Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3911
Peer-review started: April 3, 2020
First decision: April 24, 2020
Revised: May 4, 2020
Accepted: July 15, 2020
Article in press: July 15, 2020
Published online: September 6, 2020
Processing time: 154 Days and 2.8 Hours
Mixed infection of hepatic cystic and alveolar echinococcosis is extremely rare. This article reveals the typical imaging manifestations of cystic and alveolar echinococcosis and investigates the diagnosis and surgical experience of mixed infection of hepatic cystic and alveolar echinococcosis.
From January 2017 to May 2019, 4 cases with rare mixed infection of hepatic cystic and alveolar echinococcosis were admitted and treated by the Division of General Surgery of Qinghai Provincial People's Hospital. Three of the patients occasionally had upper abdominal discomfort, but it did not affect their daily lives. However, hepatic echinococcosis was found in one patient by physical examination, and the patient had no discomfort. All 4 cases were Tibetans who had lived in pastoral areas of southern Qinghai for a long time. Enzyme-linked immunosorbent assay for echinococcosis was positive for all patients. Moreover, abdominal computed tomography showed typical imaging manifestations of cystic and alveolar echinococcosis including coexisting “honeycomb sign,” and “spotted calcification.” Three of the patients underwent radical resection, and 1 case underwent palliative resection. All 4 patients developed different types of surgical complications after the operation, but all of them recovered and were discharged after symptomatic treatment.
There are no problems diagnosing mixed infection of hepatic cystic and alveolar echinococcosis. The difficulties involve preoperative evaluation and treatment of surgical complications.
Core tip: Mixed infection of hepatic cystic and alveolar echinococcosis is extremely rare. This article reveals the typical imaging manifestations of cystic and alveolar echinococcosis including coexisting “honeycomb sign” and “spotted calcification.”
- Citation: A JD, Chai JP, Wang H, Gao W, Peng Z, Zhao SY, A XR. Diagnosis and treatment of mixed infection of hepatic cystic and alveolar echinococcosis: Four case reports. World J Clin Cases 2020; 8(17): 3911-3919
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3911.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3911
Echinococcosis is a zoonotic parasitic disease caused by larvae of echinococcus and is mainly caused by the following five types of echinococcus: Echinococcosis granulosus, E. multilocularis, E. oligarthrus, E. vogli, and E. shqucus[1]. Cystic echinococcosis caused by E. granulosus is distributed globally. Alveolar echinococcosis caused by E. multilocularis is only distributed in the northern hemisphere. The two populations are the main causes of human echinococcosis[2]. In China, echinococcosis is usually cystic or alveolar. Cystic echinococcosis accounts for approximately 95% of all echinococcosis, while hepatic cystic echinococcosis accounts for approximately 75% of all cystic echinococcosis[3]. To date, there are numerous reports about single infections of cystic or alveolar echinococcosis in different organs[4,5], but there reports about mixed infections of cystic and alveolar echinococcosis in the same organ are rare. In this study, a typical case was chosen from the 4 patients with mixed infection of hepatic cystic and alveolar echinococcosis who were treated in the Division of General Surgery of Qinghai Provincial People's Hospital. The cases were reported in detail as follows.
The typical case was a 56-year-old Tibetan man who was admitted to our hospital on March 5, 2019, due to “swelling and pain in the right upper abdomen for over 2 years, and yellow skin for 2 mo.” The other 3 cases were all Tibetan women, and their ages were 26-, 42- and 49-years-old. They were admitted to our hospital due to “right upper abdominal distension and pain discontinuity for more than 1 year,” “right upper abdominal distension and pain discontinuity for more than 2 years,” and “physical examination found hepatic hydatidosis for more than 10 years.”
The 4 patients all lived in a pastoral area in southern Qinghai Province for a long time, and they had a history of close contact with cattle, sheep, and dogs.
The patient with a typical case suffered from severe yellow skin and yellow sclera, and the palpebral conjunctiva was not pale. The abdomen of the patient was flat, there was tenderness and rebound tenderness of the whole abdomen, muscular tension was positive, there was no abnormal mass by touching, no swelling was appreciated in the liver and spleen, and shifting dullness was negative. However, no positive physical signs were found in the other 3 patients.
Enzyme-linked immunosorbent assay for echinococcosis was positive for all patients. Indicators of liver functions in the typical case suggested that the synthesis and reserve functions of the liver were severely impaired. The specific data were as follows: alanine transaminase was 227 U/L (normal range, 7 to 45 U/L), aspartate aminotransferase was 242 U/L (normal range, 13 to 40 U/L), total bilirubin was 379.6 μmol/L (normal range, 5.0 to 21.0 μmol/L), direct bilirubin was 199.5 μmol/L (normal range, 0 to 3.4 μmol/L), indirect bilirubin was 180.1 μmol/L (normal range, 1.3 to 18.7 μmol/L), albumin was 25.2 g/L (normal range, 40 to 55 g/L), and serum cholinesterase was 2920 U/L (normal range, 5300 to 11300 U/L). The indicators of liver functions in the other 3 cases were normal.
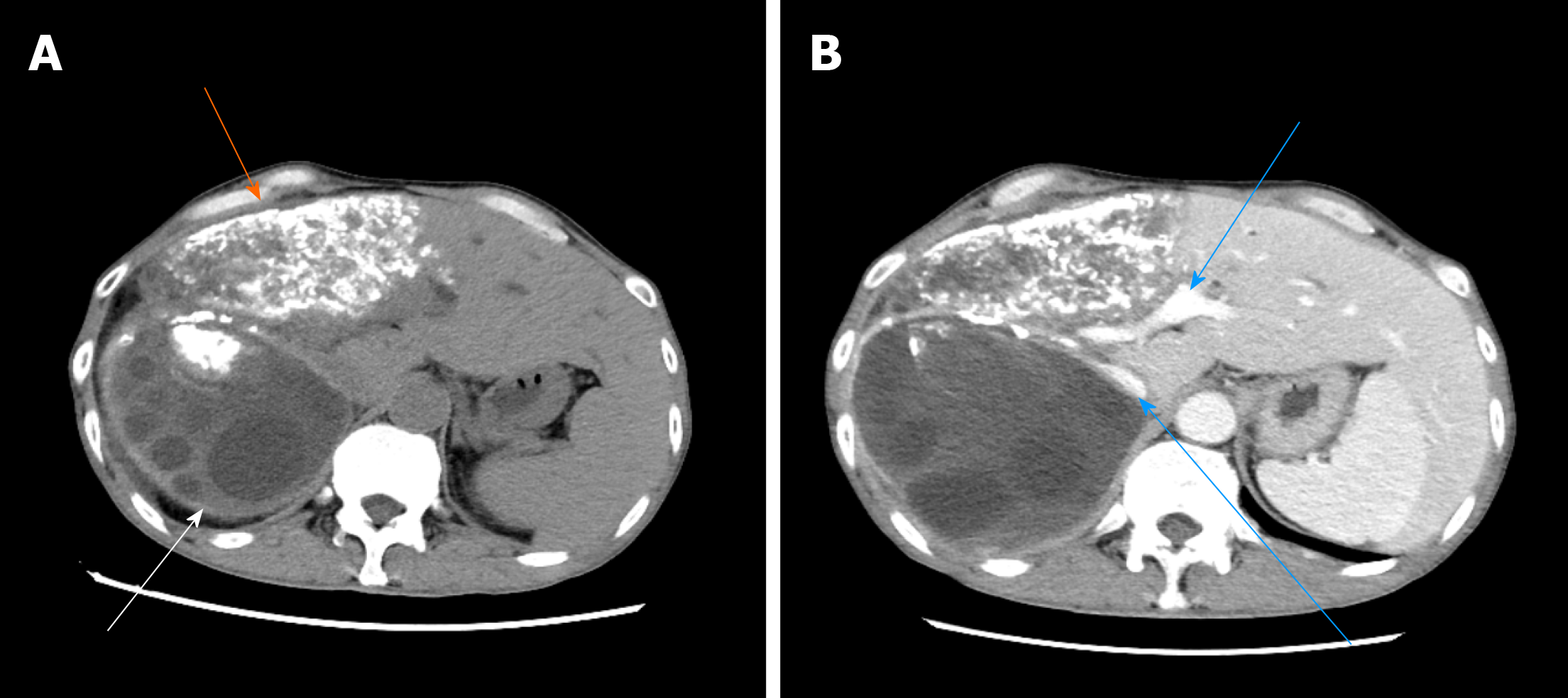
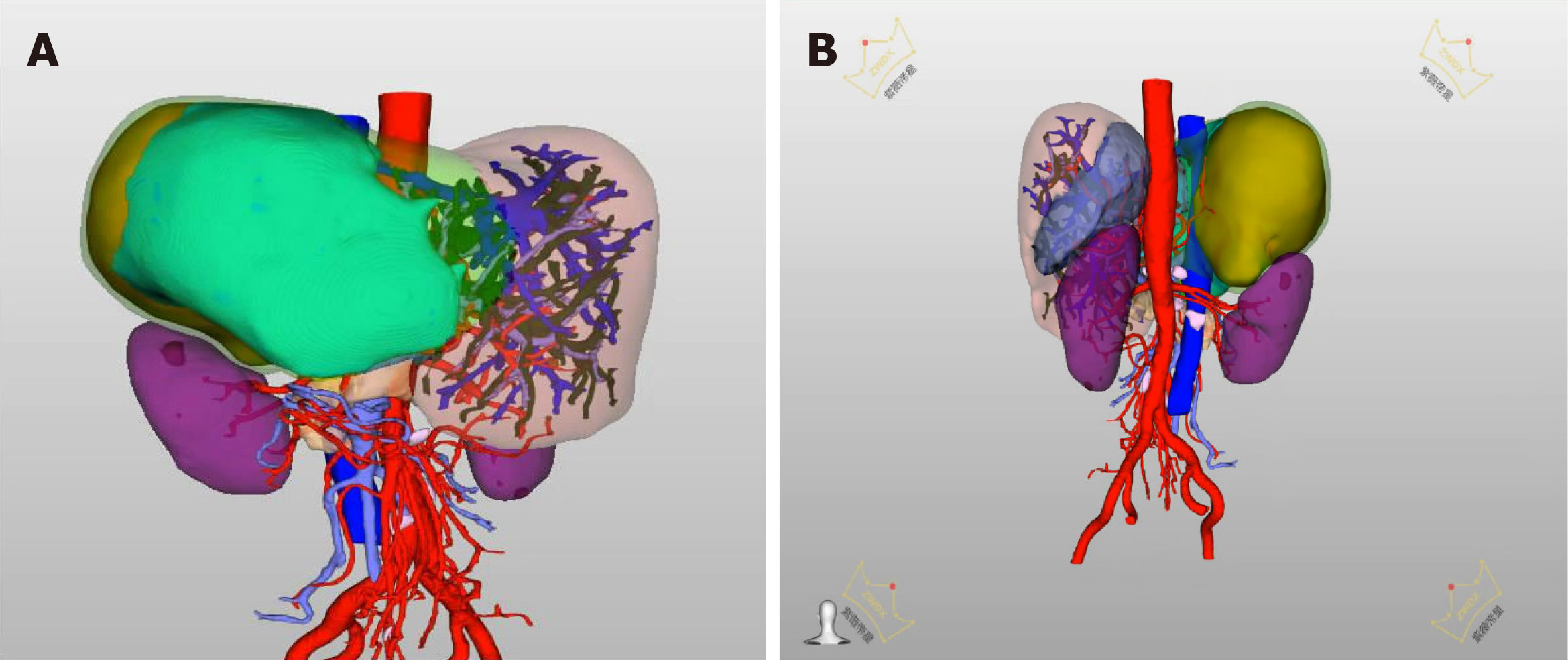
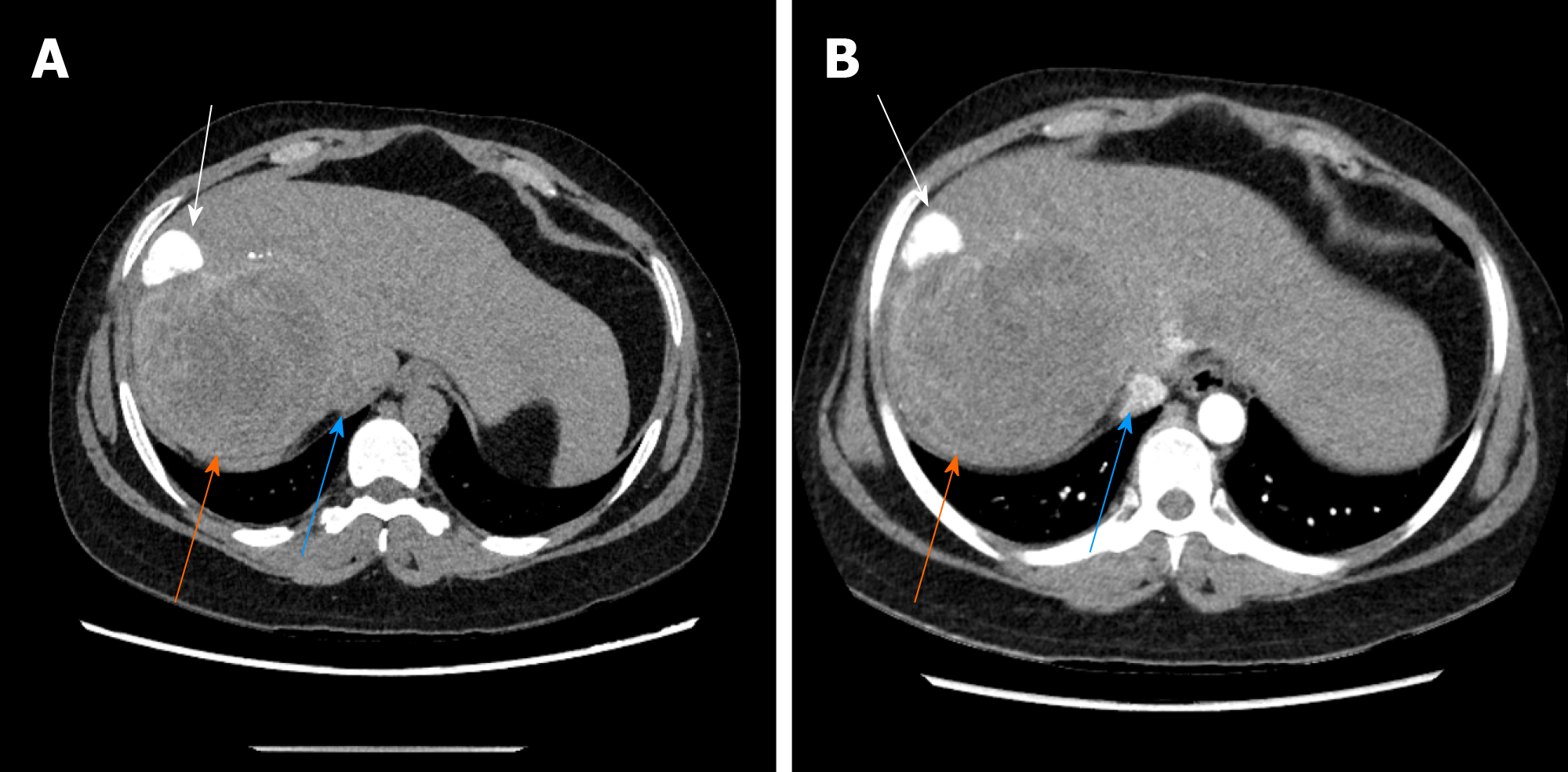
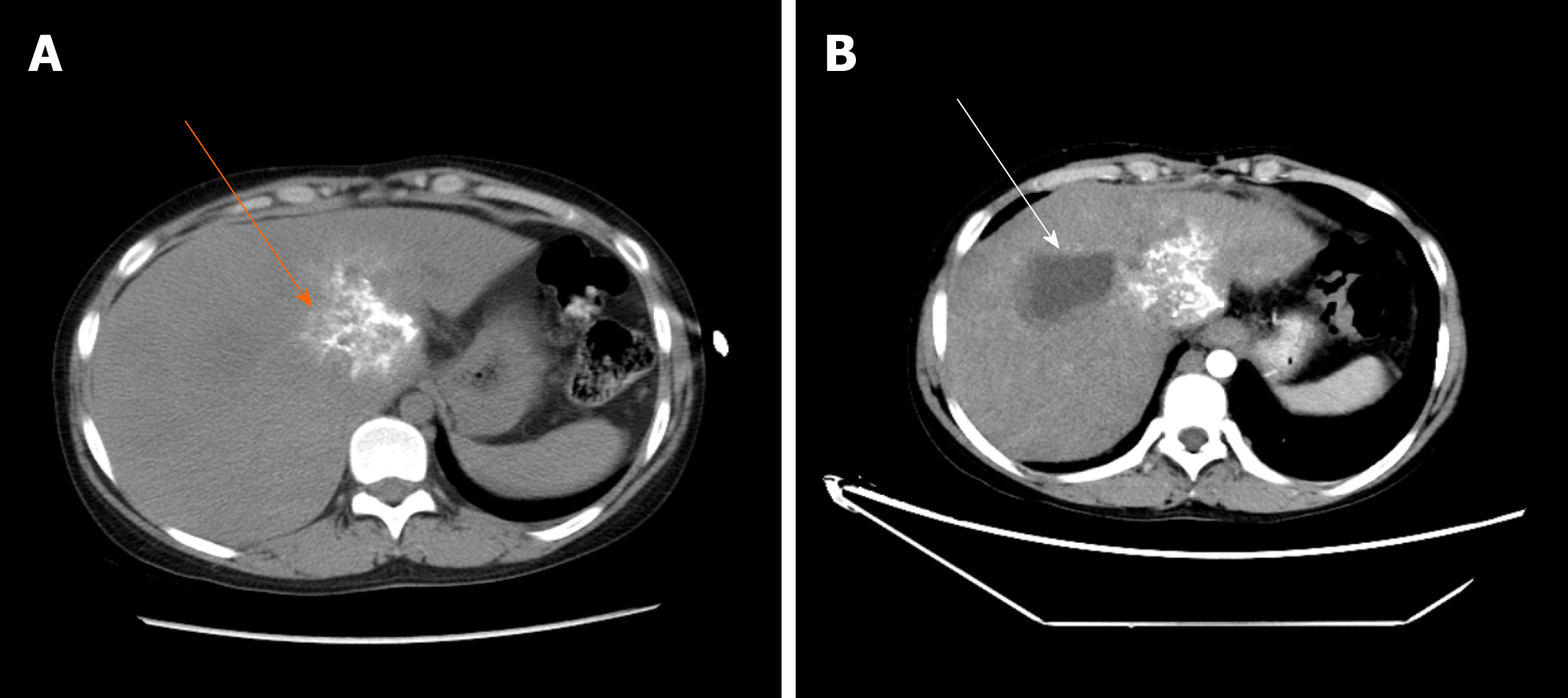
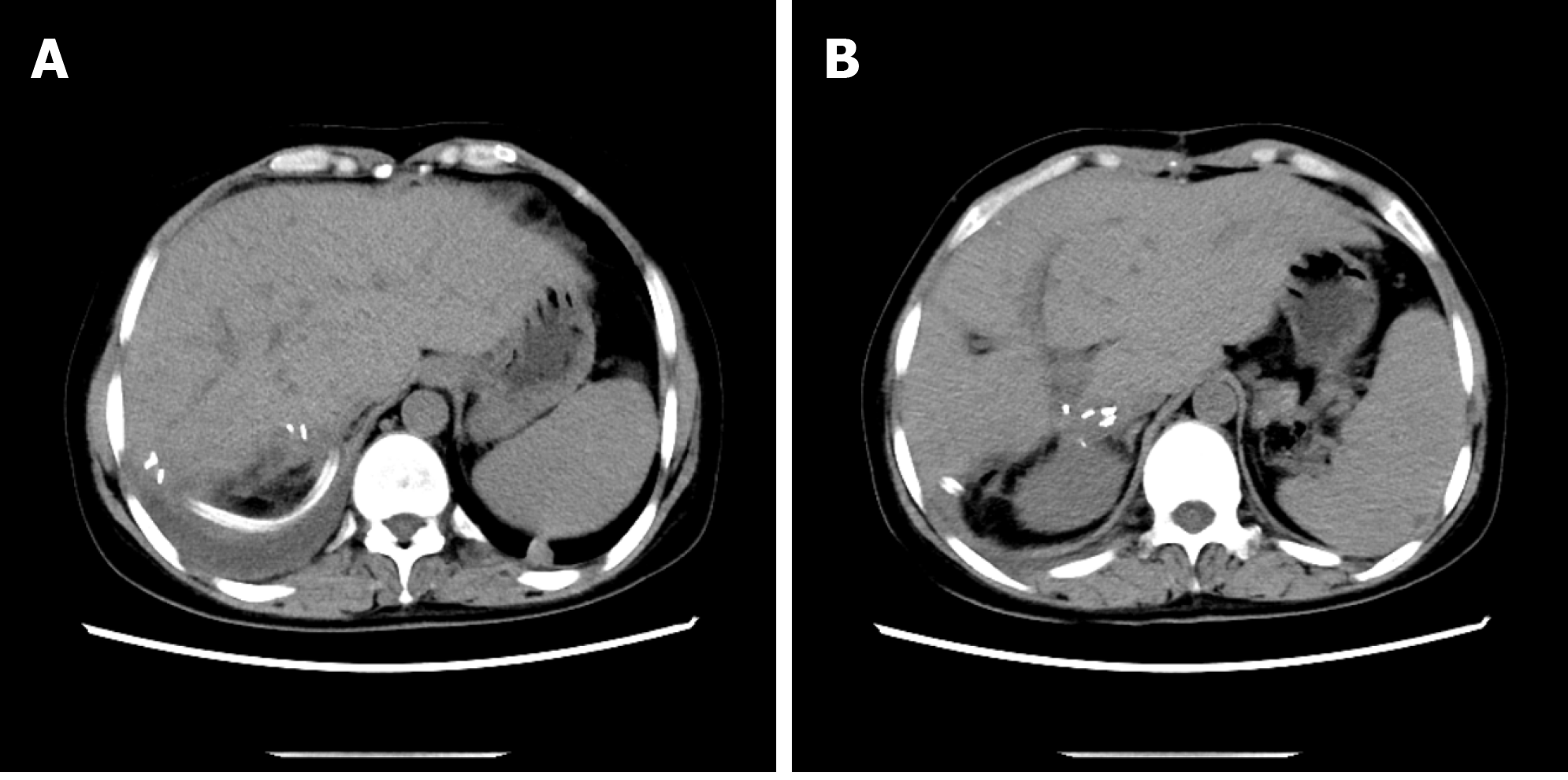
The typical case of abdominal enhanced CT (Figure 1) and preoperative three-dimensional reconstruction (Figure 2) showed that there were multiple lesions of echinococcosis in the liver, approximately 13.2 cm × 7.0 cm in size in the S4 segment and approximately 13.9 cm × 12.2 cm in size in the S5-8 segments. The other 3 cases of abdominal enhancement CT are shown in Figure 3, Figure 4, and Figure 5.
Preoperative assessment of the typical case showed Child-Pugh classification of liver functions of grade C, PNM standard classification[6] of P4N1M0, and PIVM classification[7] of PI, IV-VIII I1V1M1. The preoperative assessments of the other 3 showed Child-Pugh classifications of liver functions that were respectively grade B, grade A, and grade A; PNM standard classifications that were respectively P4N1M0, P3N0M0, and P3N0M0; and PIVM classifications that were respectively PI, VI, VIII I1V1M1, PV-VIII I1V1M1, and PV-VIII I1V1M1.
The final diagnoses of the 4 cases were mixed infection of hepatic cystic and alveolar echinococcosis, but the supplementary diagnoses of the typical case were obstructive jaundice, liver function impairment, and hypoproteinemia.
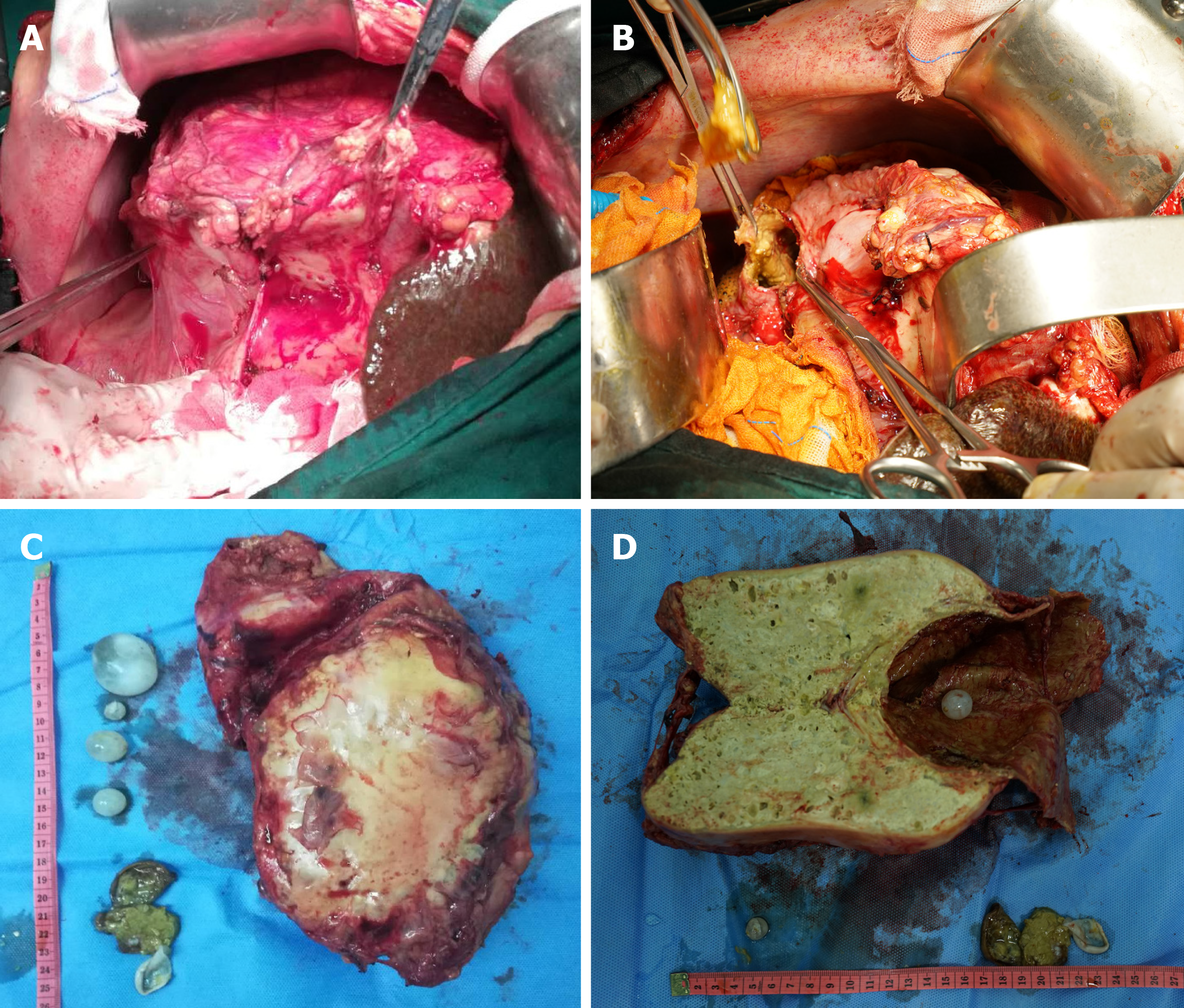
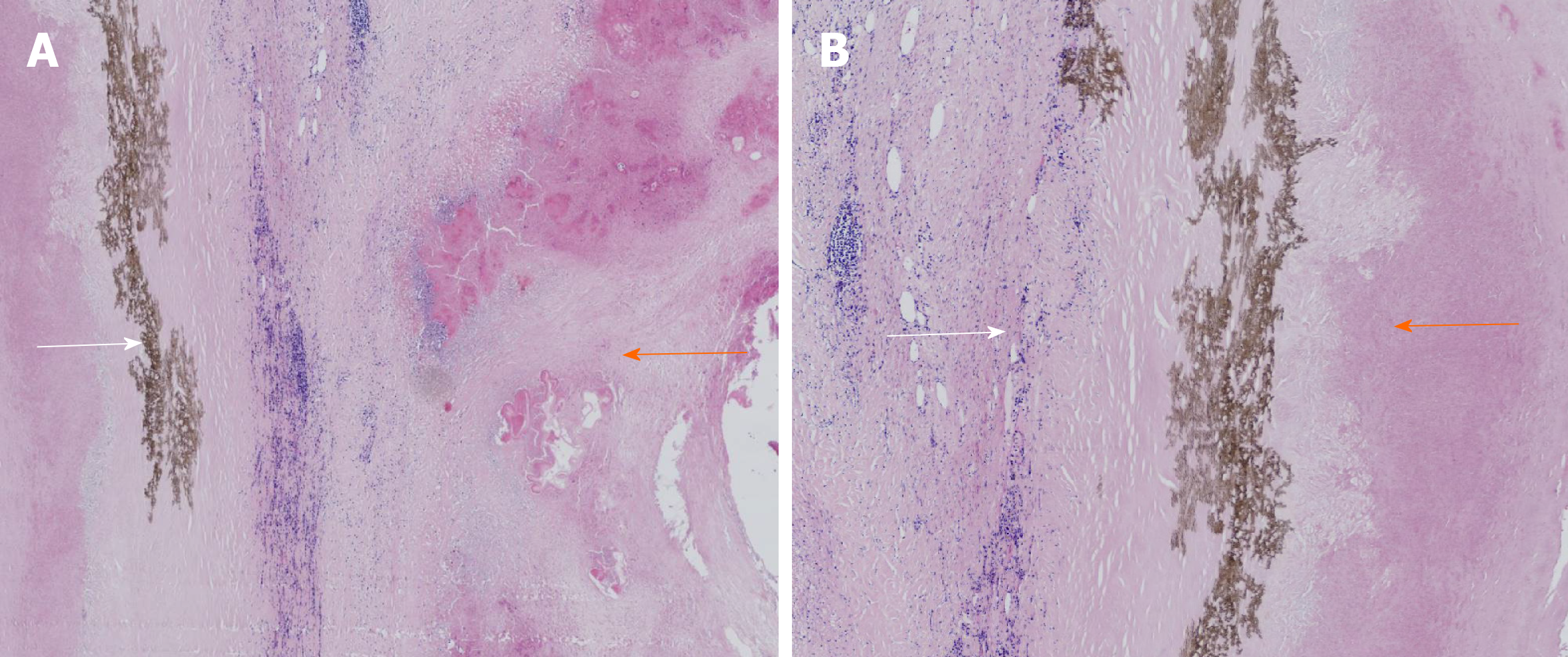
The typical case had clear surgical contraindications; thus, the complications of hepatic echinococcosis were treated first. On March 12, 2019, the patient underwent percutaneous transhepatic cholangial drainage guided by ultrasound for biliary drainage. Preoperative assessment was performed once again on the 26th day after admission, and the results showed that cardiopulmonary functions were normal, and the Child-Pugh classification was grade B. We decided to perform surgical resection after preoperative discussion. On April 8, 2019, extended right hepatectomy was performed (Figure 6). The surgery took approximately 11 h. Intraoperative bleeding was approximately 3000 mL, 1600 mL suspended red blood cells and 740 mL plasma were infused, and the hepatic portal was blocked for 55 min during the operation using the Pringle maneuver. After the operation, the patient was transferred to the intensive care unit for life support, and the next day, was transferred to a specialized ward for rehabilitation treatment. Postoperative pathology showed that the patients suffered from hepatic cystic and alveolar echinococcosis (Figure 7). However, 1 of the other 3 cases showed hepatic hydatid lesions invading the blood vessels of the second hepatic hilum after entering the abdomen, and the patient could not undergo routine radical resection of the lesions. The liver was enlarged and heavily congested. Considering the establishment of collateral circulation, it was decided that only hepatic cystic hydatid cystectomy should be performed. The other 2 patients underwent radical excision. After the operation, drainage of abdominal abscesses guided by ultrasound was performed, antibiotic was replaced by vancomycin, and fresh plasma was infused. On the postoperative 30th day, all drainage tubes were removed, and the patient was discharged after recovery. The patients started to take oral albendazole after discharge, with the daily dose of 15 mg/kg. The medicine was discontinued after 2-3 wk, and indicators of liver functions were tested. If indicators of liver functions were normal, then the medicine was continued after 2-3 d, but if the liver damage was severe, it was recommended to start hepatoprotective therapy after discontinuing the medicine. The next cycle of drug-assisted therapy continued after all indicators of liver functions had recovered to normal levels. The first review was performed once every 6 mo, and abdominal color Doppler ultrasound or abnormal CT was repeated once 1 year later and every year thereafter.
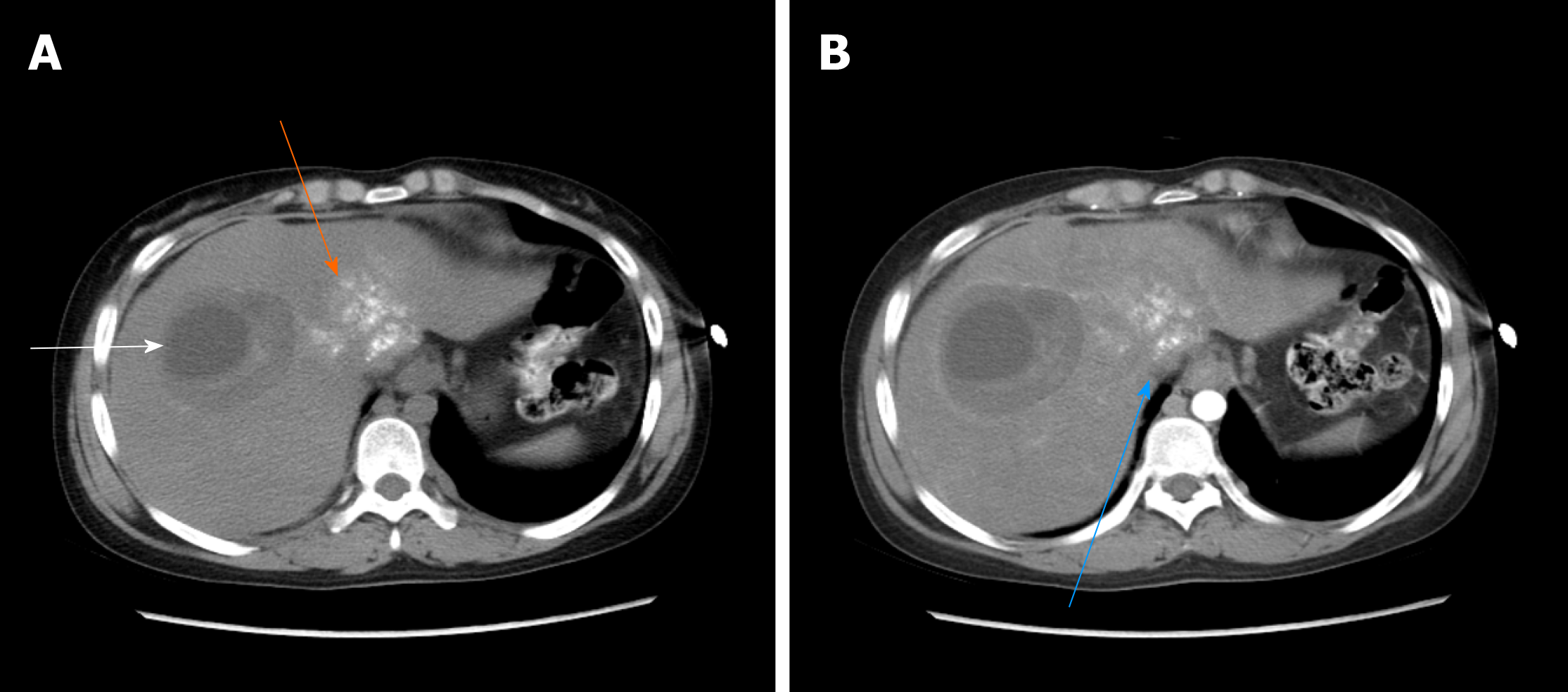
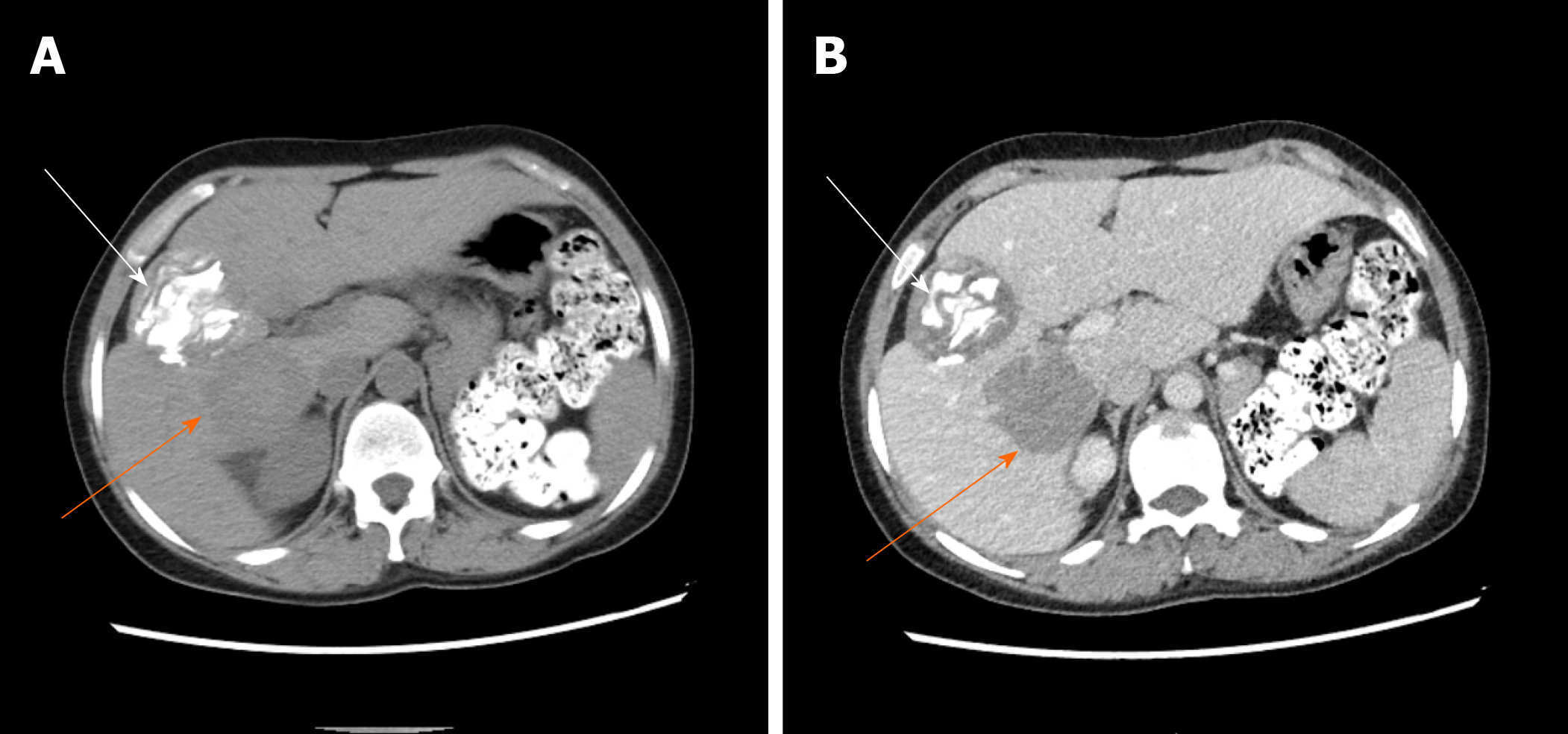
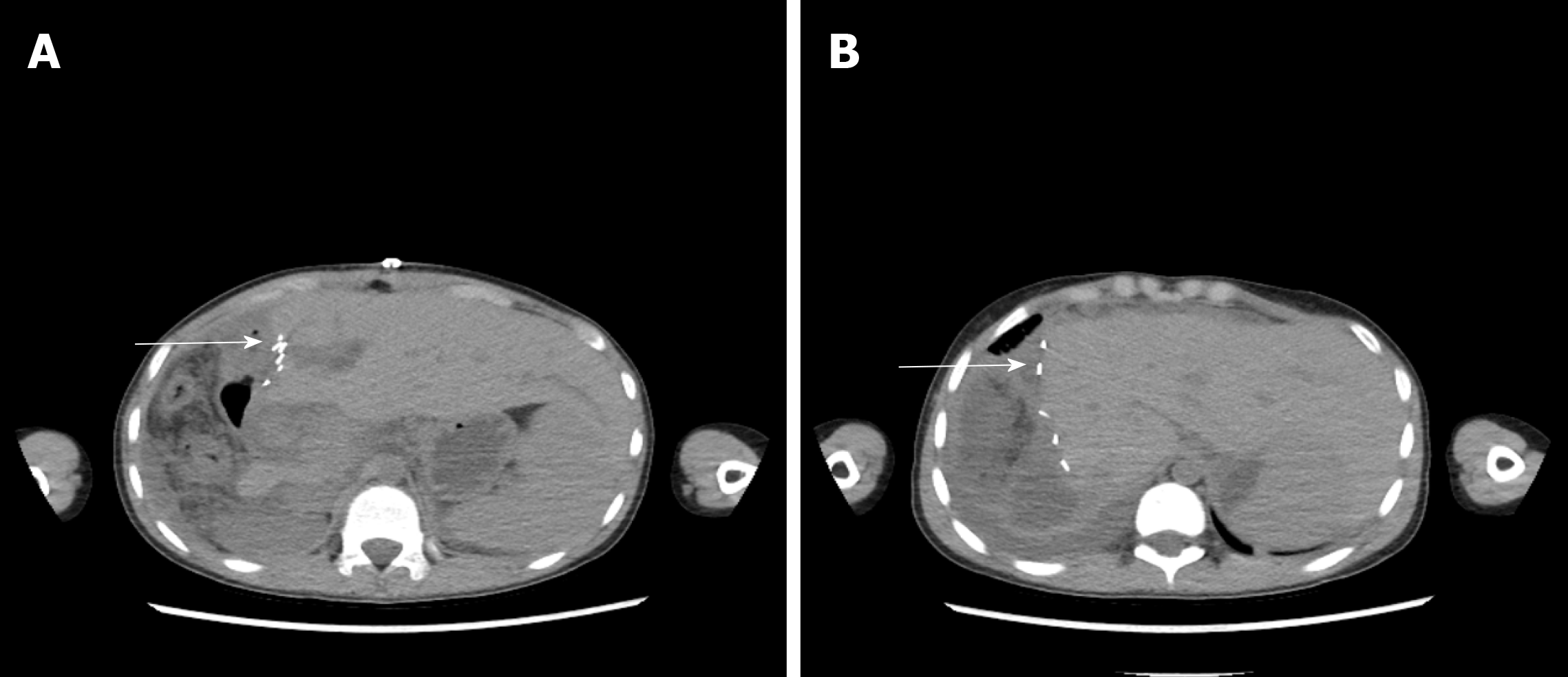
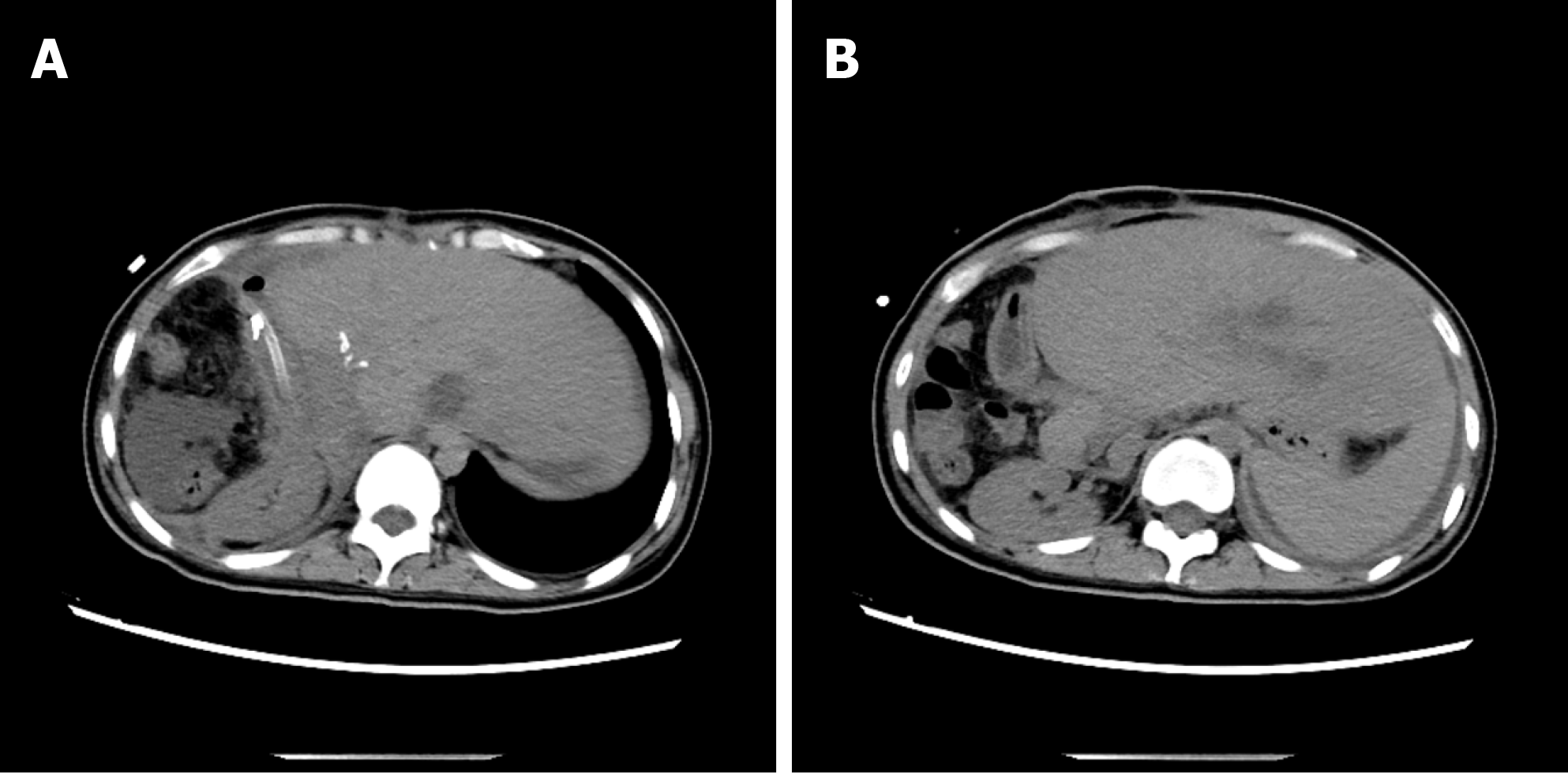
The 4 patients had different types of postoperative complications such as septic shock, hemorrhagic shock, gastrointestinal hemorrhage, abdominal infection, abdominal abscess, postoperative biliary fistula, hepatic insufficiency, pulmonary infection, pleural effusion, and hypoproteinemia. After symptomatic treatment, such as abdominal drainage, adjustment of antibiotic use and diuresis, all patients recovered or were discharged. For the typical case, almost all of these complications occurred, and after the operation, drainage of abdominal abscesses guided by ultrasound was performed, antibiotic was replaced by vancomycin, and fresh plasma was infused. The patient was discharged after complete recovery on the 30th day after treatments, such as drainage of abdominal abscess guided by ultrasound, replacement of advanced antibiotics (meropenem) and enhanced intravenous nutrition. At the follow-up visit. The typical case patient’s follow-up was conducted 2 mo after discharge, and review of the abdominal CT showed that there was residual liver compensation and hyperplasia, with no recurrence of echinococcosis (Figure 8). Moreover, this patient reported that he had good recovery, he could take care of himself, and there was no discomfort such as abdominal pain. The follow-up is still in progress. The other 3 patients were followed up for a period of 1 mo to 1.5 years. One of the 3 patients underwent hepatic hydatid lesion resection again at 1.5 years after operation because she did not have a radical resection (Figure 9). The prognoses of the remaining 2 patients were good. CT examination of the abdomen showed (Figures 10 and 11) compensatory and proliferative remnants in the liver and no recurrence of hydatid cysts.
Mixed infection of hepatic cystic and alveolar echinococcosis is extremely rare, but based on the four aspects, including major symptoms, living history in the pastoral area, manifestations of abdominal CT and enzyme-linked immunosorbent assay evidence of echinococcosis[8,9], there were essentially no problems in diagnosing this disease. The difficult problems occurred during surgical treatment of this disease, including during the preoperative evaluation and treatment of surgical compli-cations[10,11]. First, preoperative evaluation is the key of the whole surgery, as it could determine the involved vessels and bile ducts, as well as prediction of the resectability of the lesion. For instance, for 1 of the other 3 patients in this study, even if a systemic evaluation of the lesion from multiple aspects and multiple perspectives was performed before the operation, the fact that the echinococcosis had invaded the second hepatic hilum so severely was not found. Furthermore, we also had not observed carefully that the three haptic venous outflows were completely obstructed, hepatic congestion was severe, collateral circulation was rich, and there was spongy tortuosity in the portal veins. In addition, radical resection of the lesion was difficult, and the intraoperative bleeding was heavy. We finally decided that only hepatic cystic hydatid cystectomy should be performed. It has been reported that[12] even for patients with echinococcosis invading the vessels and bile ducts and who were at an advanced stage, radical resection of the lesions and biliary duct reconstruction should be performed if there are no heart and pulmonary dysfunctions or other contraindications. However, it was found in our clinical practice that if radical resection or reconstruction of the outflow tracts could not be ensured for these patients, and perihepatic ligaments containing an abundance of collateral vessels were separated, then the compensatory mechanism of the human body was broken, thus leading to severe postoperative complications, such as intractable ascites and gastrointestinal bleeding. Therefore, routine hepatectomy is difficult for patients with severe hepatic congestion, and allogeneic liver transplantation may be the only choice. Second, there were numerous problems in treating postoperative complications. For instance, our typical case had many complications after the operation, such as biliary leakage, abdominal infection, massive ascites and hepatic insufficiency. Moreover, the case also developed severe complications, such as massive gastrointestinal hemorrhage, hemorrhagic shock and septic shock on the 7th day after the operation. The causes for these complications may include the following: (1) The physique of the patient was poor, and his surgical tolerance was poor; (2) The patient was combined with complications such as obstructive jaundice, liver damage and hypoproteinemia before the surgery, and the first-time Child-Pugh liver function grade was C, which was improved to grade B after the surgery; (3) The surgical method was extended right hepatectomy, which had a large range in radical resection, a small volume of the residual liver, and severe impairment of the synthesis function of the liver after the surgery; and (4) The patient had capillary biliary fistula of the hepatic section. Finally, such cases should be followed in centers with hepatobiliary surgery experience.
In summary, diagnosis of hepatic cystic and alveolar echinococcosis is easy; the difficulties include preoperative evaluation and treatment of surgical complications, especially the precise prediction of hepatic congestion or outflow tract obstruction. Some dilemmas in the surgery may be avoided if surgeons can thoroughly complete the above two points. In this study, the sample size was small, and there was a lack of multicenter communication. Therefore, if there are similar cases in the future, we should expand the sample size to follow up the long-term efficacies of the patients and strengthen the multicenter communication and data sharing.
Patients in this study were willing to share their medical data, and they tried their best to provide informed consent.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Xing YX
| 1. | Can H, İnceboz T, Caner A, Atalay Şahar E, Karakavuk M, Döşkaya M, Çelebi F, Değirmenci Döşkaya A, Gülçe İz S, Gürüz Y, Korkmaz M. [Detection of Echinococcus granulosus and Echinococcus multilocularis in cyst samples using a novel single tube multiplex real-time polymerase chain reaction]. Mikrobiyol Bul. 2016;50:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, Rokni MB, Zhou XN, Fèvre EM, Sripa B, Gargouri N, Fürst T, Budke CM, Carabin H, Kirk MD, Angulo FJ, Havelaar A, de Silva N. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12:e1001920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 491] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 3. | Gandhiraman K, Balakrishnan R, Ramamoorthy R, Rajeshwari R. Primary Peritoneal Hydatid Cyst Presenting as Ovarian Cyst Torsion: A Rare Case Report. J Clin Diagn Res. 2015;9:QD07-QD08. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Gavriilidis P, Ananiadis A, Theodoulidis V, Barbanis S. Primary retroperitoneal echinococcal cyst. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G, Magambo J, Thompson RC, Jenkins EJ. Global Distribution of Alveolar and Cystic Echinococcosis. Adv Parasitol. 2017;95:315-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 6. | McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 717] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 7. | Chinese College of Surgeons of the Chinese Medical Doctor Association. Expert consensus on diagnosis and treatment of hepatic echinococcosis (2015 edition). Zhonghua Xiaohua Waike Zazhi. 2015;14:253-264. [DOI] [Full Text] |
| 8. | Jerraya H, Khalfallah M, Osman SB, Nouira R, Dziri C. Predictive factors of recurrence after surgical treatment for liver hydatid cyst. Surg Endosc. 2015;29:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Rossi P, Tamarozzi F, Galati F, Pozio E, Akhan O, Cretu CM, Vutova K, Siles-Lucas M, Brunetti E, Casulli A; HERACLES extended network. The first meeting of the European Register of Cystic Echinococcosis (ERCE). Parasit Vectors. 2016;9:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Akbulut S, Cicek E, Kolu M, Sahin TT, Yilmaz S. Associating liver partition and portal vein ligation for staged hepatectomy for extensive alveolar echinococcosis: First case report in the literature. World J Gastrointest Surg. 2018;10:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Akbulut S. Parietal complication of the hydatid disease: Comprehensive literature review. Medicine (Baltimore). 2018;97:e10671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Joliat GR, Melloul E, Petermann D, Demartines N, Gillet M, Uldry E, Halkic N. Outcomes After Liver Resection for Hepatic Alveolar Echinococcosis: A Single-Center Cohort Study. World J Surg. 2015;39:2529-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |