Published online Apr 26, 2019. doi: 10.12998/wjcc.v7.i8.972
Peer-review started: December 19, 2018
First decision: January 18, 2019
Revised: February 10, 2019
Accepted: February 26, 2019
Article in press: February 26, 2019
Published online: April 26, 2019
Processing time: 129 Days and 23.7 Hours
Hepatic epithelioid angiomyolipoma (HEAML) is a rare liver disease and is easily misdiagnosed. Enhanced recognition of HEAML is beneficial to the differential diagnosis of rare liver diseases.
We presented two cases of HEAML in Changzheng Hospital, Naval Medical University, and then collected and analyzed all reports about HEAML recorded in PubMed, MEDLINE, China Science Periodical Database, and VIP database from January 2000 to March 2018. A total of 409 cases of HEAML in 97 reports were collected, with a ratio of men to women of 1:4.84 and an age range from 12 years to 80 years (median 44 years). Among the patients with clinical symptoms mentioned, 61.93% (205/331) were asymptomatic, 34.74% (115/331) showed upper or right upper quadrant abdomen discomfort, while a few of them showed abdominal mass, gastrointestinal symptoms, low fever, or weight loss. The misdiagnosis rate of HEAML was as high as 40.34% (165/409) due to its nonspecific imaging findings. Most of the tumors were solitary and round in morphology, with clear boundaries. Ultrasound scan indicated low echo with internal nonuniformity and rich blood supply in most cases. Computer tomography/magnetic resonance imaging enhanced scan showed varied characteristics. The ratio of fast wash-in and fast wash-out, fast wash-in and slow wash-out, and delayed enhancement was roughly 4:5:1. A definite diagnosis of HEAML depended on the pathological findings of the epithelioid cells in lesions and the expression of human melanoma black 45, smooth muscle actin, melanoma antigen, and actin by immunohistochemical staining. HEAML had a relatively low malignant rate of 3.91%. However, surgical resection was the main treatment for HEAML, due to the difficulty diagnosing before operation.
HEAML is a rare and easily misdiagnosed disease, and it should be diagnosed carefully, taking into account clinical course, imaging, pathological ,and immunohistochemical findings.
Core tip: Hepatic epithelioid angiomyolipoma (HEAML) is a rare and easily misdiagnosed disease, with a misdiagnosis rate as high as 40.34% and a relatively low malignant rate of 3.91%. In this work, we presented two cases of primary and secondary HEAML and analyzed the 409 cases of HEAML recorded in PubMed, MEDLINE, China Science Periodical Database, and VIP database from January 2000 to March 2018. This pooled analysis of HEAML, in view of clinical course, imaging, pathological and immunohistochemical findings, may provide a better understanding of HEAML.
- Citation: Mao JX, Teng F, Liu C, Yuan H, Sun KY, Zou Y, Dong JY, Ji JS, Dong JF, Fu H, Ding GS, Guo WY. Two case reports and literature review for hepatic epithelioid angiomyolipoma: Pitfall of misdiagnosis. World J Clin Cases 2019; 7(8): 972-983
- URL: https://www.wjgnet.com/2307-8960/full/v7/i8/972.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i8.972
Hepatic epithelioid angiomyolipoma (HEAML) is a rare subtype of hepatic angiomyolipoma (AML). It is a hepatic mesenchymal neoplasm with malignant potential that is primarily composed of epithelioid cells. Retrieved from major databases, a total of 409 cases of HEMAL have been reported with a misdiagnosis rate as high as 40.34% (165/409) due to its non-specific manifestations. Here we presented two cases of HEAML in Changzheng Hospital, Naval Medical University, Shanghai and made pooled analysis on the diagnosis and prognosis of HEAML. This work was performed in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Changzheng Hospital. Written informed consents were obtained from the 2 patients for using their data for clinical research and publication.
History and physical examination: A 40-year-old female patient was admitted to hospital because "health examination revealed hepatic space-occupying lesion 1 wk ago". The patient had no obvious positive clinical manifestations and positive signs.
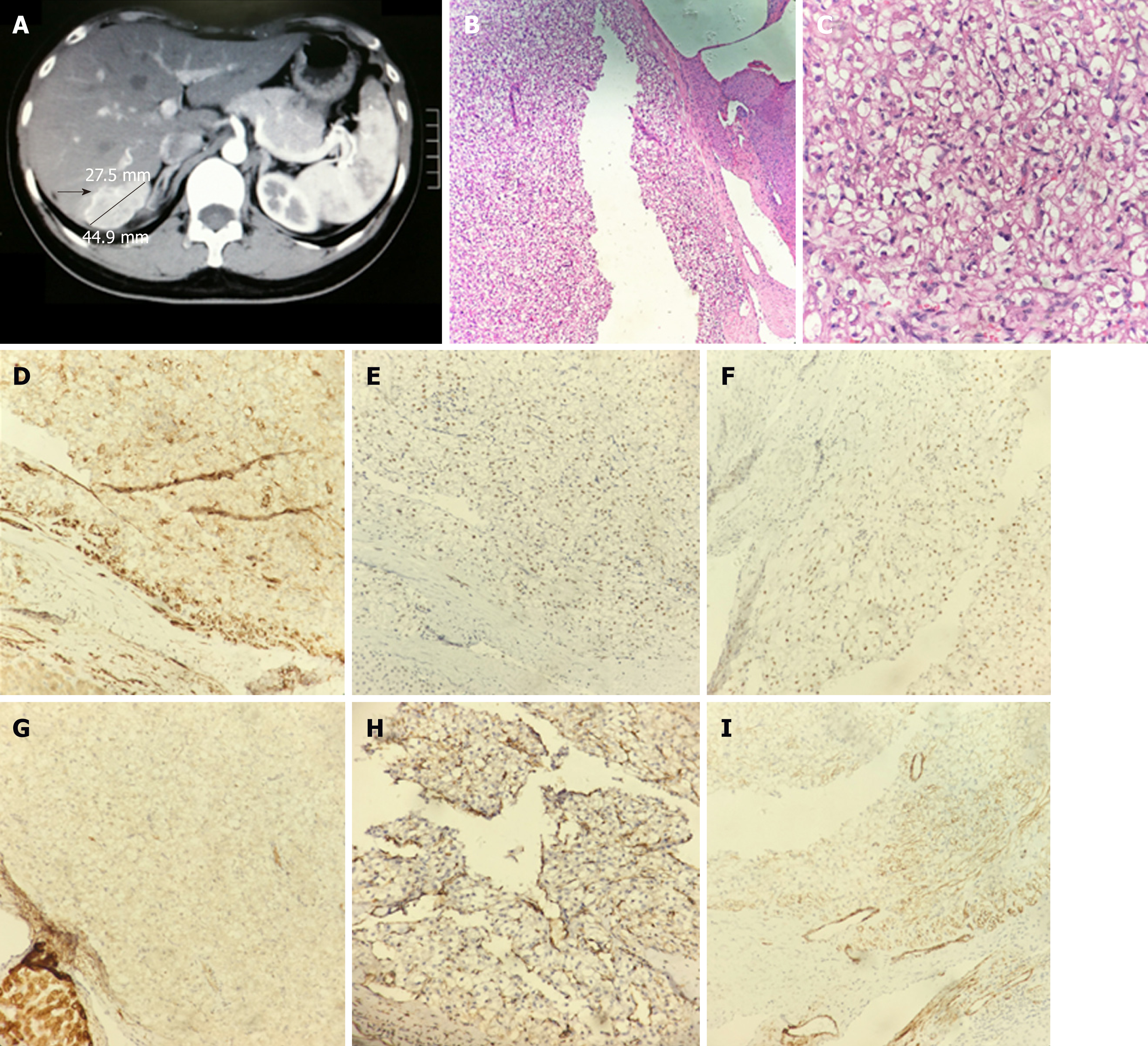
Diagnostic imaging: Ultrasound and computed tomography (CT) scan suggested a mass of 5 cm × 3 cm in the right lobe of liver (Figure 1A). The hepatitis B surface antigen and tumor markers, such as alpha fetoprotein (AFP), carcino-embryonic antigen (CEA), carbohydrate antigen (CA) 199, and CA125, were all negative.
Histopathology: The postoperative pathological diagnosis was HEAML (potentially malignant) (Figure 1B-C). Immunohistochemistry results were as follows: Antigen Ki67 (Ki67) (1% positive), human melanoma black 45 (HMB45) (positive in some cells), smooth muscle actin (SMA) (weak positive), soluble protein-100 (S100) (-), cluster of differentiation (CD) 34 (positive in vessels), calponin (++) (Figure 1D), estrogen receptor (positive in some cells) (Figure 1E), progesterone receptor (+) (Figure 1F), Keratin-pan (Kpan) (weak positive) (Figure 1G), epithelial membrane antigen (EMA) (-), vimentin (partially positive) (Figure 1H), neuron-specific enolase (-), actin (partially positive) (Figure 1I), AFP (weak positive), and hepatocyte paraffin-1 (HepPar)-1 (-).
History of illness: A 39-year-old female patient underwent left nephrectomy due to a left kidney space-occupying lesion in October, 2009. Postoperative pathological examination suggested epithelioid AML. In April 2010, abdominal CT revealed left retroperitoneal lymph node enlargement, which was biopsied afterwards by surgery. Pathological examination prompted a diagnosis of AML. The patient was treated by radiotherapy and high intensity focused ultrasound during the next few years but did not achieve a complete cure.
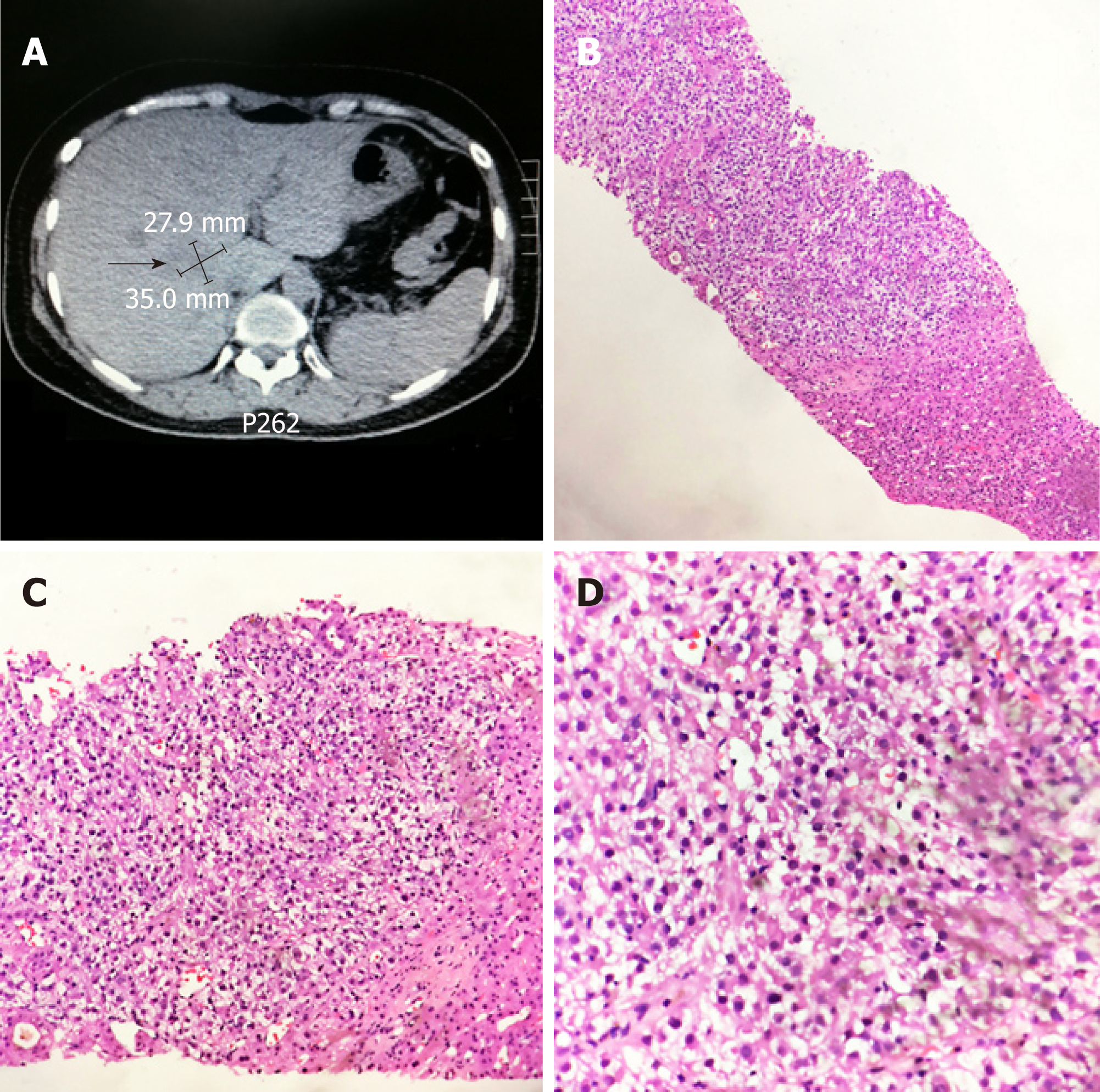
Imaging examination, histopathology: In routine review on 8 July 2014, ultrasound and CT scan revealed a mass with a maximum diameter of about 6 cm in liver. A CT-guided liver tumor puncture biopsy was performed (Figure 2A), and the pathological diagnosis suggested metastatic HEAML (Figure 2B-D). Immunohistochemistry results were as follows: Ki67 (1% positive), HMB45 (+), melanoma antigen (Melan-A) (+), SMA (+), S100 (-), EMA (-), vimentin (partially positive), AFP (-), and HepPar-1 (-). On 25 July 25 2014, chest CT revealed multiple micronodules in the right lung, the largest one of which was approximately 4 mm and located in the superior lobe.
The patient was diagnosed with primary HEAML.
This patient was diagnosed as a secondary HEAML of renal origin.
The patient underwent liver resection on the third day after admission. During intraoperative exploration, a mass with medium texture and clear boundaries was found located in segment 6, protruding from the liver surface, while no satellite lesions were discovered.
The patient underwent transcatheter arterial chemoembolization six times between 28 July 28 2014 and 10 March 2016. During that period, chest CT and abdominal magnetic resonance imaging (MRI) was performed repeatedly and confirmed the presence of bilateral lung nodules, liver space-occupying lesion, and retroperitoneal lymph node enlargement.
The patient recovered well after surgery and was discharged on the postoperative day 9. There was no relapse during the 3 years of follow-up.
The patient ultimately died on 15 October 2016 due to systemic metastasis of the tumor and multiple organ failure.
General data: We collected all reports related to HEAML recorded in the PubMed, MEDLINE, China Science Periodical Database, and VIP database from January 2000 to March 2018. A total of 64 articles were enrolled into analysis after excluding 32 articles without valuable data and five articles with repeated data[1-64]. In total, there were 409 cases, of which 386 presented as single tumor and 23 presented as multiple tumors. The male to female ratio was 1:4.84, with 70 males and 339 females. The median age was 44 years old, ranging from 12 years to 80 years old. Of the patients with symptoms mentioned in articles, 61.93% (205/331) were asymptomatic when ultrasound or imaging examination discovered the tumors, while 34.74% (115/331) present with discomfort of the upper abdomen. Fourteen patients had a history of hepatitis B. Seven patients had tuberous sclerosis complex (TSC). The amino-transferases were abnormal in 4 patients. The CA199 level was elevated in 4 patients. Two patients had a history of breast cancer, while one patient was comorbid with cholangiocarcinoma and had elevated CA125 level. Two patients were comorbid with hepatic hemangioma, one patient was comorbid with gallstone disease, and one patient was comorbid with cirrhosis due to schistosomiasis.
The tumor was located in the right lobe in 181 cases, left lobe in 153 cases, and caudate lobe in 12 cases. The maximum diameter of the tumor ranged from 1 cm to 20 cm, with a median of 5.9 cm. Among the cases with tumor morphology described, tumors were round in 86 cases (85.15%) and lobular or irregular in 15 cases (14.85%), while tumors were well defined in 116 cases (84.67%) and ill-defined in 21 cases (15.33%).
Ultrasonography: Ultrasound usually indicated low echo on HEAML, with clear boundary, internal nonuniformity, and rich blood supply. A low echo halo presented in 27.66% cases (13/47). Contrast-enhanced ultrasonography revealed that all lesions appeared homogeneous hyperechoic during arterial phase, and most lesions appeared homogeneous isoechoic during portal and delayed phase (Table 1).
| Items | Cases |
| Ultrasound | |
| Echo (low/mixed/high) | 54/8/24 |
| Internal uniformity (heterogeneity/homogeneity) | 37/38 |
| Boundary (clear/indistinct) | 55/5 |
| Hypoechoic halo (Y/N) | 13/34 |
| Blood supply (rich/abundant/rare) | 22/6/7 |
| Contrast-enhanced ultrasonography | |
| Arterial phase (high/middle/low) | 15/0/0 |
| Portal phase (high or slightly high/middle/low or slightly low) | 4/8/3 |
| Delay phase (high or slightly high/middle/low or slightly low) | 3/7/5 |
CT: HEAML mostly showed as slightly low density with nonuniformity on CT plain scan. The lesions were obviously or moderately enhanced in arterial phase, and the enhancement decreased during portal and delayed phase in most cases. The enhanced scan showed varied characteristics. The ratio of fast wash-in and fast wash-out, fast wash-in and slow wash-out, and delayed enhancement was roughly 4:5:1. The central vessel sign could be seen in 75.80% of the cases (119/157), and the early drainage veins, which returned into branches of hepatic vein or portal vein, could be seen in 61.48% of the cases (75/122). HEAML usually had no capsule. When the tumor was large enough, it might oppress the surrounding liver parenchyma to form an incomplete pseudocapsule (Table 2).
| Items | Cases |
| Computed tomography | |
| Plain scan phase | |
| Uniformity (uniform/non-uniform) | 15/24 |
| Density (low density/equidensity) | 63/9 |
| Arterial phase (Obvious uniform enhancement/apparent or moderate non-uniform enhancement) | 54/53 |
| Portal phase (obvious enhancement/enhancement reduction) | 33/42 |
| Delayed phase (sustained moderate enhancement/enhancement reduction) | 23/30 |
| Characteristic manifestations | |
| Central vessel sign (Y/N) | 119/38 |
| Early drainage veins (Y/N) | 75/47 |
| Enhancement mode (fast wash-in and fast wash-out/fast wash-in and slow wash-out/delayed enhancement) | 67/83/15 |
| Pseudocapsule (Y/N) | 65/73 |
MRI: MRI scan usually suggested inhomogenous and low T1 weighted imaging (T1WI) signal and an increase in T2WI and diffusion weighted imaging (DWI) signal. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid enhanced scan usually showed marked enhancement in the arterial phase, which was decreased during portal and delayed phase (Table 3).
| Items | Cases |
| Magnetic resonance imaging | |
| T1WI | |
| Low, slightly low signal/equisignal/high signal | 107/1/1 |
| Slightly uniform/non-uniform | 13/26 |
| T2WI | |
| Slightly high signal/high signal | 39/70 |
| Slightly uniform/non-uniform | 3/29 |
| DWI | |
| Slightly high signal/high signal | 13/51 |
| Slightly uniform/non-uniform | 4/15 |
| Gd-EOB-DTPA | |
| Arterial phase | |
| Homogeneous enhancement/heterogeneous enhancement | 29/21 |
| Obvious enhancement/Medium enhancement | 47/7 |
| Portal phase | |
| Continuous enhancement/enhancement weakened | 22/29 |
| Delayed phase | |
| Continuous enhancement/enhancement weakened | 1/2 |
Diagnosis and treatment: Preoperative diagnosis of HEAML was difficult. Among the 409 cases, 59 cases were preoperatively misdiagnosed as benign diseases, including 16 cases of focal nodular hyperplasia, 15 cases of hepatocellular adenoma (HCA), 11 cases of AML, five cases of hemangiomas, one case of hamartoma, and 11 cases of unclassified tumors. One hundred and four cases were misdiagnosed as malignant diseases, including 71 cases of hepatocellular carcinoma, one case of cholangiocarcinoma, five cases of metastatic cancer, three cases of mesenchymal tissue-derived tumors (angiosarcoma, liposarcoma, etc), and 24 cases of unclassified tumors. Another two cases were not diagnosed clearly preoperatively. The misdiagnosis rate was thus as high as 40.34% (165/409)[5-12,18-19,22-24,30-39,43-47,51-53,56,62-63]. Surgical resection was the main treatment for HEAML, due to the difficulty diagnosing before operation. For those not suitable for hepatectomy, ablation or interventional therapy could be the alternative treatment after puncture biopsy for pathological diagnosis.
Gross appearance: In general, the section of HEAML was incanus and grayish yellow or grayish red, and the texture was soft mostly. Most of the HEAML had no capsule, with clear or relatively clear boundaries. For HEAMLs, 40.27% (60/149) were a-ccompanied by hemorrhage and necrosis, and 33.33% (23/69) were comorbid with fat lesions. Cystic degeneration occurred in 14.04% (16/114) of the tumors (Table 4).
| Items | Cases or manifestations |
| Section | Mostly incanus, grayish yellow or grayish red |
| Capsule (Y/N) | 10/53 |
| Boundary | Clear |
| Texture (slightly soft/medium/slightly dense) | 65/6/18 |
| Hemorrhage or necrosis (Y/N) | 60/89 |
| Fat lesions (Y/N) | 23/46 |
| Cystic degeneration (Y/N) | 16/98 |
Microscope appearance: Epithelioid tumor cells could be observed under the microscope and arranged irregularly in nodules or flaky structures, with relatively obvious atypia. Sometimes multinucleated giant cells and ganglion-like large cells could be observed. Generally, epithelioid tumor cells were round, polygonal, or short-spindle in shape and were radially distributed around thin-walled blood vessels or muscular arteries. The cytoplasm was abundant and slightly eosinophilic staining. Sometimes the cytoplasm was clear and contained adipose vacuoles. The centrally located nuclei were large, round, or oval in shape. The nucleoli were obvious and mitotic figures were rare.
Immunohistochemistry: Immunohistochemical examination revealed that the expressions of HMB45, SMA, Actin, Melan-A, macrophage marker 387, melanoma antigen recognized by T-cells 1, A103, etc were positive. The expressions of CD31, CD34 on vascular wall, vimentin, pp70S6K, and MyoD1 were positive in about half of the cases. In a few cases, the fat S-100, CD68, desmin, muscle specific actin, human myeloperoxidase (MPO), E-cadherin, and b-cadherin was positive or weakly positive. The Kpan, AFP, HepPar-1, EMA, CEA, CD117, and p53 was basically negative (Table 5). The Ki-67 expression ranged from < 1% to 15%, with a median of 1.96%.
| Markers | Positive/weakly positive/negative (+/±/-) | Positive rate |
| HMB45 | 219/2/5 | 97.35% |
| S-100 | 19/5/63 | 24.71% |
| SMA | 128/15/32 | 77.43% |
| MSA | 4/10/1 | 60% |
| Actin | 32/1/5 | 85.53% |
| Melan-A | 91/1/22 | 80.70% |
| CD31 | 2/0/2 | 50% |
| CD34 | 38/0/34 | 52.78% |
| CD68 | 2/6/5 | 33.33% |
| CD117 | 0/0/13 | 0% |
| Vimentin | 23/11/17 | 55.88% |
| Kpan | 0/0/101 | 0% |
| HepPar-1 | 0/0/52 | 0% |
| MAC387 | 6/0/0 | 100% |
| EMA | 0/0/30 | 0% |
| Desmin | 0/20/30 | 20% |
| MART-1 | 24/0/1 | 96% |
| CEA | 1/0/9 | 10% |
| AFP | 0/1/66 | 0.75% |
| MPO | 0/1/1 | 25% |
| p53 | 0/0/9 | 0% |
| E-cadherin, b-cadherin | 0/1/4 | 10% |
| pp70S6K | 1/2/1 | 50% |
| MyoD1 | 2/2/1 | 60% |
Follow-up: The duration of follow-up was 2 mo to 180 mo, with a median of 31 mo. The rate of malignancy was 3.96% (16/409). Notably, among the 16 malignant cases, only 1 case was diagnosed potential malignancy on pathology, other cases were identified malignancy because of intrahepatic recurrence (6 cases) and distant metastases (9 cases). Among those metastatic cases, 2 cases were lung metastasis and 1 case was bone metastasis, while other cases were not specified the sites of metastasis. The time of postoperative relapse was 5 mo to 108 mo, with a median of 42.5 mo.
Since Bonetti and colleagues first described AML in 1992, it has gradually been increasingly recognized as a relatively rare mesenchymal tumor. AML was most commonly found in the kidney and less often in the liver. Most patients with hepatic AML were female and asymptomatic, and the masses usually occurred in non-cirrhotic liver without serological abnormalities. In most cases, hepatic AML was discovered incidentally during regular health check-ups or examinations for other diseases. The pathogenesis of hepatic AML has not yet been clarified. There was an association with TSC in more than 50% of the AML in the kidney, but this association had been estimated to be only 5%-15% of the patients presenting with solitary liver tumors. In our review, TSC was found in 7 patients (1.7%), which might be under-estimated due to incomplete information. However, TSC was probably a risk factor for malignant behavior of epithelioid AML[65]. Histologically, it was composed of blood vessels, adipose tissue, and smooth muscle. Compared with typical AML, HEAML was histologically dominated by epithelioid cells and contained much less adipose cells.
HEAML occurred primarily in young and middle-aged females, with an average age of 45 years old at the time of diagnosis. The male to female ratio was about 1:4.84. Approximately two-thirds of patients were found to be asymptomatic on physical examination, while one-third of patients presented with discomfort or swelling pain of the epigastrium or right upper quadrant. A few patients presented with an abdominal mass, poor appetite, nausea and vomiting, anemia, fatigue, low fever, weight loss, changes in bowel habits, etc[1]. Patients usually had no hepatitis, cirrhosis, or family history of this type of tumor. The common tumor markers were almost all negative.
HEAML imaging findings included: (1) Most of the tumors were solitary and round in morphology, while a few were lobulated or irregular, with clear boundaries; (2) Ultrasound scan indicated low echo with internal nonuniformity, clear boundary, and rich blood supply in most cases, sometimes with low echo halo. Contrast-enhanced ultrasound showed that all lesions appeared homogeneous hyperechoic during arterial phase, and most lesions appeared homogeneous isoechoic during portal and delayed phase; (3) CT plain scan usually showed low density lesions, which was obviously or moderately enhanced in arterial phase, and the enhancement decreased during portal and delayed phase in most cases; (4) Most of the lesions were a-ccompanied by central vessels and early drainage veins, which afflux into branches of the hepatic vein or portal vein; (5) The enhanced scan showed varied characte-ristics. The ratio of fast wash-in and fast wash-out, fast wash-in and slow wash-out, and delayed enhancement was roughly 4:5:1; (6) HEAML usually had no capsule. When the tumor was large enough, it might oppress the surrounding liver parenchyma to form an incomplete pseudocapsule. Recognizing the imaging features of no capsule, and hypervasularity with central punctiform or filiform vessels as a characteristic enhancement may distinguish Epi-HAML from other hepatic tumors[30]; and (7) MRI scan usually suggested inhomogenous and low T1WI signal, and increase in T2WI and DWI signal. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid enhanced scan usually showed marked enhancement in arterial phase, which was decreased during portal and delayed phase.
The preoperative misdiagnosis rate was as high as 40.34% (165/409) because of the non-specific clinical and imaging manifestations of HEAML. It was necessary to differentiate with hepatocellular carcinoma, focal nodular hyperplasia, HCA, hemangiomas, and metastatic cancers when making diagnosis. Currently, the major treatment strategy was surgical resection. In general, HEAML had no capsule, but presented with clear boundaries. HEAML demonstrated expansive growth and squeezed the adjacent liver parenchyma. Internal hemorrhage and necrosis was more common than typical AML. The definitive diagnosis of HEAML depended on pathological findings of epithelioid cells in the lesions, and the immunohistochemical findings of melanoma specific markers (HMB45, Melan-A) and myogenic markers (SMA, muscle specific actin, Actin).
Most of the HEAMLs were benign, with a relatively low malignant rate of 3.91% (16/409). Notably, 15 cases of malignancy were identified because of intrahepatic recurrence or distant metastasis, while the pathological examination did not demonstrate malignancy distinctly on the first operation. The malignant HEAML mainly metastasize to the lung and bones and could involve multiple organs in severe cases. The median time of postoperative relapse was 42.5 mo. Therefore, periodic reexamination was needed to prevent recurrence, especially within 5 years after surgery, just like gastrointestinal tumors. In addition, the HEAML could be secondary, with primary lesions mostly originated from kidney. The second case reported in this article initially presented as retroperitoneal lymph node enlargement 1 year after renal EAML resection, and eventually exhibited liver and lung metastases 5 years after the surgery. Secondary HEAML has rarely been reported. Among the 17 secondary cases reported[8,37,66-71], 5 cases turned to multiple metastases. Secondary HEAML had no significant differences from primary HEAML in terms of clinical manifestations, imaging findings, pathology, and immunohistochemistry.
We thank the patients for their cooperation. We thank Dr. Qing-Zi Zhu (Department of Pathology, Changzheng Hospital) for supplying HEAML tissue.
Conflict-of-interest statement: All authors have no conflict of interest related to the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Abenavoli L, Fujino Y, Gassler N, Hashimoto N, Nakano H S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Ma ZP, Lin GS, Chen BY, Wang C, Zhou JJ. MRI and clinical analysis of hepatic epithelioid angiomyolipoma. Zhonghua Shiyong Zhenduan Yu Zhiliao Zazhi. 2018;32:172-175. [DOI] [Full Text] |
| 2. | Lee SY, Kim BH. Epithelioid angiomyolipoma of the liver: a case report. Clin Mol Hepatol. 2017;23:91-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Yang XG, Liu GJ, He ML, Hu YF, Tong QY, Qiu WJ. CT and MRI characteristics of hepatic epithelial angiomyolipoma. Shiyong Fangshexue Zazhi. 2017;33:1545-1548. [DOI] [Full Text] |
| 4. | Zhang J, Wang C, Ma ZP. Manifestation of CT in epithelioid angiomyolipoma of liver. Gandanyi Waike Zazhi. 2017;29:123-127. [DOI] [Full Text] |
| 5. | Tan Y, Xie X, Lin Y, Huang T, Huang G. Hepatic epithelioid angiomyolipoma: clinical features and imaging findings of contrast-enhanced ultrasound and CT. Clin Radiol. 2017;72:339.e1-339.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Fan PL, Wang WP, Mao F, Ji Y, Xu C, Ji ZB, Huang BJ. Ultrasound Characteristics of Hepatic Epithelioid Angiomyolipoma. Zhongguo Chaosheng Yixue Zazhi. 2017;33:147-149. |
| 7. | Liu J, Zhang CW, Hong DF, Tao R, Chen Y, Shang MJ, Zhang YH. Primary hepatic epithelioid angiomyolipoma: A malignant potential tumor which should be recognized. World J Gastroenterol. 2016;22:4908-4917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Ortiz S, Tortosa F. Epithelioid angiomyolipoma of the liver: Clinicopathological correlation in a series of 4 cases. Rev Esp Enferm Dig. 2016;108:27-30. [PubMed] |
| 9. | Liu W, Meng Z, Liu H, Li W, Wu Q, Zhang X, E C. Hepatic epithelioid angiomyolipoma is a rare and potentially severe but treatable tumor: A report of three cases and review of the literature. Oncol Lett. 2016;11:3669-3675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Fukuda Y, Omiya H, Takami K, Mori K, Kodama Y, Mano M, Nomura Y, Akiba J, Yano H, Nakashima O, Ogawara M, Mita E, Nakamori S, Sekimoto M. Malignant hepatic epithelioid angiomyolipoma with recurrence in the lung 7 years after hepatectomy: a case report and literature review. Surg Case Rep. 2016;2:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Liu LN, Xie LQ. Diagnosis of hepatic epithelioid angiomyolipoma: one case report and review of literature. Yixue Yingxiangxue Zazhi. 2016;26:753-755. |
| 12. | Wu RD, Yang XF, Wang M, Zhou LS, Huang K, Fang ZN, Zhen KG, Feng ST. Hepatic Epithelioid Angiomyolipoma MSCT Findings. Linchuang Fangshexue Zazhi. 2016;35:395-398. [DOI] [Full Text] |
| 13. | Song X, Xu SL, Xiao WB. CT and MRI manifestations of hepatic epithelioid angiomyolipomas. Zhongguo Yixue Yingxiang Jishu. 2016;32:87-90. [DOI] [Full Text] |
| 14. | Yu YH, Wu JT, Wang SA. Imaging features of hepatic epithelioid angiomyolipoma. Yiliao Weisheng Zhuangbei. 2016;37:75-77. [DOI] [Full Text] |
| 15. | Huang SC, Chuang HC, Chen TD, Chi CL, Ng KF, Yeh TS, Chen TC. Alterations of the mTOR pathway in hepatic angiomyolipoma with emphasis on the epithelioid variant and loss of heterogeneity of TSC1/TSC2. Histopathology. 2015;66:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Sun J, Wang S, Chen W, Wu J. Gd-EOB-DTPA-enhanced and diffusion-weighted magnetic resonance findings in hepatic epithelioid angiomyolipoma: A case report. Oncol Lett. 2015;10:1145-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Tang YQ, Li JW, Ling WW, Ling L, Qiang LU, Yan L. Features of hepatic epithelioid angiomyolipoma on ultrasonography. Zhongguo Yixue Yingxiang Jishu. 2015;31:746-749. [DOI] [Full Text] |
| 18. | Mei HY, Wang SY. Imaging diagnosis of epithelioid angiomyolipoma of liver. Shiyong Yixue Yingxiang Zazhi. 2015;2:154-156. [DOI] [Full Text] |
| 19. | Wu QQ, Chen ZQ. Imaging findings and pathologic correlation of hepatic epithelioid angiomyolipoma. Yixue Yingxiangxue Zazhi. 2015;4:662-665. |
| 20. | Zhang JW, Xu PJ. Epithelioid Angiomyolipoma of the Liver: Analysis of Magnetic Resonance Imaging Features. Zhongguo Yixue Jisuanji Chengxiang Zazhi. 2015;21:124-128. [DOI] [Full Text] |
| 21. | Barbier L, Torrents J, Hardwigsen J. Hepatic angiomyolipoma: what management? Acta Chir Belg. 2014;114:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Dai CL, Xue LP, Li YM. Multi-slice computed tomography manifestations of hepatic epithelioid angiomyolipoma. World J Gastroenterol. 2014;20:3364-3368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Tajima S, Suzuki A, Suzumura K. Ruptured hepatic epithelioid angiomyolipoma: a case report and literature review. Case Rep Oncol. 2014;7:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Xu H, Wang H, Zhang X, Li G. [Hepatic epithelioid angiomyolipoma: a clinicopathologic analysis of 25 cases]. Zhonghua Bing Li Xue Za Zhi. 2014;43:685-689. [PubMed] |
| 25. | Zhao Y, Ouyang H, Wang X, Ye F, Liang J. MRI manifestations of liver epithelioid and nonepithelioid angiomyolipoma. J Magn Reson Imaging. 2014;39:1502-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Zhou Y, Chen F, Jiang W, Meng Q, Wang F. Hepatic epithelioid angiomyolipoma with an unusual pathologic appearance: expanding the morphologic spectrum. Int J Clin Exp Pathol. 2014;7:6364-6369. [PubMed] |
| 27. | Zhu H, Zheng XL, Wei M, Center MI. MRI Diagnosis of Hepatic Epithelioid Angiomyolipoma. Zhongguo CT He MRI Zazhi. 2014;3:75-77. [DOI] [Full Text] |
| 28. | Occhionorelli S, Dellachiesa L, Stano R, Cappellari L, Tartarini D, Severi S, Palini GM, Pansini GC, Vasquez G. Spontaneous rupture of a hepatic epithelioid angiomyolipoma: damage control surgery. A case report. G Chir. 2013;34:320-322. [PubMed] |
| 29. | Kang SL, He B, Han D. One case: lipid-poor hepatic epithelioid angiomyolipoma of the male. Shiyong Fangshexue Zazhi. 2013;29:338-339. |
| 30. | Ji JS, Lu CY, Wang ZF, Xu M, Song JJ. Epithelioid angiomyolipoma of the liver: CT and MRI features. Abdom Imaging. 2013;38:309-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Lo RC. Epithelioid angiomyolipoma of the liver: a clinicopathologic study of 5 cases. Ann Diagn Pathol. 2013;17:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Saito Y, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Sugimoto K, Iwahashi S, Yamada S, Asanoma M, Ishibashi H. Hepatic epithelioid angiomyolipoma with arterioportal venous shunting mimicking hepatocellular carcinoma: report of a case. J Med Invest. 2013;60:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Li FQ, Qian HF, Zhu YM, Ding Y, Hu XH. Diagnosis of Hepatic Epithelioid Angiomyolipoma by MRI. Yixue Yanjiu Zazhi. 2013;42:164-167. [DOI] [Full Text] |
| 34. | Wu ZJ, Hua H, Chen JJ, Jiang G, Li XF, Li SK, Xu WJ. CT characteristics of hepatic epithelial angiomyolipoma. Zhongguo Yixue Yingxiang Jishu. 2013;29:84-87. [DOI] [Full Text] |
| 35. | Agaimy A, Vassos N, Croner RS, Strobel D, Lell M. Hepatic angiomyolipoma: a series of six cases with emphasis on pathological-radiological correlations and unusual variants diagnosed by core needle biopsy. Int J Clin Exp Pathol. 2012;5:512-521. [PubMed] |
| 36. | Limaiem F, Korbi S, Lahmar A, Bouraoui S, Aloui S, Jedidi S, Miloudi N, Mzabi-Regaya S. A misleading hepatic tumour: epithelioid angiomyolipoma. Acta Gastroenterol Belg. 2012;75:443-445. [PubMed] |
| 37. | Xie L, Jessurun J, Manivel JC, Pambuccian SE. Hepatic epithelioid angiomyolipoma with trabecular growth pattern: a mimic of hepatocellular carcinoma on fine needle aspiration cytology. Diagn Cytopathol. 2012;40:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Liu H, Zhou BY, Lin H. Hepatic Epithelioid Angiomyolipoma One Case Report of Ultrasonography and Review of Literature. Hanshao Jibing Zazhi. 2012;19:34-37. [DOI] [Full Text] |
| 39. | Liu JP, Chen ZJ, Huang YY, Sun ZS, Lu CY. Imaging and pathological features of hepatic monotypic epithelioid angiomyolipoma. Yixue Yingxiangxue Zazhi. 2012;22:219-221. [DOI] [Full Text] |
| 40. | Wang ZH, Wu D, Hou J, Zhang LT, Kong QK. MRI diagnosis of epithelioid angiomyolipoma of liver. Zhonghua Fangshexue Zazhi. 2012;46:803-806. [DOI] [Full Text] |
| 41. | Zhang LP, Tang BH, Li LC, Li FY, He YQ, Wu RG, Huang H, Huang DC. CT Features of Epithelioid Angiomyolipoma in Liver: Report of 2 Cases and Literature Review. Linchuang Fangshexue Zazhi. 2012;31:1805-1807. |
| 42. | Talati H, Radhi J, Popovich S, Marcaccio M. Hepatic Epithelioid Angiomyolipoma: Case Series. Gastroenterology Res. 2010;3:293-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Ma HL, Yang CB, Chen WB, Zhang WJ, Li L. Hepatic epithelioid angiomyolipoma: an analysis of two cases. Shijie Huaren Xiaohua Zazhi. 2011;19:1071-1074. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Zhang B, LV ZL, Qin X, Peng MH, Peng T. Hepatic epithelioid angiomyolipoma: A case report and literature review. Zhongguo Aizheng Fangzhi Zazhi. 2011;3:120-125. [DOI] [Full Text] |
| 45. | Wen MC, Jan YJ, Li MC, Wang J, Lin A. Monotypic epithelioid angiomyolipoma of the liver with TFE3 expression. Pathology. 2010;42:300-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Fu LP, Bao YW, Ji JS, Zhao ZW, Xu M, Wang ZF, Fan XX. Imaging Diagnosis of Epithelioid Angiomyolipoma of Liver. Shiyong Fangshexue Zazhi. 2010;26:973-976. [DOI] [Full Text] |
| 47. | Alatassi H, Sahoo S. Epithelioid angiomyolipoma of the liver with striking giant cell component: fine-needle aspiration biopsy findings of a rare neoplasm. Diagn Cytopathol. 2009;37:192-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Yamasaki S, Tanaka S, Fujii H, Matsumoto T, Okuda C, Watanabe G, Suda K. Monotypic epithelioid angiomyolipoma of the liver. Histopathology. 2000;36:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Leenman EE, Mukhina MS, Nasyrov AR. [Monophasic angiomyolipoma (PEComa) of the liver]. Arkh Patol. 2009;71:44-46. [PubMed] |
| 50. | Xu PJ, Shan Y, Yan FH, Ji Y, Ding Y, Zhou ML. Epithelioid angiomyolipoma of the liver: cross-sectional imaging findings of 10 immunohistochemically-verified cases. World J Gastroenterol. 2009;15:4576-4581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Yuan Y, Zhang J, Huang YN. One cases report of epithelioid angiomyolipoma (EAML) of liver. Xiandai Zhongliu Yixue. 2009;17:1776-1780. |
| 52. | Deng YF, Lin Q, Zhang SH, Ling YM, He JK, Chen XF. Malignant angiomyolipoma in the liver: a case report with pathological and molecular analysis. Pathol Res Pract. 2008;204:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Khalbuss WE, Fischer G, Bazooband A. Imprint cytology of epithelioid hepatic angiomyolipoma: mimicry of hepatocellular carcinoma. Acta Cytol. 2007;51:670-672. [PubMed] |
| 54. | Parfitt JR, Bella AJ, Izawa JI, Wehrli BM. Malignant neoplasm of perivascular epithelioid cells of the liver. Arch Pathol Lab Med. 2006;130:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 55. | Rouquie D, Eggenspieler P, Algayres JP, Béchade D, Camparo P, Baranger B. [Malignant-like angiomyolipoma of the liver: report of one case and review of the literature]. Ann Chir. 2006;131:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Mizuguchi T, Katsuramaki T, Nobuoka T, Nishikage A, Oshima H, Kawasaki H, Kimura S, Satoh M, Hirata K. Growth of hepatic angiomyolipoma indicating malignant potential. J Gastroenterol Hepatol. 2004;19:1328-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Tryggvason G, Blöndal S, Goldin RD, Albrechtsen J, Björnsson J, Jónasson JG. Epithelioid angiomyolipoma of the liver: case report and review of the literature. APMIS. 2004;112:612-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Hino A, Hirokawa M, Takamura K, Sano T. Imprint cytology of epithelioid angiomyolipoma in a patient with tuberous sclerosis. A case report. Acta Cytol. 2002;46:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Lin Y, Shi Q, Zhou X. Hepatic monotypic epithelioid angiomyolipoma:two cases report and review of literature. Zhenduan Binglixue Zazhi. 2002;9:281-276. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Mai KT, Yazdi HM, Perkins DG, Thijssen A. Fine needle aspiration biopsy of epithelioid angiomyolipoma. A case report. Acta Cytol. 2001;45:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Croquet V, Pilette C, Aubé C, Bouju B, Oberti F, Cervi C, Arnaud JP, Rousselet MC, Boyer J, Calès P. Late recurrence of a hepatic angiomyolipoma. Eur J Gastroenterol Hepatol. 2000;12:579-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Dalle I, Sciot R, de Vos R, Aerts R, van Damme B, Desmet V, Roskams T. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Flemming P, Lehmann U, Becker T, Klempnauer J, Kreipe H. Common and epithelioid variants of hepatic angiomyolipoma exhibit clonal growth and share a distinctive immunophenotype. Hepatology. 2000;32:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Savastano S, Piotto M, Mencarelli R, Spanio P, Rubaltelli L. [A monotypic variant of hepatic angiomyolipoma completely composed of perivascular epithelioid cells. A case]. Radiol Med. 2000;100:79-81. [PubMed] |
| 65. | Klompenhouwer AJ, Verver D, Janki S, Bramer WM, Doukas M, Dwarkasing RS, de Man RA, IJzermans JNM. Management of hepatic angiomyolipoma: A systematic review. Liver Int. 2017;37:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 66. | Guo B, Song H, Yue J, Li G. Malignant renal epithelioid angiomyolipoma: A case report and review of the literature. Oncol Lett. 2016;11:95-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Neofytou K, Famularo S, Khan AZ. PEComa in a Young Patient with Known Li-Fraumeni Syndrome. Case Rep Med. 2015;2015:906981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Vicens RA, Jensen CT, Korivi BR, Bhosale PR. Malignant renal epithelioid angiomyolipoma with liver metastasis after resection: a case report with multimodality imaging and review of the literature. J Comput Assist Tomogr. 2014;38:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Nese N, Martignoni G, Fletcher CD, Gupta R, Pan CC, Kim H, Ro JY, Hwang IS, Sato K, Bonetti F, Pea M, Amin MB, Hes O, Svec A, Kida M, Vankalakunti M, Berel D, Rogatko A, Gown AM, Amin MB. Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: A clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol. 2011;35:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 70. | Sato K, Ueda Y, Tachibana H, Miyazawa K, Chikazawa I, Kaji S, Nojima T, Katsuda S. Malignant epithelioid angiomyolipoma of the kidney in a patient with tuberous sclerosis: an autopsy case report with p53 gene mutation analysis. Pathol Res Pract. 2008;204:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Lin WC, Wang JH, Wei CJ, Pan CC, Chang CY. Malignant renal epithelioid angiomyolipoma with aggressive behavior and distant metastasis. J Chin Med Assoc. 2003;66:303-306. [PubMed] |