | Gender, Age | Site and pathological TNM stage | LELC type | Treatment | Documented follow-up period |
| Lopez-Beltran et al[3] | 10 | 68 yr old (Range: 54-85); male: 8; female: 2 | All high grade; T stage: pT1: 2 (20%) pT2: 2 (20%) pT3: 6 (20%); renal pelvis: 4 (pT3N1: 3); ureter: 6 (pT3N1: 2,pT3N0: 1) | Pure type: 3 (pT3: 1); predominant type: 7 (pT3: 5) | Radical nephrectomy + adjuvant chemotherapy: 4 (all pT3); radical nephrectomy: 1; nephroureterectomy+ chemotherapy: 1(pT3); nephroureterectomy:2(pT3:1); ureterectomy: 2; all pT3N1predominant LELC at renal pelvis received radical nephrectomy+ adjuvant chemotherapy | Maximal 4.8 yr; cancer-related death: 4 (40%) (all pT3); overall survival of pT3N1 predominant LELC at renal pelvis: 7 ± 3 mo (range: 4-10 mo) |
| Haga et al[4] | 1 | 75-year-old female | Left renal pelvis; pT1N1M0 | Pure | Laparoscopic nephroureterectomy; no adjuvant therapies | 3 yr, no recurrence |
| Yamada et al[5] | 1 | 75-year-old female | Left renal pelvis; pT3N0M0 | Not mentioned | Left nephrectomy; no adjuvant therapies | 6 mo; no recurrence |
| Modi et al[6] | 1 | 75 year-old female | Right renal pelvis; pT3N1M0 | Mixed; predominant | Right radical nephroureterectomy; no adjuvant therapies | 6 mo; no recurrence |
| Ahn et al[7] | 1 | 65-year-old female | Right renal pelvis; pT3N0M0 | Mixed; predominant | Laparoscopic right radical nephroureterectomy; no adjuvant therapies | 6 mo; no recurrence |
| Valverde Martínez et al[8] | 2 (one is at low urinary tract) | 74-year-old female | Left renal pelvis; pT4N1M0 | pure | Left radical nephrectomy + adjuvant chemotherapy( cisplatin, gemcitabine) | Recurrent at the 5th yr |
| Wen et al[9] | 1 | 64-year-old male | Right middle ureter | Mixed, predominant | Rght radical nephroureterectomy with bladder cuff excision; no adjuvant therapies | 6 mo; no recurrence |
| Terai et al[10] | 1 | 73-year-old male | Right ureter; pT2N0M0 | Pure | Right laparoscopic nephroureterectomy; no adjuvant therapies | 30 mo; no recurrence |
| Tamas et al[11] | 30 (only 1 at renal pelvis; the rests are at low urinary tract) | Not mentioned | Renal pelvis; pT3Nx | Mixed | Radical nephrectomy + adjuvant chemotherapy | 34 mo; no recurrence |
Among most publications, very few are documented with follow-ups long enough to represent meaningful survival analysis[3,4,8,10,11]. In article by Lopez-Beltran et al[3], radical nephrectomy with adjuvant chemotherapy is performed to all pT3N1 predominant LELC, which is similar to our case, at renal pelvis but they all die from cancer within 7 ± 3 mo. Judging from our case, literature from Lopez-Beltran et al[3] and case reports from Valverde et al[8] and Tamas et al[11], patients can benefit more from the operative method of nephroureterectomy than nephrectomy in terms of recurrence-free survival and overall survival. Adjuvant chemotherapy and palliative RT, by comparing our case with T1 from Haga et al[4] and T2 from Terai et al[10], might provide beneficial credits to prolong the recurrence-free survival for those with unfavorable advanced stage. However, there are still some examples with radical nephrectomy and adjuvant chemotherapy[8] that demonstrated patients could live without recurrence to a maximal 5-year interval. This phenomenon of prognostic variations might imply that some other factors will further affect recurrence-free survival.
From our experiences and published literature, operative methods still possess definite roles, and locally advanced LELC at the upper urinary tract could benefit from nephroureterectomy and bladder cuff excision over nephrectomy in terms of recurrence-free survival and overall survival. Adjuvant chemotherapy and palliative RT might have positive roles for locally advanced LELC at upper urinary tract to extend the recurrence-free survival parallel to those with T1 and T2 ones, but a large-scale analysis is necessary to further prove the hypothesis.
We thank Chung Hsiao Chin (Pathologist, Tungs’ Taichung MetroHarbor Hospital, Taiwan) for helping re-stain the pathological specimen.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Thanindratarn P S-Editor: Zhang H L-Editor: A P-Editor: Xing YX
| 1. | Fukunaga M, Ushigome S. Lymphoepithelioma-like carcinoma of the renal pelvis: a case report with immunohistochemical analysis and in situ hybridization for the Epstein-Barr viral genome. Mod Pathol. 1998;11:1252-1256. [PubMed] |
| 2. | Yang CH, Lin YS, Weng WC, Ou YC, Hsu CY, Tung MC. Simultaneous upper and lower urinary tract invasive Lymphoepithelioma-like carcinoma with programmed death-ligand 1 full expression on combined positive score. Urol Case Rep. 2020;31:101201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Lopez-Beltran A, Paner G, Blanca A, Montironi R, Tsuzuki T, Nagashima Y, Chuang SS, Win KT, Madruga L, Raspollini MR, Cheng L. Lymphoepithelioma-like carcinoma of the upper urinary tract. Virchows Arch. 2017;470:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Haga K, Aoyagi T, Kashiwagi A, Yamashiro K, Nagamori S. Lymphoepithelioma-like carcinoma of the renal pelvis. Int J Urol. 2007;14:851-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Yamada Y, Fujimura T, Yamaguchi T, Nishimatsu H, Hirano Y, Kawamura T, Teshima S, Takeuchi T, Kitamura T. Lymphoepithelioma-like carcinoma of the renal pelvis. Int J Urol. 2007;14:1093-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Modi H, Beckley I, Bhattarai S, Spencer J, Cartledge J. Lymphoepithelioma-like carcinoma of the renal pelvis: Pathological and therapeutic implications. Can Urol Assoc J. 2013;7:E590-E593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Ahn H, Sim J, Kim H, Yi K, Han H, Chung Y, Rehman A, Paik SS. Lymphoepithelioma-like Carcinoma of the Renal Pelvis: A Case Report and Review of the Literature. Korean J Pathol. 2014;48:458-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Valverde Martínez S, Salcedo Mercado W, Rodríguez Cruz I, Prieto Nogal SB, Martín Hernández M, Gómez Tejeda LM. [Urinary tract lymphoepithelial carcinoma. Report of two cases and bibliographic review.]. Arch Esp Urol. 2017;70:361-366. [PubMed] |
| 9. | Wen SC, Shen JT, Jang MY, Tsai KB, Chang SF, Tsai LJ, Wu WJ. Lymphoepithelioma-like carcinoma of ureter-a rare case report and review of the literature. Kaohsiung J Med Sci. 2012;28:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Terai A, Terada N, Ichioka K, Matsui Y, Yoshimura K, Wani Y. Lymphoepithelioma-like carcinoma of the ureter. Urology. 2005;66:1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Tamas EF, Nielsen ME, Schoenberg MP, Epstein JI. Lymphoepithelioma-like carcinoma of the urinary tract: a clinicopathological study of 30 pure and mixed cases. Mod Pathol. 2007;20:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |