Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4303
Peer-review started: April 30, 2020
First decision: May 21, 2020
Revised: June 1, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: October 6, 2020
Processing time: 150 Days and 17.5 Hours
In December 2019, an outbreak of unexplained pneumonia was reported in Wuhan, China. The World Health Organization officially named this disease as novel coronavirus disease 2019 (COVID-19). Liver injury was observed in patients with COVID-19, and its severity varied depending on disease severity, geographical area, and patient age. Systemic inflammatory response, immune damage, ischemia-reperfusion injury, viral direct damage, drug induce, mechanical ventilation, and underlying diseases may contribute to liver injury. Although, in most cases, mild liver dysfunction is observed, which is usually temporary and does not require special treatment, the importance of monitoring liver injury should be emphasized for doctors. The risk of COVID-19 infection of liver transplantation recipients caused more and more concerns. In this article, we aimed to review the available literature on liver injury in COVID-19 to highlight the importance of monitoring and treating liver injury in COVID-19.
Core Tip: Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2. Liver injury was observed in COVID-19 patients especially in severe and critical types. Liver injury presented differentially based on disease severity, geographical areas, and patient age. It may be related to systemic inflammatory response, immune damage, ischemia-reperfusion injury, viral direct damage, drug induce, mechanical ventilation, and underlying diseases. Special treatment is unnecessary in most mild liver injury cases and only one or two types of hepatoprotective drugs should be used to treat significant liver injury. Monitoring liver function is important parameter during the treatment of COVID-19 as it affects survival and recovery of patients, and its significance should be emphasized for doctors treating the disease.
- Citation: Zhao JN, Fan Y, Wu SD. Liver injury in COVID-19: A minireview. World J Clin Cases 2020; 8(19): 4303-4310
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4303.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4303
An outbreak of unexplained pneumonia occurred in Wuhan, China beginning in December 2019. The causative pathogen, a novel coronavirus, was subsequently identified by high-throughput sequencing technology[1,2]. The novel coronavirus was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[3], and the disease was named 2019 novel coronavirus disease (COVID-19) by the World Health Organization. COVID-19 is transmitted by droplets and aerosols via mouth mucus membranes[4]. Because of its worldwide spread, the World Health Organization declared COVID-19 a pandemic, and it has since spread to at least 210 countries and infected more than 2700000 people globally as of the end of April 2020. Fever and dry cough were the main clinical characteristics of COVID-19. Diarrhea was also reported in some patients. Liver injury was observed in several cases with gastrointestinal (GI) symptoms. It may be related to liver injury in COVID-19 as different degrees of liver dysfunction were observed in a proportion of patients with COVID-19[5-7]. This article aimed to review the available literature on liver injury in COVID-19.
Nausea, vomiting, and diarrhea are the main GI clinical manifestations of COVID-19. The incidence of liver injury in patients with COVID-19 with GI symptoms were significantly higher than that in those without GI symptoms (17.57% vs 8.84%, P = 0.035)[8]. Similarly, the rate of chronic liver disease in patients with COVID-19 with GI symptoms was also significantly higher than that of those without GI symptoms (10.81% vs 2.95%, P = 0.004)[8]. It might indicate that chronic liver disease was closely related to GI symptoms. Meanwhile, chronic liver disease increased the level of alanine transaminase and aspartate transaminase to aggravate the liver injury in the patients with COVID-19. The incidence of liver injury in COVID-19, which was mainly indicated by abnormal alanine transaminase/aspartate transaminase levels, ranged from 14.8% to 53%[9]. The severity of liver injury was most obvious in the 2nd wk of the course of disease, and it may be related to the viral infection of the regenerated liver cells from the bile duct with high angiotensin-converting enzyme 2 (ACE2) expression[10].
The degree of liver injury varied with different types of COVID-19. The incidence of liver injury in patients with severe COVID-19 was significantly higher than that in patients showing mild symptoms[4,5,11]. Additionally, incidence of liver injury was reported in 58.06%[12] and 78%[13] of the total COVID-19 related deaths. Wan et al[14] found that liver injury was more significant in severe patients as compared to those with mild symptoms. Likewise, Ma et al[15] also reported a significant difference between the liver function of patients in the intensive care unit and those out of the intensive care unit. Using multiple regression analysis, Yao et al[10] found that the liver injury was only associated with critical type patients, and they concluded that a higher proportion of liver injury was detected in severe patients, and critical type was an independent risk factor for liver injury. Moreover, Jin et al[8] reported that the incidence of the severe and critical types was also significantly increased in patients with COVID-19 with GI symptoms than in those without GI symptoms (22.97% vs 8.14%, P < 0.001), indicating increased incidence of liver injury in severe and critical types.
Liver injury in COVID-19 also varies based on geographical area and age of patients. A study revealed that liver dysfunction was detected in only three patients (3.75%) in Jiangsu province, which was lower than that observed in Wuhan[16]. As the place where the outbreak of COVID-19 occurred, the number of patients in Wuhan surged, which exceeded the existing capacity of treatment in the early stage. So a proportion of patients managed in Wuhan were severe and critical types whose liver injury was significant. In other places such as Jiangsu province, the number of patients was fewer, and early detection and treatment made the liver injury mild when compared with patients from Wuhan. Zhu et al[17] observed thrombocytopenia along with liver dysfunction in two out of ten neonates. Additionally, in a single center observational study, Sun et al[18] found liver dysfunction in four out of eight pediatric patients with severe COVID-19. Thus, the management of severe and critical pediatric patients should be valued.
Liver injury in COVID-19 can cause characteristic pathological changes that can be revealed during patient autopsy. Xu et al[19] reported that moderate microvesicular steatosis and mild lobular and portal activity were detected in the postmortem liver biopsies of a patient with COVID-19; this indicated that either SARS-CoV-2 infection or drug induction caused the liver injury. Minimally invasive autopsies of several organs or tissues from three patients who died due to COVID-19 were performed[20]. Hepatocyte degeneration, focal necrosis, and cholestasis of cholangioles were observed along with bile suppository in the small bile duct, which might have been the secondary injury caused by viral infection. However, in both these studies, viral inclusions were not observed, and SARS-CoV-2 was not detected in the liver tissue.
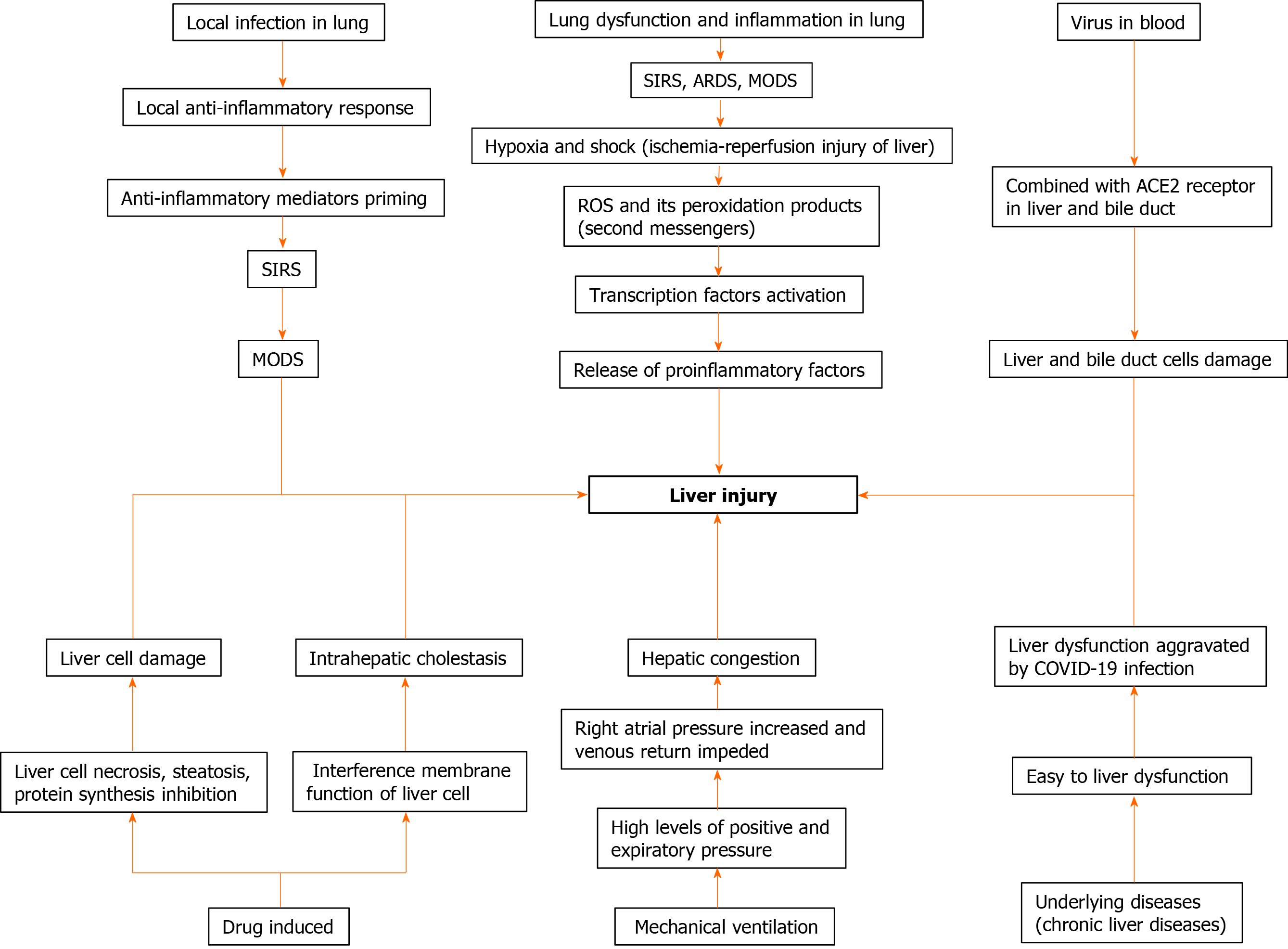
Liver injury may be related to systemic inflammatory response and immune damage. Xu et al[19] found decreased peripheral CD4 and CD8 T cell counts with hyperactivated status, which may be indicative of severe immune injury in the patient with COVID-19. Other studies have also observed that liver injury was not dangerous during the early stage of the disease; however, liver deterioration suddenly occurred during the late stage, which was considered to be related to systemic inflammatory response syndrome (SIRS) and activated immune system due to viral infection[21]. SIRS and immune damage can cause multiple organ damage including the liver. Yao et al[10] found that patients with lower absolute value of lymphocyte were more likely to suffer liver dysfunction, which suggested that immune damage might cause liver injury (Figure 1).
Ischemia-reperfusion injury can also contribute to liver injury. Hypoxia and shock caused by the complications of COVID-19, such as acute respiratory distress syndrome, SIRS, and multiple organ dysfunction syndrome, can lead to ischemia-reperfusion injury of the liver[21]. Another study found that hepatocyte necrosis and inflammatory cell infiltration caused by ischemia and hypoxia can be observed not only in the liver transplantation specimens but also in vivo and in vitro models of liver ischemia and hypoxia[22]. Under conditions of shock and hypoxia, there is a continuous increase in reactive oxygen species. Reactive oxygen species and its peroxidation products act as second messengers to activate transcription factors sensitive to redox reactions, and further initiate the release of various proinflammatory factors, leading to liver damage[23].
The novel coronavirus may damage the liver function directly. As the viral RNA can be detected in the blood specimens of the patients with COVID-19[24], and the liver is an organ with abundant blood supply, thus providing the virus with an opportunity to cause direct liver injury. It is now known that ACE2 is the receptor for cell entry of SARS-CoV-2[25]. ACE2 was found to be expressed in both liver and bile duct cells[26]. Up-regulated expression of ACE2 in hepatic cells due to compensatory hyperplasia of bile duct epithelial cells that was observed in patients with COVID-19 might be related to viral infection of neoplastic hepatocytes[27]. However, the ACE2 expression was found to be higher in bile duct cells than in liver cells[9] indicating that damage and dysfunction of bile duct cells induced hepatocyte dysfunction and subsequent liver injury. Thus, liver injury is caused by bile duct damage and dysfunction rather than hepatocyte damage. However, as there is no significant increase of markers, such as alkaline phosphatase and gamma-glutamyl transpeptidase[15], associated with bile duct cell injury reported in patients with COVID-19, direct virus damage to hepatocytes and biliary duct cells may be limited.
Liver injury may be induced by medication, which is a likely reason for the varied extent of liver dysfunction observed in patients with COVID-19 based on different types and geographical areas[28]. Until now, no “wonder drug” has been developed to treat COVID-19, and supporting therapy is the mainstay of its management. Fever is the main symptom of COVID-19, and acetaminophen, a common constituent of antipyretic drugs, is recognized as a common liver damage-causing drug and may be one of the important causes of liver dysfunction[21]. In addition, antivirals, antibiotics, steroids, herbal medications, as well as hormones are widely used for the treatment of COVID-19[29,30]. Antivirals such as oseltamivir, abidor, lopinavir, and ritonavir were found to have adverse reactions such as liver injury[21]. Moreover, similar findings were observed in a recent study where lopinavir/ritonavir caused liver injury in patients with COVID-19[31]. In detail, the odds of liver injury using lopinavir/ritonavir was increased by four fold[30]. Another study reported that liver injury was more likely to occur in patients treated with multiple kinds of drugs as well as large amounts of hormones (P = 0.002, P = 0.031, respectively)[10]. This may explain that liver injury was more commonly observed in severe and critical types because less drugs are usually administered to patients with mild disease. As drug metabolism occurs in the liver and kidneys, liver injury may impair their metabolism, excretion, dosing, and effective concentrations, leading to increased risk of toxicity due to the use of antiviral drugs[32]. Therefore, we should attach importance to the monitoring of liver function in COVID-19.
In the cases of patients receiving mechanical ventilation, high levels of positive and expiratory pressure can increase the right atrial pressure and impede venous return and subsequently cause hepatic congestion, which may participate in liver injury. However, clinical data show that many patients with COVID-19 who do not require mechanical ventilation also have liver dysfunction[33]. This observation may support the higher incidence of liver injury in severe and critical patients than in other mild cases.
Furthermore, underlying diseases may contribute to liver injury. Individuals suffering from underlying diseases, such as chronic liver disease, are more likely to be infected by the SARS-CoV-2 as they have lower immune function. Similarly, a guideline proposed by the Asian-Pacific Association for the Study of the Liver suggested that patients with advanced cirrhosis, hepatic cell cancer, autoimmune liver diseases, and post liver transplantation were more susceptible to COVID-19 infection because of being immuno-compromised[34]. A study on COVID-19, found that out of 22 patients with liver injury, the proportion of injury was significantly higher (P = 0.011) in 16 patients (72.7%) with underlying diseases than that in patients without underlying diseases[10]. Half of the patients with abnormal liver test results also tended to have chronic liver diseases, including nonalcoholic fatty liver disease, alcoholic liver disease, and chronic hepatitis B (P = 0.001)[30]. Another study about GI symptoms in COVID-19 reported that the incidence of chronic liver disease (10.81%) in patients with GI symptoms was significantly higher than that (2.95%) in patients without GI symptoms (P = 0.004)[8], which indicates a potential relationship between chronic liver disease and liver injury. However, few studies reported the occurrence of liver failure in the chronic liver disease patients with COVID-19[9].
The mainstay for management of liver injury in COVID-19 is to inhibit the inflammatory response and correct the hypoxemia in order to prevent the SIRS and multiple organ dysfunction syndrome[21]. Most cases with COVID-19 show mild liver damage, which is usually temporary and returns to normal without any special treatment[28] (Table 1). Targeted hepatoprotective therapy is necessary in patients with severe liver injury[21]. Clinically used liver protective drugs include polyene phosphatidylcholine, glycyrrhizin preparation, ursodeoxycholic acid, and s-adenosine methionine. One or two of these drugs were used according to the liver function of the patient. As we have previously discussed, the use of several drug types is not recommended. As prevention is preferable to management, the monitoring of liver function to avoid liver damage is a key role in the treatment of COVID-19.
| Ref. | Object | Group 1 | Group 2 | P value |
| Jin et al[8] | GI symptoms | Without GI symptoms | ||
| Incidence of liver injury | 17.57% | 8.84% | 0.035 | |
| Rate of chronic liver disease | 10.81% | 2.95% | 0.004 | |
| Incidence of the severe and critical types | 22.97% | 8.14% | < 0.001 | |
| Yao et al[10] | Critical type patients (%) | Liver injury group (77.3%) | Non-liver injury group (27.8%) | 0.002 |
| Wan et al[14] | Mild cases | Severe cases | ||
| Aspartate aminotransferase, U/L (≤ 40) | 84% | 62.5% | 0.0005 | |
| Lactate dehydrogenase, U/L (> 250) | 29% | 75% | 0.0055 |
With the outbreak of COVID-19, the risk of COVID-19 infection and management of liver transplantation (LT) recipients have recently begun to attract increased attention. The impacts of COVID-19 on LT recipients may different. The infection of COVID-19 may be more severe in the perioperative or early post-transplant period because of high immunosuppressive levels. As for long-term post-transplant patients, diabetes, hypertension, and other comorbidities may be associated with severe COVID-19[12,35]. A study suggested that immunosuppressed patients did not have an increased risk of severe complications of COVID-19 when compared with the general population[36]. Just as mentioned above, a reactive innate immune response might cause severe clinical manifestations, such as liver injury, SIRS, and multiple organ damage, and immunosuppression might have a protective effect[37]. Compared with non-transplant patients, it was observed that COVID-19 infection was no worse in LT patients, and they had lower risks of hyperinflammation and cytokine storm[38], which might indicate that the host response to infection may be modified by immuno-suppression[34]. Based on current studies, some recommendations were given below[39]. For LT recipients without COVID-19, there is no need to adjust the immunosuppressive dosage. Keep current immunosuppressant dosage and monitor the condition of patients with mild to moderate COVID-19. Reducing the dosage of calcineurin inhibitor and stopping anti-metabolic drugs can be considered in the patients with severe or rapidly progressing COVID-19.
The novel infectious disease, COVID-19, is known to mainly present with fever, dry cough, and in some cases GI symptoms. Liver injury is observed in all types of COVID-19, especially in severe and critical types, which may be related to systemic inflammatory response, immune damage, ischemia-reperfusion injury, viral direct damage, drug induce, mechanical ventilation, and underlying diseases. Therefore, more attention should be paid to the monitoring of liver function in the management of COVID-19. However, until now, the mechanism of liver injury in COVID-19 has not been elucidated. Therefore, further studies need to be carried out. Diagnostic criteria, pathogenesis, clinical characteristics, treatment, and prognosis of liver injury in COVID-19 should be clarified in further clinical trials[40]. Finally, while facing this new unknown infectious disease, the importance of prevention by wearing masks, washing hands, and staying at home needs to be emphasized for the public.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Milovanovic T, Rossi RE S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
| 1. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8473] [Cited by in RCA: 7582] [Article Influence: 1516.4] [Reference Citation Analysis (0)] |
| 2. | Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6893] [Cited by in RCA: 7472] [Article Influence: 1494.4] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance, 28 January 2020. Available from: https://apps.who.int/iris/handle/10665/330893. |
| 4. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30057] [Article Influence: 6011.4] [Reference Citation Analysis (3)] |
| 5. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18844] [Article Influence: 3768.8] [Reference Citation Analysis (7)] |
| 6. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12962] [Article Influence: 2592.4] [Reference Citation Analysis (1)] |
| 7. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14751] [Article Influence: 2950.2] [Reference Citation Analysis (0)] |
| 8. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 868] [Article Influence: 173.6] [Reference Citation Analysis (0)] |
| 9. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 574] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 10. | Yao N, Wang SN, Lian JQ, Sun YT, Zhang GF, Kang WZ, Kang W. [Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 11. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 12. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18175] [Article Influence: 3635.0] [Reference Citation Analysis (0)] |
| 13. | Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 324] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 14. | Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, Huang X, Lv J, Luo Y, Shen L, Yang H, Huang G, Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 643] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 15. | Ma K, Chen T, Han MF, Guo W, Ning Q. [Management and clinical thinking of Coronavirus Disease 2019]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:E002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 16. | Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, Xu W, Zhang C, Yu J, Jiang B, Cao H, Li L. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. 2020;71:706-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 413] [Article Influence: 82.6] [Reference Citation Analysis (1)] |
| 17. | Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, Xia S, Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 771] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 18. | Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, Liu ZS. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 19. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5774] [Article Influence: 1154.8] [Reference Citation Analysis (2)] |
| 20. | Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 461] [Reference Citation Analysis (0)] |
| 21. | Hu LL, Wang WJ, Zhu QJ, Yang L. [Novel coronavirus pneumonia-related liver injury: etiological analysis and treatment strategy]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 22. | Yang L, Wang W, Wang X, Zhao J, Xiao L, Gui W, Fan H, Xia J, Li Z, Yan J, Alasbahi A, Zhu Q, Hou X. Creg in Hepatocytes Ameliorates Liver Ischemia/Reperfusion Injury in a TAK1-Dependent Manner in Mice. Hepatology. 2019;69:294-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Zhang XJ, Cheng X, Yan ZZ, Fang J, Wang X, Wang W, Liu ZY, Shen LJ, Zhang P, Wang PX, Liao R, Ji YX, Wang JY, Tian S, Zhu XY, Zhang Y, Tian RF, Wang L, Ma XL, Huang Z, She ZG, Li H. An ALOX12-12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nat Med. 2018;24:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 24. | Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 25. | Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-1 coronavirus receptor2 ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020;. [DOI] [Full Text] |
| 26. | Chai XQ, Hu LF, Zhang Y, Han WY, Lu Z, Ke AW, Zhou J, Shi GM, Fang N, Fan J, Cai JB, Fan J, Lan F. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv. 2020;. [DOI] [Full Text] |
| 27. | Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, Zhang T, Chen XM, Lu FM. [Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 28. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1293] [Article Influence: 258.6] [Reference Citation Analysis (4)] |
| 29. | National Health Commission of the People's Republic of China. Diagnosis and Treatment of Coronavirus disease 2019 (Trial Version 7). Accessed March 6, 2020. Available from: http://www.nhc.gov.cn/yzygj/s7652m/202003/a31191442e29474b98bfed5579d5af95.shtml. |
| 30. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 31. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 32. | Rismanbaf A, Zarei S. Liver and Kidney Injuries in COVID-19 and Their Effects on Drug Therapy; a Letter to Editor. Arch Acad Emerg Med. 2020;8:e17. [PubMed] |
| 33. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 34. | APASL. Covid-19 Task Force; George Lau, Manoj Sharma. Clinical Practice Guidance for Hepatology and Liver Transplant Providers during the COVID-19 Pandemic: APASL Expert Panel Consensus Recommendations. Hepatol Int. 2020;1-14. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1712] [Cited by in RCA: 1698] [Article Influence: 339.6] [Reference Citation Analysis (0)] |
| 36. | D'Antiga L. Coronaviruses and Immunosuppressed Patients: The Facts during the Third Epidemic. Liver Transpl. 2020;26:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 491] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 37. | Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 38. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 39. | Liu H, He X, Wang Y, Zhou S, Zhang D, Zhu J, He Q, Zhu Z, Li G, Sun L, Wang J, Cheng G, Liu Z, Lau G. Management of COVID-19 in patients after liver transplantation: Beijing working party for liver transplantation. Hepatol Int. 2020;14:432-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 40. | Chinese Digestion Association; Chinese Medical Doctor Association; Chinese Society of Hepatology; Chinese Medical Association. [The protocol for prevention, diagnosis and treatment of liver injury in coronavirus disease 2019]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |