Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3372
Peer-review started: March 18, 2020
First decision: April 22, 2020
Revised: June 7, 2020
Accepted: July 16, 2020
Article in press: July 16, 2020
Published online: August 6, 2020
Processing time: 140 Days and 20.5 Hours
New direct-acting antivirals (DAAs)-based anti-hepatitis C virus (HCV) therapies are highly effective in patients with HCV infection. However, safety data are lacking regarding HCV treatment with DAAs and drugs for comorbidities.
Herein, we reported a case of HCV-infection in a 46-year-old man with benign prostatic hypertrophy. The patient received sofosbuvir/velpatasvir as well as methadone maintenance therapy for drug abuse. The viral load became negative at week 1 post treatment. He developed facial and bilateral lower extremity edema 48 h after starting receiving tamsulosin. Edema disappeared 10 d after treatment with oral furosemide and spironolactone.
In conclusion, this is the first case of an acute edema in the course of treatment with new DAAs, methadone and tamsulosin. These agents are useful in clinical management of patients with HCV infection, particularly in men with benign prostatic hypertrophy. Clinicians should be aware of potential drug-drug interactions in this subset of patients.
Core tip: In this manuscript, we report a case of drug-drug interaction in a 46-year-old man who was diagnosed with hepatitis C virus infection with benign prostatic hypertrophy. The patient received sofosbuvir/velpatasvir as well as methadone maintenance therapy for drug abuse. The viral load became negative at week 1 post treatment. He developed facial and bilateral lower extremity edema 48 h after starting receiving tamsulosin. Edema disappeared 10 d after treatment with oral furosemide and spironolactone. This case could be useful in clinical management of patients with hepatitis C virus infection, and clinicians should be aware of drug-drug interactions in this subset of patients.
- Citation: Li YP, Yang Y, Wang MQ, Zhang X, Wang WJ, Li M, Wu FP, Dang SS. Facial and bilateral lower extremity edema due to drug-drug interactions in a patient with hepatitis C virus infection and benign prostate hypertrophy: A case report. World J Clin Cases 2020; 8(15): 3372-3376
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3372.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3372
The emergence of second generation direct-acting antivirals (DAAs) has radically changed the landscape of treatment of chronic hepatitis C virus (HCV) infection. A once-daily, single-tablet, pange334notypic regimen comprising velpatasvir, a NS5A inhibitor of HCV viral ribonucleic acid replication and virion assembly, and sofosbuvir, a HCV NS5B polymerase inhibitor, for 12 wk has proven highly effective and is well tolerated in all patients with chronic HCV genotype 1-6 infection[1]. The single-tablet sofosbuvir/velpatasvir (Epclusa®) has well established pharmacological properties and achieves very high rates of sustained virological response at 12 wk post treatment in both treatment-naïve and treatment-experienced individuals with chronic HCV genotype 1-6 infection[2]. Sofosbuvir/velpatasvir is generally well tolerated and has low rates of adverse events. However, treatment of HCV infection in patients with comorbidities is a medical challenge. Evidence-based safety data are lacking regarding HCV treatment with DAAs and drugs for comorbidities.
In the current case report, we described the development of acute facial and bilateral lower edema in a patient with HCV infection and benign prostatic hypertrophy and a history of drug abuse who received sofosbuvir/velpatasvir, methadone and tamsulosin.
A 46-year-old man with HCV infection and dysuria for 1 wk was referred to our department for HCV therapy assessment in September 2018. Genotyping revealed HCV 3b. Initial viral load was 5.8 lgIU/mL. Liver stiffness was 8.0 kPa by liver transient elastography (Fibroscan®).
At 8 wk later, the patient started taking oral tamsulosin hydrochloride (0.2 mg/d) because of dysuria. Forty-eight hours later, the patient complained of progressive bilateral lower extremity edema and facial edema, which did not change with posture. No redness or swelling was present, and there was no skin ulceration in the lower limbs. The patient did not complain of lower limb pain, and there was no limited range of motion of the lower limbs. High-resolution Doppler ultrasound of the arteries and veins of the lower limbs showed no flow alterations.
The patient had a history of intravenous drug abuse and received methadone maintenance therapy. He also took tamsulosin hydrochloride intermittently for benign prostatic hypertrophy (BPH) during the past 3 years.
Physical examination at admission revealed no remarkable findings.
The laboratory findings (Table 1) showed alanine aminotransferase at 118 IU/L (reference range < 50 IU/L), aspartate aminotransferase at 66 IU/L (reference range < 40 IU/L), gamma glutamyl transferase at 127 IU/L (reference range < 60 IU/L) and creatinine at 53.26 μmol/L (reference range 57-111 μmol/L). Echocardiogram showed normal ejection fraction and diastolic function.
| Liver function | TBIL in μmol/L | ALT in IU/L | AST in IU/L | TP in g/L | ALB in g/L | Glb in g/L | TG in mmol/L | TCHO in mmol/L |
| Before treatment | 11.85 | 118 | 66 | 70.7 | 41.3 | 29.4 | 1.81 | 4.54 |
| 4 wk | 10.8 | 56 | 35 | 69.6 | 39.8 | 29.8 | 3.45 | 4.56 |
| 8 wk | 8.03 | 43 | 30 | 68.6 | 39.3 | 29.3 | 6.08 | 4.88 |
| 12 wk | 7.43 | 42 | 28 | 77.2 | 46.2 | 31.0 | 2.04 | 6.02 |
The final diagnosis of the presented case is chronic hepatitis C and benign prostatic hypertrophy.
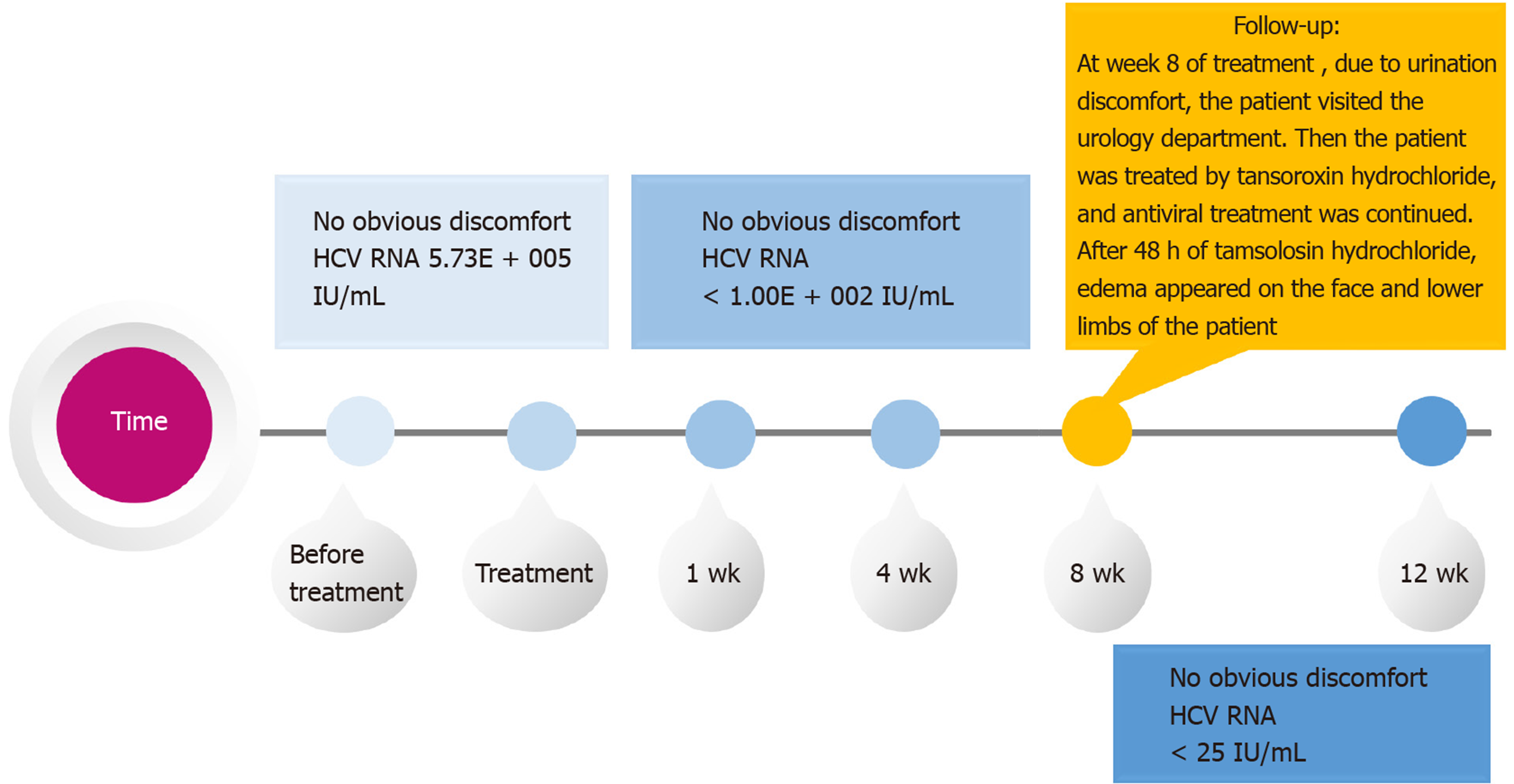
The patient was not contraindicated for methadone and sofosbuvir/velpatasvir and was started on antiviral therapy. Sofosbuvir/velpatasvir was administered at a fixed-dose (100/400 mg) as a singlet tablet once daily for 12 wk. At week 1 of sofosbuvir/ velpatasvir treatment, the viral load of the patient became negative. At week 8 of treatment (Figure 1), due to urination discomfort, the patient visited the urology department. Then, the patient was treated by tansoroxin hydrochloride, and antiviral treatment was continued. After 48 h of tamsolosin hydrochloride, edema appeared on the face and lower limbs of the patient. Tamsulosin hydrochloride was discontinued immediately. Oral furosemide (20 mg/d) and spironolactone (20 mg twice daily) were administered. The edema dissipated gradually after treatment and disappeared 10 d later.
At 1 mo after antiviral treatment, his viral load was negative, and his liver function index improved (Table 1). He was re-started with tamsulosin hydrochloride after 12 wk antiviral treatment. No signs of edema were observed.
The current case report was approved by the local ethics committee of the authors’ affiliated hospital (No. 2018-1104). Patient data were anonymized in the report.
DAAs are highly effective and well tolerated and have radically improved the treatment of chronic HCV infection. However, each DAA has a unique metabolic profile and has an important potential for drug–drug interactions (DDIs). Chronic hepatitis C patients may have comorbidities and could receive multiple drugs along with DAAs and are therefore prone to potential DDIs[3]. The current paper presents the first report of a potential DDI between DAAs and tamsulosin in a patient who developed edema of the lower limb following polypharmacy.
Many DAAs carry high DDI potential due to their metabolism by cytochrome P450 3A (CYP3A) or transport by P-glycoprotein (P-gp)[4]. Velpatasvir is a pangenotypic HCV NS5A inhibitor and is administered in a fixed dose combination with sofosbuvir. Sofosbuvir/velpatasvir and methadone have different metabolic pathways[5,6]. Sofosbuvir is a substrate of P-gp 14, and neither inhibits nor induces the CYP enzyme system, including inactive nucleoside metabolites of sofosbuvir. Velpatasvir is a substrate of P-gp, CYP2B6 and CYP3A4 and inhibits P-gp. Methadone is commonly used as an opioid substitute in patients with a history of drug abuse[7]. Methadone binds to α1-acid glycoprotein (AAG) and is metabolized almost exclusively in the liver by type I cytochrome P450 enzymes, excluded mainly by P-gp[8]. CYP3A4 and CYP2B6 are the main enzymes responsible for N-demethylation of methadone[9,10]. The area under the curve for plasma velpatasvir is increased 30% when it is concurrently used with methadone[4]. In addition, as an inhibitor of P-gp, velpatasvir may up-regulate effective concentration of methadone by restraining the transport of methadone[11]. However, sofosbuvir/velpatasvir is safe and effective for HCV infection in patients who received methadone[12]. Besides, there is no evidence that sofosbuvir/velpatasvir induces withdrawal syndrome.
Tamsulosin, an alpha-adrenoceptor blocker, is commonly used to treat BPH. Similar to methadone, tamsulosin binds to AAG and is extensively metabolized by cytochrome P450 enzymes in the liver[13]. The metabolism of tamsulosin can be reduced when used together with methadone. Consequently, when two or more drugs that are metabolic substrates of the same CYP450 enzyme are administered concurrently, the drug that has the greater affinity for that cytochrome can inhibit the metabolism of the other drugs. Tamsulosin binds to AAG with higher affinity and could increase the effective concentration of methadone[14,15].
In the present case, facial and bilateral lower extremity edema emerged 48 h after concurrent treatment of tamsulosin with sofosbuvir/velpatasvir and methadone. Edema did not occur upon completion of antiviral treatment and continued tamsulosin therapy. Therefore, we have good reasons to speculate that edema was due to DDIs among sofosbuvir/velpatasvir, methadone and tamsulosin. A previous study reported systemic edema induced by methadone in a dose-dependent manner[16]. Edema in this case could be the result of increased effective concentration of methadone by tamsulosin.
This is the first case of acute bilateral lower extremity and facial edema in the course of treatment with DAAs, methadone and tamsulosin. These agents are useful in clinical management of patients with HCV infection, particularly in men with BPH. However, clinicians should be aware of potential DDIs in this subset of patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Komatsu H, Salvadori M S-Editor: Zhang L L-Editor: Filipodia E-Editor: Liu JH
| 1. | Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 2. | Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, Ravendhran N, Vierling JM, Tran TT, Pianko S, Bansal MB, de Lédinghen V, Hyland RH, Stamm LM, Dvory-Sobol H, Svarovskaia E, Zhang J, Huang KC, Subramanian GM, Brainard DM, McHutchison JG, Verna EC, Buggisch P, Landis CS, Younes ZH, Curry MP, Strasser SI, Schiff ER, Reddy KR, Manns MP, Kowdley KV, Zeuzem S; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med. 2017;376:2134-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 417] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 3. | Garrison KL, German P, Mogalian E, Mathias A. The Drug-Drug Interaction Potential of Antiviral Agents for the Treatment of Chronic Hepatitis C Infection. Drug Metab Dispos. 2018;46:1212-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Burger D, Back D, Buggisch P, Buti M, Craxí A, Foster G, Klinker H, Larrey D, Nikitin I, Pol S, Puoti M, Romero-Gómez M, Wedemeyer H, Zeuzem S. Clinical management of drug-drug interactions in HCV therapy: challenges and solutions. J Hepatol. 2013;58:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Belema M, Lopez OD, Bender JA, Romine JL, St Laurent DR, Langley DR, Lemm JA, O'Boyle DR 2nd, Sun JH, Wang C, Fridell RA, Meanwell NA. Discovery and development of hepatitis C virus NS5A replication complex inhibitors. J Med Chem. 2014;57:1643-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Masaki T, Matsunaga S, Takahashi H, Nakashima K, Kimura Y, Ito M, Matsuda M, Murayama A, Kato T, Hirano H, Endo Y, Lemon SM, Wakita T, Sawasaki T, Suzuki T. Involvement of hepatitis C virus NS5A hyperphosphorylation mediated by casein kinase I-α in infectious virus production. J Virol. 2014;88:7541-7555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Ayanga D, Shorter D, Kosten TR. Update on pharmacotherapy for treatment of opioid use disorder. Expert Opin Pharmacother. 2016;17:2307-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Nanovskaya T, Nekhayeva I, Karunaratne N, Audus K, Hankins GD, Ahmed MS. Role of P-glycoprotein in transplacental transfer of methadone. Biochem Pharmacol. 2005;69:1869-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Weschules DJ, Bain KT, Richeimer S. Actual and potential drug interactions associated with methadone. Pain Med. 2008;9:315-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Kapur BM, Hutson JR, Chibber T, Luk A, Selby P. Methadone: a review of drug-drug and pathophysiological interactions. Crit Rev Clin Lab Sci. 2011;48:171-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Roncero C, Villegas JL, Martínez-Rebollar M, Buti M. The pharmacological interactions between direct-acting antivirals for the treatment of chronic hepatitis c and psychotropic drugs. Expert Rev Clin Pharmacol. 2018;11:999-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Grebely J, Feld JJ, Wyles D, Sulkowski M, Ni L, Llewellyn J, Mir HM, Sajed N, Stamm LM, Hyland RH, McNally J, Brainard DM, Jacobson I, Zeuzem S, Bourlière M, Foster G, Afdhal N, Dore GJ. Sofosbuvir-Based Direct-Acting Antiviral Therapies for HCV in People Receiving Opioid Substitution Therapy: An Analysis of Phase 3 Studies. Open Forum Infect Dis. 2018;5:ofy001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Soeishi Y, Matsushima H, Watanabe T, Higuchi S, Cornelissen K, Ward J. Absorption, metabolism and excretion of tamsulosin hydrochloride in man. Xenobiotica. 1996;26:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Eap CB, Cuendet C, Baumann P. Binding of d-methadone, l-methadone, and dl-methadone to proteins in plasma of healthy volunteers: role of the variants of alpha 1-acid glycoprotein. Clin Pharmacol Ther. 1990;47:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Korstanje C, Krauwinkel W, van Doesum-Wolters FL. Tamsulosin shows a higher unbound drug fraction in human prostate than in plasma: a basis for uroselectivity? Br J Clin Pharmacol. 2011;72:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Sarica A, Servettaz A, Abou Taam M, Herlem E, Carlier C, Trenque T. [Methadone-induced edema: A case report]. Presse Med. 2015;44:552-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |