Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3774
Peer-review started: April 10, 2020
First decision: June 8, 2020
Revised: June 15, 2020
Accepted: August 4, 2020
Article in press: August 4, 2020
Published online: September 6, 2020
Processing time: 146 Days and 23.8 Hours
Phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 (P-Rex1) was reported to be a risk factor in several cancers, including breast cancer, lung cancer, and melanoma, but its expression and role in hepatocellular carcinoma (HCC) have not yet been fully studied.
To explore the expression of P-Rex1 in HCC, and further evaluate its potential application in the diagnosis and prognosis of HCC, especially in hepatitis B virus (HBV)-related patients.
P-Rex1 expression in HCC was evaluated by real-time-quantitative polymerase chain reaction. The expression of P-Rex1 was subjected to correlation analysis with clinical features, such as lymph node invasion, distant metastasis, HBV infection, patient's age and gender. Receiver operating characteristic analysis was used to examine the potential role of P-Rex1 as a diagnostic biomarker in HCC. Kaplan-Meier analysis was used to determine the association between P-Rex1 expression and overall survival, progression-free survival and relapse-free survival. Bioinformatic analysis was used to validate the clinical findings.
P-Rex1 expression was significantly increased in HCC tumors than adjacent tissues. The expression of P-Rex1 was higher in HCC patients with lymph node invasion, distant metastasis, HBV infection and positive alpha-fetoprotein, respectively. The receiver operating characteristic analysis showed that P-Rex1 was a diagnostic biomarker with a higher area under the curve value, especially in patients with HBV infection. Survival analysis showed that patients with higher P-Rex1 expression had a favorable survival rate, even in early-stage patients.
P-Rex1 expression was highly increased in HCC, and the expression level of P-Rex1 was positively correlated with tumor progression. P-Rex1 has a higher efficiency in the diagnosis of HBV-related HCC, and could also be used as a favorable prognostic factor for HCC patients.
Core tip: This study revealed the overexpression of phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 (P-Rex1) in hepatocellular carcinoma (HCC), and the expression level was closely associated with the development of HCC. P-Rex1 is a new diagnostic biomarker for hepatitis B virus-related HCC patients and could serve as a favorable prognostic biomarker in the clinical prediction of HCC.
- Citation: Cai Y, Zheng Q, Yao DJ. Phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 is a diagnostic and prognostic biomarker for hepatocellular carcinoma. World J Clin Cases 2020; 8(17): 3774-3785
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3774.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3774
Hepatocellular carcinoma (HCC) is a major malignant tumor, with the 5th highest cancer-related mortality[1]. Currently, the treatments for HCC include surgery, ablation, liver transplantation, chemotherapy, targeted therapy and some novel theranostic approaches[2]. According to the Barcelona Clinic Liver Cancer staging, surgical resection, ablation, and liver transplantation are performed in patients at an early stage, and for those patients at an intermediate or advanced stage, palliative treatments are recommended, which include sorafenib targeted therapy and transarterial chemoembolization[2,3]. In addition, some novel treatments have been reported. However, immune checkpoint blocking therapy (pembrolizumab) has no significant impact on the overall survival (OS) or progression-free survival (PFS) compared with chemotherapy[4,5]. Some studies have reported novel theranostics based on the tumor microenvironment, which have a significant effect on liver tumor inhibition, but this was only found in the laboratory research stage[6-8]. Drug resistance is a common challenge in HCC treatment, which contributes to a poor prognosis. Furthermore, the diagnosis of HCC in the clinic is also poor, as due to insignificant early symptoms, many HCC patients are diagnosed at an intermediate or advanced stage, which contributes to fewer opportunities for surgical resection[9,10]. Thus, the study of novel molecular biomarkers or pharmaceutic targets is critical for HCC therapy.
Phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 (P-Rex1) is a Rho guanine nucleotide exchange factor, which activates Rac1 and plays a critical role in neutrophil and macrophage function[11,12]. P-Rex1 was first purified from the cytoplasm of neutrophils, and P-Rex1 deficiency in a mouse model resulted in impaired Rac1 activation, reactive oxygen species formation and chemotaxis[13-15]. In cancer, P-Rex1 was reported to be involved in regulation of the PI3K/AKT, mTOR and MEK/ERK pathways[16,17]. P-Rex1 could interact with Gβγ and PIP3 to mediate the process of Rac-GDP to Rac-GTP in some cancer types[18,19]. Furthermore, P-Rex1 is also involved in the progression of melanoma through the regulation of melanoblasts migration[20-22]. However, its expression and role in HCC remain unclear. This study aimed to examine the expression of P-Rex1 in HCC and analyze the potential association between P-Rex1 and the development of HCC, and further evaluate the potential value of P-Rex1 in the clinical diagnosis and prognosis of HCC.
The total ribonucleic acid (RNA) extraction reagent Trizol was purchased from Thermo Fisher Scientific Co., Ltd (Waltham, United States). The first-strand complementary DNA (cDNA) synthesis kit (with genomic deoxyribonucleic acid digester) and the Hieff quantitative polymerase chain reaction (qPCR) SYBR Green Master Mix (with high Rox) were obtained from Yeasen Technology (Shanghai, China). The specific qPCR primer was synthesized by Sangon Technology (Shanghai, China). The detailed sequence for P-Rex1 is as follows: Forward: 5’- TGG AGT ATT GTT TAC ACC CGGA-3’; Reverse: 5’-CTC GTA CAC GCA GAA CTT GTC-3’.
Ninety resected liver tumor tissues and adjacent normal liver tissues were obtained from the Department of Oncology, Hospital of Chengdu University of Traditional Chinese Medicine. Tissue collection was undertaken during surgery, and the whole resected tissues including tumor and surrounding normal tissues were washed with cold saline solution and then preserved in the refrigerator (-80°C). All patients provided informed consent, and the study was approved by the Ethics Committee of the Hospital of Chengdu University of Traditional Chinese Medicine. Patient information is as follows: Male vs female (69 vs 21), age ≤ 50 vs age > 50 (24 vs 66), hepatitis B virus (HBV) infection positive vs negative (38 vs 52), lymph node invasion positive vs negative (48 vs 42), distant metastasis positive vs negative (24 vs 66), alpha-fetoprotein (AFP) < 20 ng/mL vs 20 ng/mL ≤ AFP ≤ 200 ng/mL vs AFP > 200 ng/mL (16 vs 28 vs 46).
The resected tissues were homogenized and then lysed with Trizol for 30 min at room temperature. The supernatant was collected and treated with isopropyl alcohol. Total RNA was washed with 75% ethanol and then used as a template to synthesize the first-strand cDNA according to the first-strand cDNA synthesis kit manual. The acquired cDNA was amplified with the Hieff qPCR SYBR kit as previously reported[23]. High Rox was used as an internal control.
The messenger RNA expression of P-Rex1 in liver tumor and adjacent normal liver tissues was validated with the cancer genome atlas (TCGA, https://http://www.cancer.gov/tcga). The protein expression in liver tumor and normal liver tissues was obtained from the human protein atlas as reported previously[24,25].
Receiver operating characteristic (ROC) analysis was used to confirm the diagnostic value of P-Rex1 in liver cancer. The adjacent normal liver was used as the control group, and the corresponding tumor tissues were set as the disease group. The area under the curve (AUC) value was used to assess the diagnostic value of the indicated marker.
Survival analysis (including OS, PFS, and relapse-free survival (RFS)) was conducted with the Kaplan-Meier plotter. The median expression level of P-Rex1 was applied as the cut-off of the high or low expression patient group.
Statistical difference was determined by Graphpad Prism 8.0, and the difference between the two groups was calculated with the Student’s t-test. Pearson analysis was used to assess the correlation between AFP and P-Rex1. The survival analysis was conducted with the logrank P method.
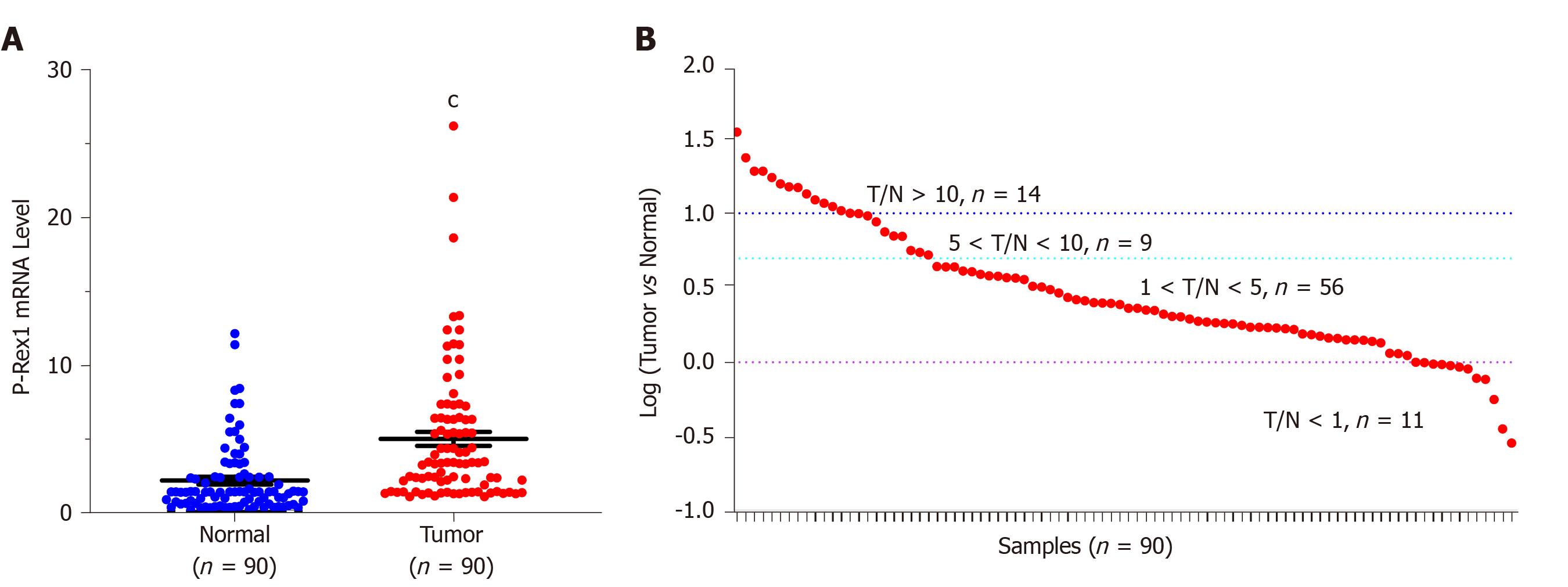
To evaluate the potential role of P-Rex1 in HCC, we firstly examined the expression of P-Rex1 using the real-time quantitative PCR assay in 90 resected HCC tumor tissues and corresponding adjacent normal liver tissues. As shown in Figure 1A, the expression level of P-Rex1 was significantly upregulated in liver tumor tissues. The details of the expression difference of P-Rex1 in liver tumor and normal tissues are shown in Figure 1B, the T/N ratio > 5 was considered statistically different (23/90, 25.6%), and the number of patients with a ratio between 1 and 5 was 56 cases (62.2%), with only 11 patients showing lower expression in tumor tissues than in adjacent tissues (12.2%). These results demonstrated that P-Rex1 was frequently overexpressed in liver tumor tissues.
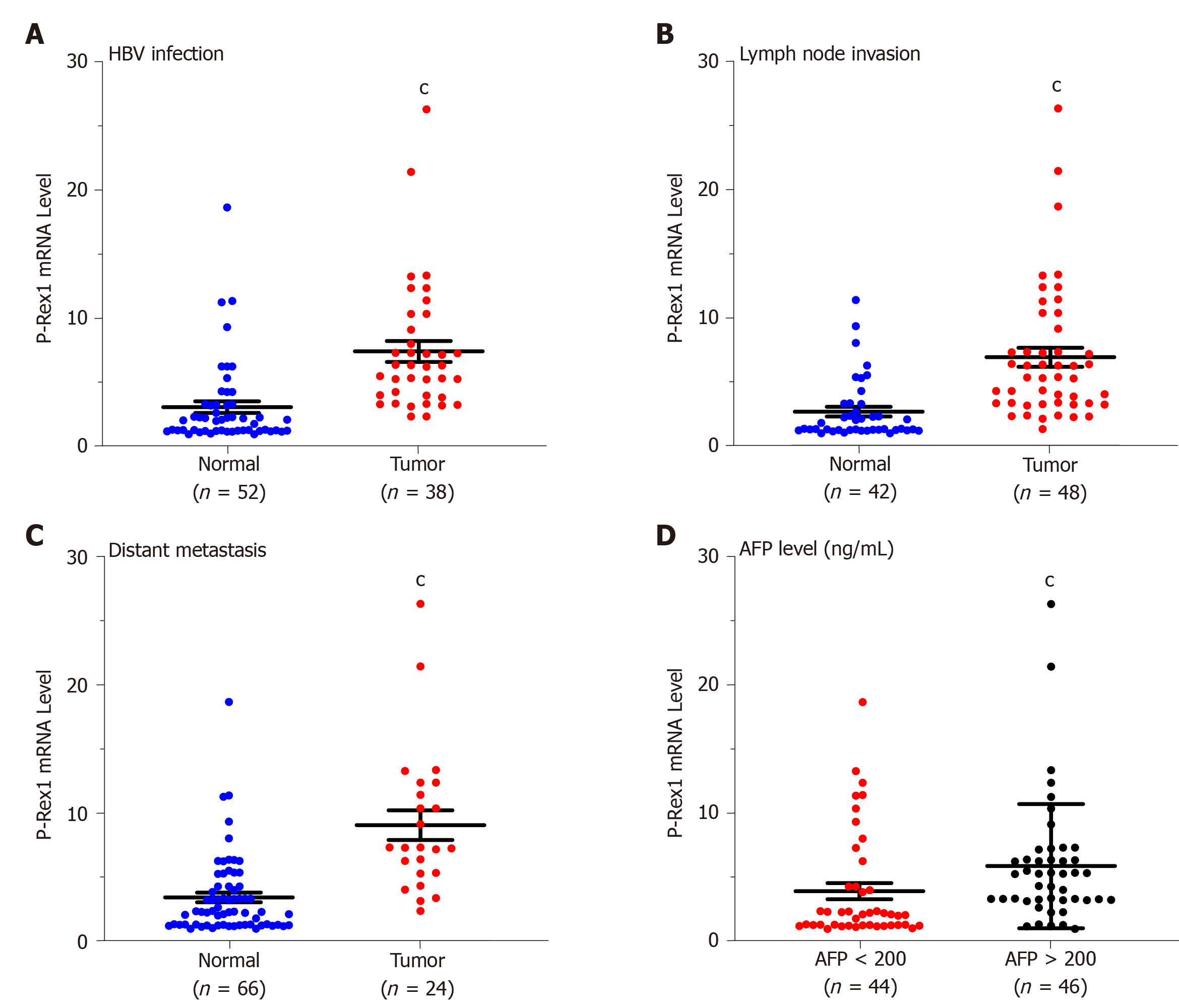
The upregulation of P-Rex1 in liver tumor tissues suggested that P-Rex1 might be involved in the development of HCC. To further demonstrate the potential role of P-Rex1 in HCC, we evaluated the association between P-Rex1 and the following clinical features, HBV infection, lymph node invasion, distant metastasis, and serum AFP level. HBV infection is an important risk factor for HCC, and results in a marked difference in the choice of treatment[26,27]. Figure 2A shows that P-Rex1 expression was significantly higher in patients with HBV infection than in those without HBV infection. Metastasis is a critical factor contributing to the poor prognosis of HCC patients in the clinic[28]; thus, we conducted a further analysis of lymph node invasion and distant metastasis. The data revealed that patients with lymph node invasion or distant metastasis had increased P-Rex1 expression in tumors (Figure 2B and C). AFP is the gold standard biomarker for HCC diagnosis, and some studies have also revealed that AFP can predict prognosis during HCC treatment[29,30]. Thus, we also evaluated the correlation between P-Rex1 and AFP. As shown in Figure 2D, patients with an AFP level more than 200 ng/mL had higher expression of P-Rex1. The detailed correlation analysis is also shown in Table 1, where the 90 HCC patients were divided into the high expression group and the low expression group according to the median value of P-Rex1 expression in liver tumor tissues. Compared with HCC patients with low P-Rex1 expression, the P-Rex1 high expression group included more patients with HBV infection (33 cases vs 5 cases), lymph node invasion (37 cases vs 11 cases), distant metastasis (22 cases vs 2 cases) and increased AFP concentration (31 cases vs 15 cases). It is noteworthy that the patients with high P-Rex1 expression had lower false negative AFP results (14/45, 31%), and patients with low P-Rex1 expression had higher false-negative AFP results (35/45, 67%). These findings revealed that P-Rex1 expression was closely associated with the clinical features of HCC and may participate in the development of HCC.
| Characteristics | P-Rex1 expression1 | P value | χ2 | |
| High | Low | |||
| Age (yr) | ||||
| ≤ 50 | 13 | 11 | 0.634 | 0.227 |
| > 50 | 32 | 34 | ||
| Gender | ||||
| Male | 33 | 36 | 0.455 | 0.559 |
| Female | 12 | 9 | ||
| HBV infection | ||||
| Yes | 33 | 5 | < 0.001 | 35.709 |
| No | 12 | 40 | ||
| Lymph node invasion | ||||
| Yes | 37 | 11 | < 0.001 | 30.179 |
| No | 8 | 34 | ||
| Distant metastasis | ||||
| Yes | 22 | 2 | < 0.001 | 22.727 |
| No | 23 | 43 | ||
| AFP level (ng/mL) | ||||
| < 20 | 8 | 8 | < 0.001 | 14.708 |
| 20 ≤ AFP ≤ 200 | 6 | 22 | ||
| > 200 | 31 | 15 | ||
| Total | 45 | 45 | ||
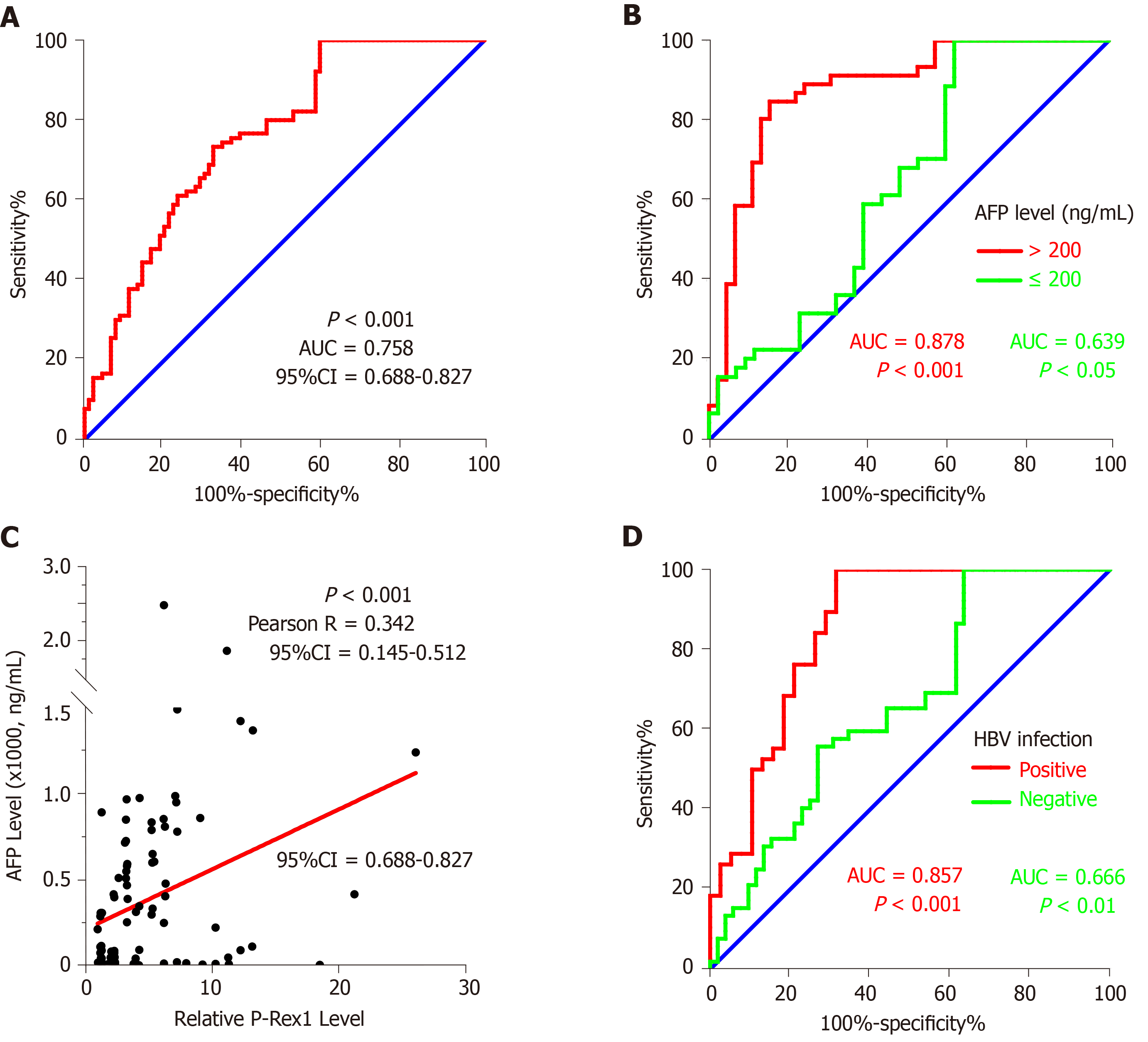
The marked difference in P-Rex1 between HCC tumor and liver normal tissues as mentioned above was closely associated with some pathological features of HCC. Considering the significant upregulation of P-Rex1 in liver tumor, and poor diagnosis in the clinic, we hypothesized that the upregulation of P-Rex1 may be used as a diagnostic biomarker for HCC. To confirm this hypothesis, ROC analysis was conducted on all 90 HCC patients. The results are shown in Figure 3A, and the AUC value was high at 0.758 (P < 0.001, 95%CI = 0.688-0.827). Based on this, P-Rex1 could be a potential biomarker for the diagnosis of HCC. It is necessary to fully understand the role of P-Rex1 in the diagnosis of HCC, and the importance and shortcomings of AFP in clinical practice. In the clinic, a concentration of AFP over 200 ng/mL is highly suspicious of liver cancer[31]. To evaluate the diagnostic potential of P-Rex1 in HCC, we also examined the ROC analysis in patients with AFP > 200 ng/mL and AFP < 200 ng/mL, respectively. As shown in Figure 3B, the ROC analysis in patients with higher serum AFP showed a higher AUC value (0.878, P < 0.001) than patients with low AFP levels (0.639, P < 0.05). Combined with the results of Figure 2D, P-Rex1 expression was closely associated with serum AFP concentration. We further tested the correlation between serum AFP level and P-Rex1 expression in liver tumor, and a positive correlation was observed between P-Rex1 and AFP (Figure 3C); therefore, P-Rex1 may be a new AFP-related factor in HCC. Considering the significance of HBV on P-Rex1 expression (Figure 2A), we further examined HCC patients with and without HBV infection, respectively, using ROC analysis. The ROC analysis is shown in Figure 3D, and the AUC value in HBV positive patients was higher than that in HBV negative patients (0.857 vs 0.666). Thus, P-Rex1 as a diagnostic biomarker could be more effective for HBV-related HCC patients.
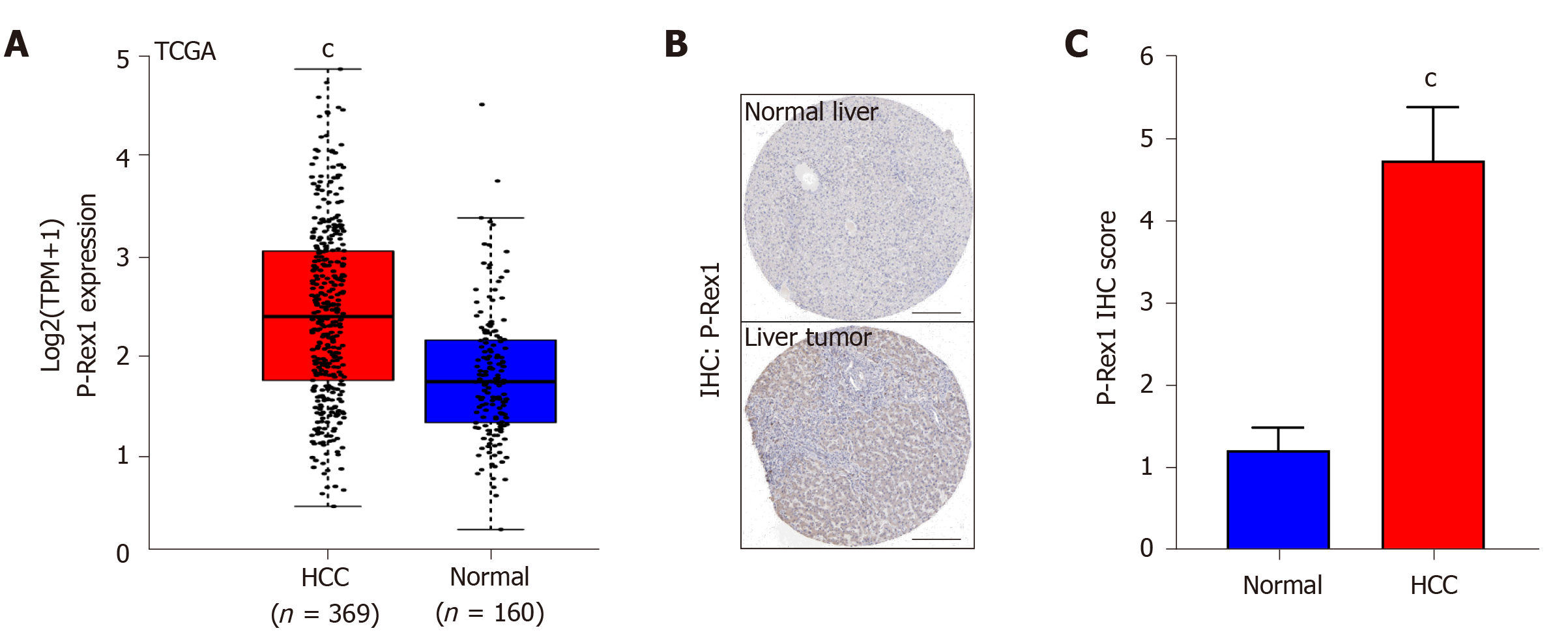
In the above-mentioned results, we revealed the overexpression of P-Rex1 in liver tumor, and the significant difference in P-Rex1 could be a diagnostic biomarker for HCC patients, especially in patients with HBV infection. Here, we further validated the overexpression of P-Rex1 by bioinformatics analysis. As shown in Figure 4A, samples from TCGA liver cancer were included, and the GTEx value of the liver tumor and adjacent normal liver were also included. The results showed that P-Rex1 was significantly increased in liver tumor tissues, which was consistent with our clinical HCC samples. Moreover, we also evaluated the protein expression of P-Rex1 in the human protein atlas database. Immunohistochemistry staining of P-Rex1 was increased in liver tumor cells compared with normal liver tissues, and the main expression of P-Rex1 was in the cytoplasm (Figure 4B and C). The bioinformatics analysis validated the overexpression of P-Rex1 in liver tumor, which confirmed the results of the clinical HCC samples.
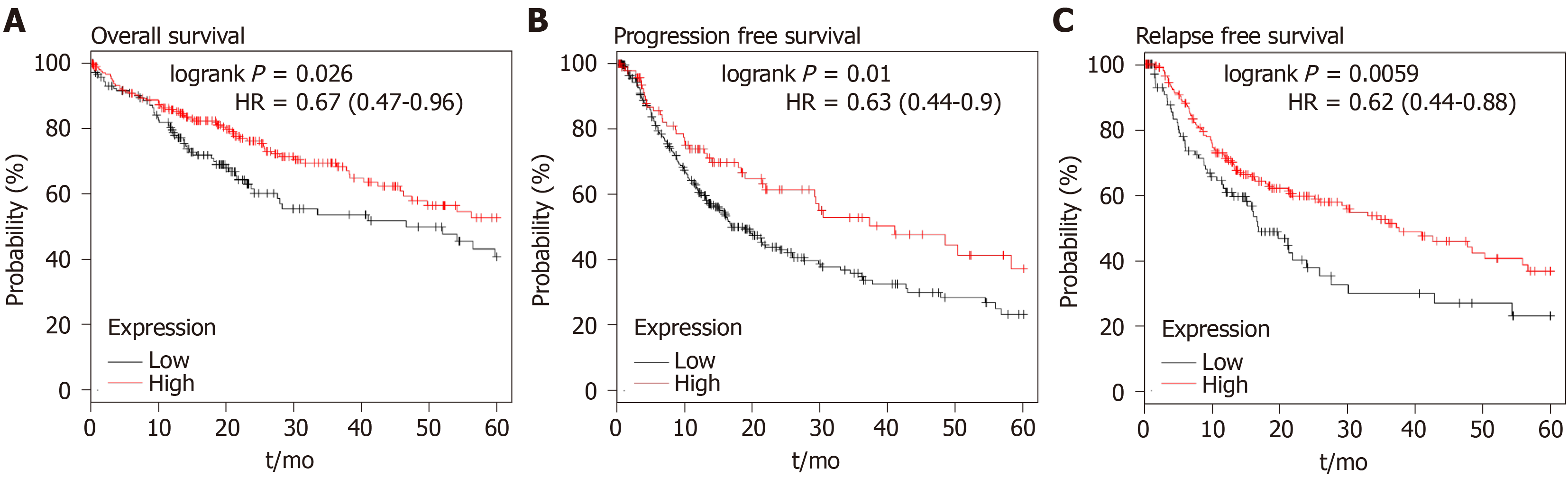
Our data showed the upregulation of P-Rex1 in liver tumor tissues, and P-Rex1 expression was further enhanced in patients with HBV infection, lymph node invasion or distant metastasis, suggesting that P-Rex1 was closely associated with the clinical-pathological features of HCC. The ROC analysis revealed the potential of P-Rex1 as a diagnostic biomarker for HCC patients, especially for HBV positive patients. P-Rex1 expression was closely associated with the development of HCC. To understand the role of P-Rex1 in HCC, the survival analysis was applied in this study. As shown in Figure 5, HCC patients with high P-Rex1 had a higher OS, PFS and RFS. Table 2 shows that the median survival time of the P-Rex1 high expression group was significantly longer than patients with low expression of P-Rex1 (OS 25.5 mo vs 14 mo), PFS (40.97 mo vs 18.7 mo), RFS (37.23 mo vs 16.83 mo).
| Survival type | P-Rex1 expression1 | P value | HR | |
| High | Low | |||
| OS | ||||
| Total | 25.5 | 14 | 0.026 | 0.67 (0.47-0.96) |
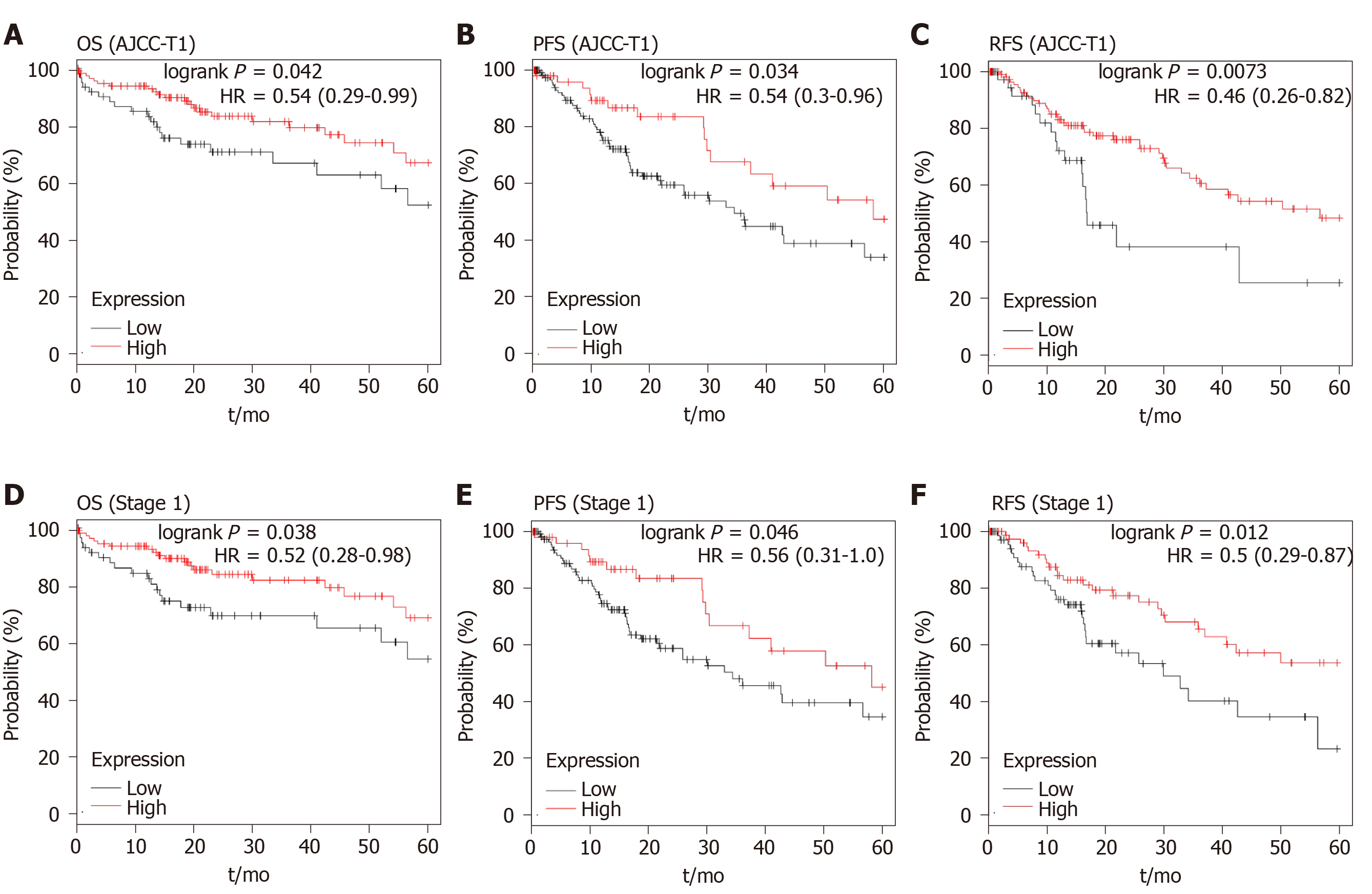
| AJCC-T1 | 45.7 | 17.8 | 0.0421 | 0.54 (0.29-0.99) |
| Stage 1 | 54.1 | 14.6 | 0.038 | 0.52 (0.28-0.98) |
| PFS | ||||
| Total | 40.97 | 18.7 | 0.01 | 0.63 (0.44-0.90) |
| AJCC-T1 | 58.17 | 34.4 | 0.034 | 0.54 (0.30-0.96) |
| Stage 1 | 58.17 | 34.4 | 0.046 | 0.56 (0.31-1.00) |
| RFS | ||||
| Total | 37.23 | 16.83 | 0.0059 | 0.62 (0.44-0.88) |
| AJCC-T1 | 56.67 | 16.83 | 0.0073 | 0.46 (0.26-0.82) |
| Stage 1 | 25.87 | 13.03 | 0.012 | 0.50 (0.29-0.87) |
Higher P-Rex1 expression was revealed as a favorable factor in the analysis of OS, PFS, and RFS, suggesting that P-Rex1 could serve as a new prognostic biomarker for HCC patients. In clinical practice, HCC therapy showed that prognostic evaluation was poor in the early stage of HCC. Considering the prolonged survival time in patients with high P-Rex1 expression, we further examined the OS, PFS, and RFS in early stage HCC patients. In this study, the AJCC-T1 or stage 1 was considered early stage. As shown in Figure 6A-C, the higher P-Rex1 group of HCC patients with AJCC-T1 showed prolonged OS [logrank P = 0.042, HR = 0.54 (0.29-0.99)], PFS [logrank P = 0.034, HR = 0.54 (0.3-0.96)], and RFS [logrank P = 0.0073, HR = 0.46 (0.26-0.82)]. Similar results were also observed in HCC patients with stage 1 (Figure 6D and F). The analysis of median survival time in HCC patients with AJCC-T1 or stage 1 is shown in Table 2. The median survival time in the higher P-Rex1 expression group was longer than in patients with lower P-Rex1 expression [OS (P < 0.05), PFS (P < 0.05), RFS (P < 0.05)].
HCC is one of the most lethal human cancers, especially in East Asia. Many risk factors contribute to the high incidence, such as HBV infection, aflatoxin exposure, alcoholic liver injury and non-alcoholic fatty liver disease[32]. Multiple methods are applied in current HCC clinical practice, but the 5-year survival rate is not satisfactory. Poor diagnosis and drug resistance are the two major challenges in the treatment of HCC. These challenges have attracted considerable attention in order to overcome these problems, and the identification of novel biomarkers and pharmaceutic targets is critical in the management of HCC.
In this study, we identified that P-Rex1 was overexpressed in liver tumor tissues (Figures 1 and 4), which was consistent with previous reports in other cancers. Baker et al[33] reported hyperactivation of P-Rex1 in prostate cancer[33]. P-Rex1 amplification is also involved in the progression of melanoma via the PAK1/P38 pathways[34]. We hypothesized that a close association between P-Rex1 and risk factors of HCC exists, such as HBV infection, lymph node invasion and distant metastasis. Our results confirmed this hypothesis. Interestingly, we also found the P-Rex1 was further enhanced in the AFP-positive HCC patients (Figure 2D), and this positive correlation is demonstrated in Figure 3C. Considering the diagnostic value of AFP in HCC, we then tried to evaluate the potential application of P-Rex1 as a diagnostic biomarker. Our findings showed that P-Rex1 was a diagnostic biomarker with a higher AUC value (Figure 3A). We also determined the effect of HBV infection on the upregulation of P-Rex1 levels, and conducted ROC analysis in HCC patients with and without HBV infection. A significant increase in the AUC value in HBV positive infection was discovered (Figure 3D). Therefore, P-Rex1 is a novel diagnostic biomarker for HBV-related HCC patients.
The effect of the overexpression of P-Rex1 in HCC is unknown. To determine the potential function of P-Rex1 in HCC, the effect of P-Rex1 expression on survival time was assessed. Analysis of OS, PFS, and RFS demonstrated that patients with higher P-Rex1 expression had prolonged survival time (Figure 5 and Table 2). P-Rex1 was reported to be an important factor which regulates immune signaling pathways, and the complex regulatory mechanism of tumor immunity is also considered the most important factor in the development of liver cancer. Thus, high P-Rex1 correlated with the development of liver cancer, but high expression of P-Rex1 also improved survival time, suggesting that high expression of P-Rex1 might be a compensatory phenomenon. We also evaluated early stage HCC patients, as prognosis is poorly studied in these patients. Our findings revealed that even in early stage HCC, P-Rex1 was a favorable prognostic biomarker (Figure 6 and Table 2). Other researchers previously reported that P-Rex1 expression could be a marker for sensitivity to PI3K inhibitors in breast cancer[35]; thus, we believe that P-Rex1 is a critical biomarker in the diagnosis and prognosis of HCC. The overexpression of P-Rex1 in HCC will be an encouraging pharmaceutic target in the treatment of HCC.
Phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 (P-Rex1) is regarded a critical regulator in the inflammation response, and a recent study showed the significance of P-Rex1 in cancers.
P-Rex1 functions as a tumor promoter in many cancers, but the expression and the clinical significance of P-Rex1 in hepatocellular carcinoma (HCC) are still unknown.
The study aimed to evaluate the potential value of P-Rex1 in the diagnosis and prognosis of HCC.
Ninety resected HCC tissues were examined by real-time quantitative polymerase chain reaction to analyze P-Rex1 expression. The correlation between P-Rex1 expression and pathological features was determined. Bioinformatics analysis was applied to validate the results.
P-Rex1 was highly expressed in liver tumor, and P-Rex1 expression was closely correlated with lymph node invasion, hepatitis B virus (HBV) infection, distant metastasis, and alpha-fetoprotein levels. P-Rex1 acts as a diagnostic biomarker with a higher area under the curve value, especially in patients with HBV infection. P-Rex1 could also act as a favorable prognostic factor in HCC, even in patients with early stage HCC.
Our data suggest that P-Rex1 is overexpressed in HCC and is associated with HCC progression. P-Rex1 is a new diagnostic biomarker for HBV-related HCC and is also a favorable prognosis factor for HCC.
The overexpression of P-Rex1 was confirmed in this study, and P-Rex1 was closely associated with the pathological features of HCC. A positive correlation between P-Rex1 and the risk factors of HCC was also observed, suggesting that P-Rex1 is an important mediator in the development of HCC. Survival analysis showed that P-Rex1 is a favorable prognosis biomarker. Therefore, the overexpression of P-Rex1 in the development of HCC and the effect on cell proliferation, migration or differentiation will be further demonstrated in a future study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Hawary AK, Sawada K S-Editor: Zhang L L-Editor: Webster JR P-Editor: Li JH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15470] [Article Influence: 2578.3] [Reference Citation Analysis (2)] |
| 2. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1381] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 3. | Vitale A, Trevisani F, Farinati F, Cillo U. Treatment of hepatocellular carcinoma in the Precision Medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 4. | Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS; KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 998] [Article Influence: 142.6] [Reference Citation Analysis (0)] |
| 5. | Singh P, Toom S, Avula A, Kumar V, Rahma OE. The Immune Modulation Effect of Locoregional Therapies and Its Potential Synergy with Immunotherapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2020;7:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Cheng H, Wu Z, Wu C, Wang X, Liow SS, Li Z, Wu YL. Overcoming STC2 mediated drug resistance through drug and gene co-delivery by PHB-PDMAEMA cationic polyester in liver cancer cells. Mater Sci Eng C Mater Biol Appl. 2018;83:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Huang KW, Lai YT, Chern GJ, Huang SF, Tsai CL, Sung YC, Chiang CC, Hwang PB, Ho TL, Huang RL, Shiue TY, Chen Y, Wang SK. Galactose Derivative-Modified Nanoparticles for Efficient siRNA Delivery to Hepatocellular Carcinoma. Biomacromolecules. 2018;19:2330-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Fan X, Cheng H, Wang X, Ye E, Loh XJ, Wu YL, Li Z. Thermoresponsive Supramolecular Chemotherapy by "V"-Shaped Armed β-Cyclodextrin Star Polymer to Overcome Drug Resistance. Adv Healthc Mater. 2018;7:e1701143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Kong W, Zuo X, Liang H, Hu J, Zhang H, Wang X, Chen W. Prognostic Value of Lactate Dehydrogenase in Patients with Hepatocellular Carcinoma: A Meta-Analysis. Biomed Res Int. 2018;2018:1723184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Xue C, He Y, Zhu W, Chen X, Yu Y, Hu Q, Chen J, Liu L, Ren F, Ren Z, Cui G, Sun R. Low expression of LACTB promotes tumor progression and predicts poor prognosis in hepatocellular carcinoma. Am J Transl Res. 2018;10:4152-4162. [PubMed] |
| 11. | Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, Okkenhaug K, Coadwell WJ, Andrews SR, Thelen M, Jones GE, Hawkins PT, Stephens LR. P-Rex1 regulates neutrophil function. Curr Biol. 2005;15:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Wang Z, Dong X, Li Z, Smith JD, Wu D. Lack of a significant role of P-Rex1, a major regulator of macrophage Rac1 activation and chemotaxis, in atherogenesis. Prostaglandins Other Lipid Mediat. 2008;87:9-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kumar NR, Khamar P, Shetty R, Sharma A, Shetty N, Pahuja N, Abilash VG, Jhanji V, Ghosh A, Mohan RR, Vangala RK, Ghosh A. Identification of novel predictive factors for post surgical corneal haze. Sci Rep. 2019;9:16980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Liang Q, Cheng N, Zhang G, Liang Y, Qian F, Wu D, Ye RD. Identification of P-Rex1 as an anti-inflammatory and anti-fibrogenic target for pulmonary fibrosis. Sci Rep. 2016;6:25785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Lawson CD, Donald S, Anderson KE, Patton DT, Welch HC. P-Rex1 and Vav1 cooperate in the regulation of formyl-methionyl-leucyl-phenylalanine-dependent neutrophil responses. J Immunol. 2011;186:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Dillon LM, Bean JR, Yang W, Shee K, Symonds LK, Balko JM, McDonald WH, Liu S, Gonzalez-Angulo AM, Mills GB, Arteaga CL, Miller TW. P-REX1 creates a positive feedback loop to activate growth factor receptor, PI3K/AKT and MEK/ERK signaling in breast cancer. Oncogene. 2015;34:3968-3976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Hernández-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernández-García R, Calderón-Salinas JV, Reyes-Cruz G, Gutkind JS, Vázquez-Prado J. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem. 2007;282:23708-23715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Barrows D, He JZ, Parsons R. PREX1 Protein Function Is Negatively Regulated Downstream of Receptor Tyrosine Kinase Activation by p21-activated Kinases (PAKs). J Biol Chem. 2016;291:20042-20054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Pan D, Barber MA, Hornigold K, Baker MJ, Toth JM, Oxley D, Welch HC. Norbin Stimulates the Catalytic Activity and Plasma Membrane Localization of the Guanine-Nucleotide Exchange Factor P-Rex1. J Biol Chem. 2016;291:6359-6375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Srijakotre N, Man J, Ooms LM, Lucato CM, Ellisdon AM, Mitchell CA. P-Rex1 and P-Rex2 RacGEFs and cancer. Biochem Soc Trans. 2017;45:963-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Lindsay CR, Lawn S, Campbell AD, Faller WJ, Rambow F, Mort RL, Timpson P, Li A, Cammareri P, Ridgway RA, Morton JP, Doyle B, Hegarty S, Rafferty M, Murphy IG, McDermott EW, Sheahan K, Pedone K, Finn AJ, Groben PA, Thomas NE, Hao H, Carson C, Norman JC, Machesky LM, Gallagher WM, Jackson IJ, Van Kempen L, Beermann F, Der C, Larue L, Welch HC, Ozanne BW, Sansom OJ. P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat Commun. 2011;2:555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Walker G. P-REX1, a Rac guanine exchange factor, links melanocyte development and melanoma progression. Pigment Cell Melanoma Res. 2011;24:1086-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Li JB, Liu ZX, Zhang R, Ma SP, Lin T, Li YX, Yang SH, Zhang WC, Wang YP. Sp1 contributes to overexpression of stanniocalcin 2 through regulation of promoter activity in colon adenocarcinoma. World J Gastroenterol. 2019;25:2776-2787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Xu WH, Xu Y, Wang J, Tian X, Wu J, Wan FN, Wang HK, Qu YY, Zhang HL, Ye DW. Procollagen-lysine, 2-oxoglutarate 5-dioxygenases 1, 2, and 3 are potential prognostic indicators in patients with clear cell renal cell carcinoma. Aging (Albany NY). 2019;11:6503-6521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Zhong X, Kan A, Zhang W, Zhou J, Zhang H, Chen J, Tang S. CBX3/HP1γ promotes tumor proliferation and predicts poor survival in hepatocellular carcinoma. Aging (Albany NY). 2019;11:5483-5497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Tseng TC, Liu CJ, Chang CT, Su TH, Yang WT, Tsai CH, Chen CL, Yang HC, Liu CH, Chen PJ, Chen DS, Kao JH. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J Hepatol. 2020;72:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Hou L, Wei X, Zhuo Y, Qin L, Yang F, Zhang L, Song X. GC-MS-based metabolomics approach to diagnose depression in hepatitis B virus-infected patients with middle or old age. Aging (Albany NY). 2018;10:2252-2265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Zhang Y, Wang S, Dong QZ, Shen Z, Wang W, Tao S, Gu C, Liu J, Xie Y, Qin LX. Prospero-related homeobox 1 drives angiogenesis of hepatocellular carcinoma through selectively activating interleukin-8 expression. Hepatology. 2017;66:1894-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Rich NE, John BV, Parikh ND, Rowe I, Mehta N, Khatri G, Thomas SM, Anis M, Mendiratta-Lala M, Hernandez C, Odewole M, Sundaram LT, Konjeti VR, Shetty S, Shah T, Zhu H, Yopp AC, Hoshida Y, Yao FY, Marrero JA, Singal AG. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multi-center cohort of patients with cirrhosis. Hepatology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 30. | Cao L, Cheng H, Jiang Q, Li H, Wu Z. APEX1 is a novel diagnostic and prognostic biomarker for hepatocellular carcinoma. Aging (Albany NY). 2020;12:4573-4591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 31. | Sohn W, Paik YH, Cho JY, Lim HY, Ahn JM, Sinn DH, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol. 2015;62:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Hoo RL, Lee IP, Zhou M, Wong JY, Hui X, Xu A, Lam KS. Pharmacological inhibition of adipocyte fatty acid binding protein alleviates both acute liver injury and non-alcoholic steatohepatitis in mice. J Hepatol. 2013;58:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Baker MJ, Abba MC, Garcia-Mata R, Kazanietz MG. P-REX1-Independent, Calcium-Dependent RAC1 Hyperactivation in Prostate Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Wang J, Hirose H, Du G, Chong K, Kiyohara E, Witz IP, Hoon DSB. P-REX1 amplification promotes progression of cutaneous melanoma via the PAK1/P38/MMP-2 pathway. Cancer Lett. 2017;407:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Marotti JD, Muller KE, Tafe LJ, Demidenko E, Miller TW. P-Rex1 Expression in Invasive Breast Cancer in relation to Receptor Status and Distant Metastatic Site. Int J Breast Cancer. 2017;2017:4537532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |