Published online Feb 26, 2019. doi: 10.12998/wjcc.v7.i4.419
Peer-review started: November 26, 2018
First decision: December 15, 2018
Revised: December 24, 2018
Accepted: December 29, 2018
Article in press: December 30, 2018
Published online: February 26, 2019
Processing time: 93 Days and 19.1 Hours
The needs for human epidermal growth factor receptor 2 (HER-2) and/or programmed death-ligand 1 (PD-L1) evaluations in gastric cancer are dramatically increasing. Although the importance of standardization of sample fixation has been widely recognized, most of the evidence regarding the fixation duration or type of fixing solution are based on breast cancer.
To investigate the real effects of fixation conditions on HER-2 testing or PD-L1 testing for gastric cancer using gastrectomy specimens.
Thirty-two patients who underwent gastrectomy for gastric cancer were enrolled. Their resected specimens were each divided into four pieces and fixed in four strictly controlled different durations (6 h, 24 h, and 48 h, and 1 wk) by 10% formalin (n = 22) or 10% neutral buffered formalin (NBF) (n = 10). Immunohistochemistry (IHC) of HER-2 and PD-1 was performed, and a pathology examination was conducted. In the HER-2-immunoreactive cases, all four specimens were subjected to dual-color in situ hybridization (DISH). Five cases were assessed as HER-2-positive by IHC and DISH. We used the cut-off values of 1%, 10%, and 50% to assess the IHC findings of PD-L1.
No significant difference was observed in comparisons between the shorter fixation period groups (6 h, 24 h, and 48 h) and the prolonged fixation period (1 wk) group in the HER-2 and PD-L1 analyses. Although no significant difference was observed between 10% formalin and 10% NBF within 1 wk of fixation, the superiority of 10% NBF was confirmed in a long-term (> 3 mo) fixation in both the HER-2 and PD-L1 analyses.
In this small-numbered pilot study, prolonged fixation within 1 wk showed no inferiority in HER-2 or PD-L1 testing. However, a large-numbered prospective study is needed to obtain conclusive results.
Core tip: We prospectively investigated the real effects of fixation conditions on human epidermal growth factor receptor 2 (HER-2) or programmed death-ligand 1 (PD-L1) testing for gastric cancer using 32 cases of gastrectomy specimens. We analyzed these resected specimens dividing into four pieces and fixed in four strictly controlled different durations (6 h, 24 h, and 48 h, and 1 wk) by 10% formalin or 10% neutral buffered formalin. In this small-numbered pilot study, the prolonged fixation within 1 wk showed no inferiority in HER-2 or PD-L1 testing. These results will provide supporting information for the interpretation of HER-2 and PD-L1 testing for prolonged fixation cases due to unavoidable circumstances.
- Citation: Kai K, Yoda Y, Kawaguchi A, Minesaki A, Iwasaki H, Aishima S, Noshiro H. Formalin fixation on HER-2 and PD-L1 expression in gastric cancer: A pilot analysis using the same surgical specimens with different fixation times. World J Clin Cases 2019; 7(4): 419-430
- URL: https://www.wjgnet.com/2307-8960/full/v7/i4/419.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i4.419
After the results of 2010 international prospective randomized phase III trial (the ToGA study)[1], trastuzumab therapy combined with chemotherapy became the standard treatment for human epidermal growth factor receptor 2 (HER-2)-positive advanced gastric cancer. The needs for HER-2 evaluations in gastric cancer by immunohistochemistry (IHC) and/or in situ hybridization (ISH) has dramatically increased and the importance of standardization of HER-2 testing has been widely recognized.
It was noted that fixation procedures affect the results of HER-2 testing[2]. The updated 2013 American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guideline recommends the fixation of HER-2 testing specimens in 10% neutral buffered formalin (NBF) for 6 to 72 h[3,4]. However, in some cases it may be difficult to strictly control the formalin fixation time due to institutional constraints, and the fixation time may exceed 72 h. Moreover, some institutions do not use 10% NBF as a standard fixation solution. Even though some institutions strictly control the formalin fixation times and use 10% NBF in their routine work, it is sometimes necessary to perform HER-2 testing using previous formalin-fixed paraffin-embedded (FFPE) gastric cancer tissues for which the fixation solution and/or duration have not been managed.
There is not enough evidence regarding the interpretation of the results of HER-2 testing for gastric cancer conducted using fixed solutions other than 10% NBF or prolonged-fixation specimens. Most of the evidence regarding the relationship between HER-2 testing and the fixation duration or type of fixing solution was obtained in studies of breast cancer[5-11]. Although it seems reasonable that the findings obtained for breast cancer may be applicable to gastric cancer, the data that are specific to gastric cancer are needed, because characteristics of HER-2 overexpression (e.g., positivity and heterogeneity) are quite different between breast cancer and gastric cancer[12-14]. We could find only one study using a xenograft model of gastric cancer cell lines[15] and a single clinical study of gastric cancer[16] that analyzed the association between HER-2 test results and the formalin fixation status.
A significant therapeutic effect of a humanized monoclonal antibody against programmed death-1 (PD-1) was confirmed for non-small-cell lung cancer (NSCLC), which has shown a high level of programmed death-ligand 1 (PD-L1) expression[17], after which the need for the immunohistochemical evaluations of PD-L1 in NSCLC specimens has greatly increased. In gastric cancer, a multicenter, open-label, phase 1b trial recently reported the safety and efficacy of a humanized monoclonal antibody against PD-1 for patients with PD-L1-positive advanced gastric cancer[18]. Therefore, although the utility of PD-L1 IHC for the prediction of the effectiveness of anti-PD-1 therapy is under investigation[19], it is expected that the need for PD-L1 IHC in gastric cancer will increase in the near future.
In the present study, we sought to verify the effect of the duration of formalin fixation on both HER-2 and PD-L1 testing, using surgically resected gastric cancer specimens. We also attempted to determine whether prolonged fixation (we considered 1 wk as an appropriate maximum prolonged-fixation duration in a daily pathology practice) is truly inferior to a more standard fixation period. We also examined the differences between the uses of 10% NBF and 10% formalin in HER-2 and PD-L1 testing.
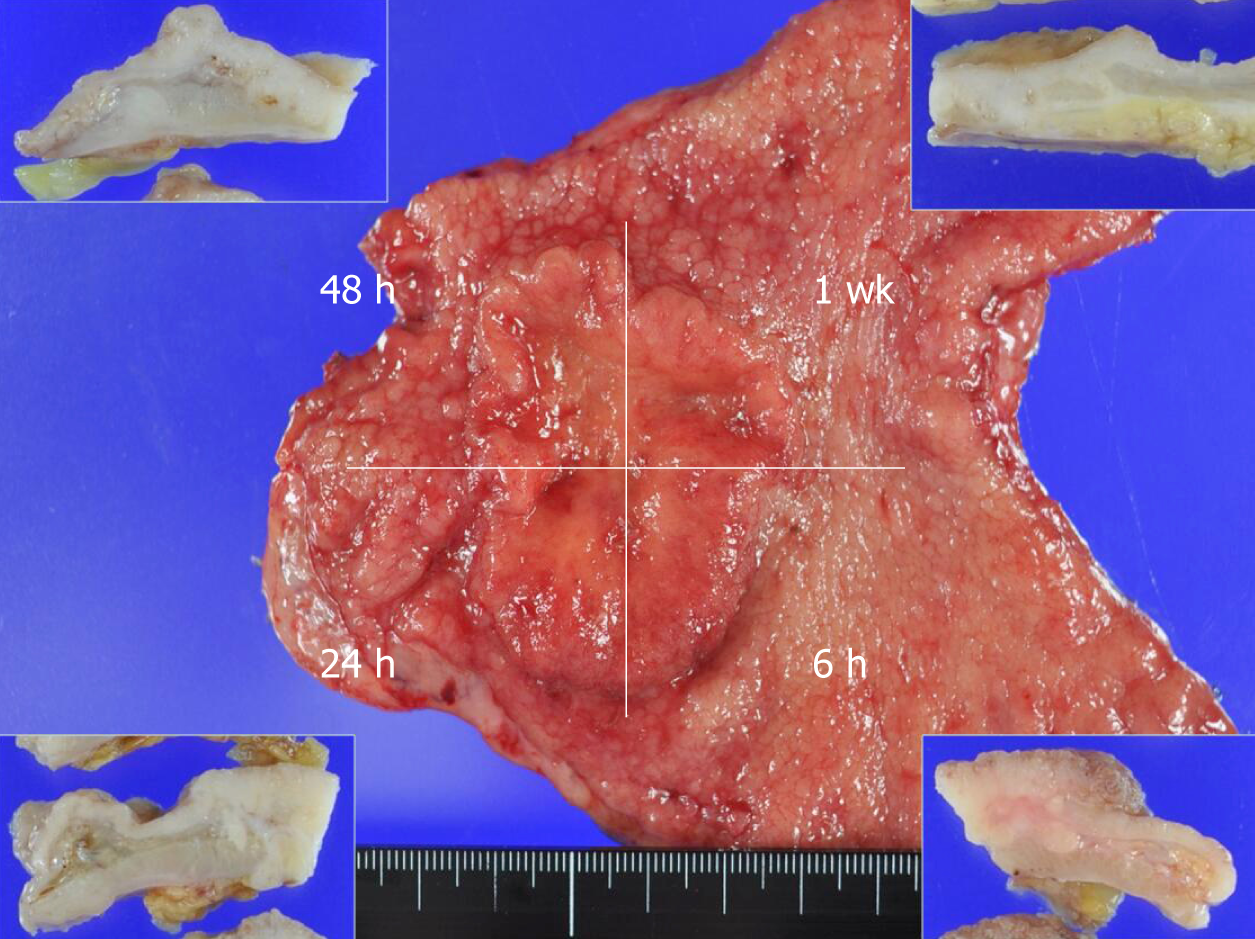
This study protocol was reviewed and approved by the Ethics Committee of the Faculty of Medicine at Saga University (approval No. 29-74), and written informed consent for the study was obtained from all of the patients. From 2014 to 2016, a total of 32 consecutive patients with gastric cancer clinically diagnosed as a Type 2 or Type 3 tumor and who underwent a gastrectomy without neoadjuvant chemotherapy were enrolled in this study. The resected tumor specimen from each patient was immediately treated by a pathologist (K.K.). Each patient’s specimen was divided into four pieces and fixed by fixing solution for a strictly controlled duration (6 h, 24 h, 48 h, or 1 wk) (Figure 1). For the first 22 cases, 10% formalin was used as the fixing solution. For the remaining 10 cases, 10% NBF was used.
Paraffin-embedded specimens were sectioned at a thickness of 3 μm and subjected to IHC and DISH. The HER-2 IHC was performed using the HercepTest II (Agilent Technologies, Santa Clara, CA) on an Autostainer Link 48 platform (Agilent Technologies) per the manufacturer's instructions. We outsourced the DISH to a clinical test company (BML, Kurume Laboratory, Saitama, Japan) and they performed DISH using a Ventana INFORM Dual ISH HER2 kit (Roche Diagnostics, Tokyo).
Our evaluation of the IHC results (score 0, 1+, 2+, and 3+) and DISH results were based on the Updated 2013 ASCO/CAP guideline[3,4]. If at least one specimen was score 1+ or more for all four specimen-pieces with different fixation times, all four pieces were subjected to the DISH analysis.
The assessment of the HER-2 IHC and DISH results was performed by consensus of two pathologists (K.K. and S.A.). When at least one specimen-piece was revealed to be positive by IHC or by DISH among the same specimen's four pieces examined with different fixation times, we considered the result HER-2-positive.
For the PD-L1 IHC, we prepared a TMA using a JF-4 Tissue Microarrayer (Sakura Finetek Japan, Tokyo). A tissue core (2.0 mm) from each of a patient specimen’s four fixation pieces was selected and used for the TMA. We performed IHC of PD-L1 using the PD-L1 IHC 22C3 pharmDx kit (Agilent Technologies) on an Autostainer Link 48 platform (Agilent Technologies) per the manufacturer's instructions. Membranous expression of tumor cells irrespective of its intensity was considered PD-L1-positivity. The same two pathologists (K.K. and S.A.) assessed the IHC results and achieved a consensus when necessary. The IHC specimens were categorized into 0%, 1%-9%, and every 10% of PD-L1-positive cells. We set cut-offs as 1%, 10%, and 50% PD-L1-positive cells. When at least one of a specimen's four pieces was positive at a given cut-off in a fixation time, we considered this result PD-L1 positivity.
All statistical analyses were performed using JMP ver. 12.2 software (SAS, Cary, NC) or R version 3.4.4 (free software). The findings from pairs of groups were compared by Student's t-test or Pearson's chi-square test, as appropriate. We compared the “proper” fixation periods (6 h, 24 h, and 48 h) and the prolonged fixation period (1 wk) was performed by simultaneous tests for linear hypotheses (the difference of the average of the proper fixation period and the prolonged fixation period) based on a linear mixed effects model. In this analysis, we used the HER2/CEP17 ratio for the assessment of DISH findings, and we regarded the finding of “0%-9%” of PD-L1 as “5%”. P-values < 0.05 were accepted as significant. All statistical analyses were supervised by the co-author statistician (A. K.).
The clinicopathological features of the 32 gastric cancer cases are summarized in Table 1. The mean age of all patients is 74.5 years old; 18 males and 14 females were enrolled. Based on pathological examinations, six cases were classified as T1b2, nine cases were T2, five cases were T3, and 12 cases were T4a according to TNM classification. Eighteen cases were classified as intestinal type, and the other 14 cases were classified as diffuse/mixed type by Lauren's classification. No significant difference was observed between the 10% formalin-fixed group (n = 22) and the 10% NBF-fixed group (n = 10) in the comparisons of age, gender, T-stage, Lauren's classification, HER-2 positivity, or PD-L1 positivity.
| All cases (n = 32) | 10% formalin (n = 22) | 1% NBF (n = 10) | P | |
| Age (mean ± SD) | 74.5 ± 7.8 | 73.3 ± 8.0 | 77.1 ± 7.0 | 0.2020 |
| Gender | 0.7731 | |||
| Male | 18 (56.3) | 12 (54.6) | 6 (60.0) | |
| Female | 14 (43.7) | 10 (45.4) | 4 (40.0) | |
| T-Stage | 0.6793 | |||
| T1b2 | 6 (18.8) | 3 (13.6) | 3 (30.0) | |
| T2 | 9 (28.1) | 6 (27.3) | 3 (30.0) | |
| T3 | 5 (15.6) | 4 (18.2) | 1 (10.0) | |
| T4a | 12 (37.5) | 9 (40.9) | 3 (30.0) | |
| Lauren's classification | ||||
| Intestinal | 18 (56.2) | 12 (54.6) | 6 (60.0) | 0.7731 |
| Diffuse / mixed | 14 (43.8) | 10 (45.4) | 4 (40.0) | |
| HER-2 IHC | ||||
| Complete negative1 | 24 (75) | 18 (81.8) | 6 (60.0) | 0.1966 |
| Final positive by DISH | 5 (15.6) | 2 (9.1) | 3 (30.0) | 0.1311 |
| PD-L1 positivity | ||||
| Cut off 1% | 20 (62.5) | 14 (63.4) | 6 (60.0) | 0.8439 |
| Cut off 10% | 13 (40.6) | 8 (36.4) | 5 (50.0) | 0.4666 |
| Cut off 50% | 7 (21.9) | 4 (18.2) | 3 (30.0) | 0.4535 |
The whole data of IHC, i.e., both the HER-2 and PD-L1 results, are summarized in Table 2. Of the 32 cases, 24 (75%) were completely negative (score 0) for HER-2 IHC, and the remaining eight cases showed positive HER-2 staining of score 1+ or more in at least one of the four specimens pieces (subjected to the four different fixation times). No significant difference was observed in the comparisons between the three proper fixation periods (6 h, 24 h, and 48 h) and the prolonged fixation period (1 wk) groups (P = 0.7713).
| Case No. | Formalin | HER-2 IHC score; P = 0.7713 (6 h-48 h vs 1 wk)1 | PD-L1 expression (%); P = 0.46 (6 h-48 h vs 1 wk)1 | ||||||
| 6 h | 24 h | 48 h | 1 wk | 6 h | 24 h | 48 h | 1 wk | ||
| 1 | 10% F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 10% F | 0 | 0 | 0 | 0 | 10 | 10 | 10 | 1-9 |
| 3 | 10% F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 10% F | 0 | 0 | 0 | 0 | 80 | 80 | 90 | 90 |
| 5 | 10% F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1-9 |
| 6 | 10% F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 10% F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 10% F | 0 | 0 | 0 | 0 | 0 | 0 | 1-9 | 1-9 |
| 9 | 10% F | 0 | 0 | 0 | 0 | 0 | 1-9 | 0 | 1-9 |
| 10 | 10% F | 0 | 0 | 0 | 0 | 40 | 30 | 30 | 40 |
| 11 | 10% F | 0 | 0 | 0 | 0 | 1-9 | 0 | 10 | 10 |
| 12 | 10% F | 3+ | 3+ | 3+ | 3+ | 1-9 | 1-9 | 1-9 | 0 |
| 13 | 10% F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 10% F | 1+ | 2+ | 0 | 0 | 100 | 1-9 | 30 | 60 |
| 15 | 10% F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 10% F | 0 | 0 | 0 | 0 | 1-9 | 10 | 1-9 | 1-9 |
| 17 | 10% F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 10% F | 0 | 0 | 0 | 0 | 20 | 60 | 20 | 40 |
| 19 | 10% F | 0 | 0 | 0 | 0 | 1-9 | 1-9 | 1-9 | 0 |
| 20 | 10% F | 2+ | 0 | 0 | 0 | 80 | 80 | 20 | 1-9 |
| 21 | 10% F | 0 | 0 | 0 | 0 | 1-9 | 0 | 1-9 | 1-9 |
| 22 | 10% F | 1+ | 1+ | 2+ | 2+ | 0 | 0 | 0 | 0 |
| 23 | 10% NBF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 10% NBF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 10% NBF | 0 | 1+ | 2+ | 1+ | 10 | 0 | 10 | 0 |
| 26 | 10% NBF | 2+ | 2+ | 2+ | 2+ | 0 | 0 | 0 | 0 |
| 27 | 10% NBF | 2+ | 1+ | 1+ | 3+ | 20 | 70 | 30 | 1-9 |
| 28 | 10% NBF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 29 | 10% NBF | 0 | 0 | 0 | 0 | 1-9 | 1-9 | 0 | 1-9 |
| 30 | 10% NBF | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 |
| 31 | 10% NBF | 3+ | 3+ | 3+ | 3+ | 0 | 1-9 | 10 | 1-9 |
| 32 | 10% NBF | 0 | 0 | 0 | 0 | 70 | 80 | 90 | 90 |
Twelve cases (37.5%) were completely negative for PD-L1 IHC, and the remaining 20 cases showed positive staining of ≥ 1% tumor cells in at least one of the four specimen pieces. No significant difference was revealed in our comparisons between the proper fixation period group and the prolonged-fixation group (P = 0.46).
The details of the HER-2 IHC of the eight cases which showed HER-2 immunoreactivity and the DISH results are summarized in Table 3. As the HER-2 amplification could not be confirmed by DISH analysis, three cases (case nos. 14, 20 and 25) were assessed as “HER-2-negative”. A final total of five cases were assessed as “HER-2-positive”. Among these five cases, two cases (case nos. 12 and 31) diffusely expressed HER-2 irrespective of the fixation duration. No significant difference was observed in the comparisons between the proper fixation period groups and the prolonged-fixation group in the analysis of HER-2 IHC (P = 1.000) or the HER2/CEP17 ratio in the DISH analysis (P = 0.6989).
| Case No. | Formalin | HER-2 IHC score; P = 1.000 (6 h-48 h vs 1 wk)1 | DISH; HER2/CEP17ratio (average HER2 copy number); P = 0.6989 (6 h-48 h vs 1 wk)1 | Final assessment | ||||||
| 6 h | 24 h | 48 h | 1 wk | 6 h | 24 h | 48 h | 1 wk | |||
| 12 | 10% F | 3+ | 3+ | 3+ | 3+ | 11.4 (33.7) | 10.9 (28.5) | 7.1 (20.4) | 4.8 (17.4) | + |
| 14 | 10% F | 1+ | 2+ | 0 | 0 | 1.2 (3.0) | 1.3 (3.3) | 1.3 (2.2) | 1.3 (2.2) | - |
| 20 | 10% F | 2+ | 0 | 0 | 0 | 0.8 (2.1) | 1.2 (2.8) | 1.3 (2.1) | 1.3 (2.0) | - |
| 22 | 10% F | 1+ | 1+ | 2+ | 2+ | 1.6 (3.4) | 1.9 (4.4) | 2.1 (2.5) | 2.1(2.8) | + |
| 25 | 10% NBF | 0 | 1+ | 2+ | 1+ | 1.0 (1.9) | 1.1 (2.2) | 1.8 (3.1) | 1.4 (2.5) | - |
| 26 | 10% NBF | 2+ | 2+ | 2+ | 2+ | 2.6 (5.2) | 3.7 (6.5) | 3.5 (6.6) | 3.3 (5.7) | + |
| 27 | 10% NBF | 2+ | 1+ | 1+ | 3+ | 1.1 (2.9) | 1.4 (2.8) | 1.3 (2.1) | 2.2 (4.9) | + |
| 31 | 10% NBF | 3+ | 3+ | 3+ | 3+ | 4.7 (19.8) | 6.7 (21.2) | 4.7 (15.7) | 7.2 (21.2) | + |
Among the five HER-2-positive cases, two showed a discrepant expression of HER-2. In case 22, the 6 h-fixation piece was confirmed as negative by both IHC (1+) and DISH, and the 24 h-fixation piece was indicated as negative by IHC (1+) but equivocal by DISH (HER2/CEP17: 1.9, average copy number: 4.4), and both the 48 h- and 1 wk-fixed pieces were equivocal by IHC but positive by DISH.
In case 27, only the 1 wk-fixed piece was assessed as positive by both IHC (3+) and DISH; the other three pieces (6 h-, 24 h-, and 48 h-fixed) were negative or equivocal by IHC and all negative by DISH. We speculate that these discrepant results could be explained by the heterogeneity of the HER-2 expression of the tumor tissues.
The details of the PD-L1 IHC of the 20 cases (excluding the 12 completely PD-1-negative cases) are summarized in Table 4. In the assessment using the 1% cut-off, all 20 cases were determined as PD-L1-positive. With the 10% cut-off, 13 cases were PD-L1-positive, and with the 50% cut-off, seven cases were PD-L1-positive. No significant difference was revealed by our comparisons of the three proper fixation period groups and the prolonged-fixation group in the analysis of PD-L1 IHC (P = 0.4605).
| Case No. | Used formalin | PD-L1 expression; P = 0.4605 (6 h-48 h vs 1 wk) 1 | Assessment (Cut-off 1%) | Assessment (Cut-off 10%) | Assessment (Cut-off 50%) | |||
| 6 h | 24 h | 48 h | 1 wk | |||||
| 2 | 10% F | 10 | 10 | 10 | 1-9 | + | + | - |
| 4 | 10% F | 80 | 80 | 90 | 90 | + | + | + |
| 5 | 10% F | 0 | 0 | 0 | 1-9 | + | - | - |
| 8 | 10% F | 0 | 0 | 1-9 | 1-9 | + | - | - |
| 9 | 10% F | 0 | 1-9 | 0 | 1-9 | + | - | - |
| 10 | 10% F | 40 | 30 | 30 | 40 | + | + | - |
| 11 | 10% F | 1-9 | 0 | 10 | 10 | + | + | - |
| 12 | 10% F | 1-9 | 1-9 | 1-9 | 0 | + | - | - |
| 14 | 10% F | 100 | 1-9 | 30 | 60 | + | + | + |
| 16 | 10% F | 1-9 | 10 | 1-9 | 1-9 | + | + | - |
| 18 | 10% F | 20 | 60 | 40 | 40 | + | + | + |
| 19 | 10% F | 1-9 | 1-9 | 1-9 | 0 | + | - | - |
| 20 | 10% F | 80 | 80 | 20 | 1-9 | + | + | + |
| 21 | 10% F | 1-9 | 0 | 1-9 | 1-9 | + | - | - |
| 25 | 10% NBF | 10 | 0 | 10 | 0 | + | + | - |
| 27 | 10% NBF | 20 | 70 | 30 | 1-9 | + | + | + |
| 29 | 10% NBF | 1-9 | 1-9 | 0 | 1-9 | + | - | - |
| 30 | 10% NBF | 100 | 100 | 100 | 100 | + | + | + |
| 31 | 10% NBF | 0 | 1-9 | 10 | 1-9 | + | + | - |
| 32 | 10% NBF | 70 | 80 | 90 | 90 | + | + | + |
We re-assessed the five cases that received the final assessment of HER-2-positivity and the three of the seven cases assessed as PD-L1-positive with the 50% cut-off (long-fixed tissue of four cases were unavailable) by HER-2 or PD-L1 IHC using cancer tissue that had been fixed for very long durations, i.e., 3 mo to 28 mo. The fixation durations and the IHC results for each case are summarized in Table 5.
| Case No. | Formalin | Min. expression (6 h - 1 wk fixation) | Max. expression (6 h - 1 wk fixation) | Fixation period (mo) | Results after long fixation | |
| HER-2: | ||||||
| 12 | 10% F | Score 3+ | Score 3+ | 19 | Score 2+ | |
| 22 | 10% F | Score 1+ | Score 2+ | 3 | Score 0 | |
| 26 | 10% NBF | Score 2+ | Score 2+ | 20 | Score 0 | |
| 27 | 10% NBF | Score 1+ | Score 3+ | 18 | Score 0 | |
| 31 | 10% NBF | Score 3+ | Score 3+ | 16 | Score 3+ | |
| PD-L1: | ||||||
| 20 | 10% F | 1%-9% | 80% | 28 | 0% | |
| 30 | 10% NBF | 100% | 100% | 16 | 90% | |
| 32 | 10% NBF | 70% | 90% | 14 | 70% |
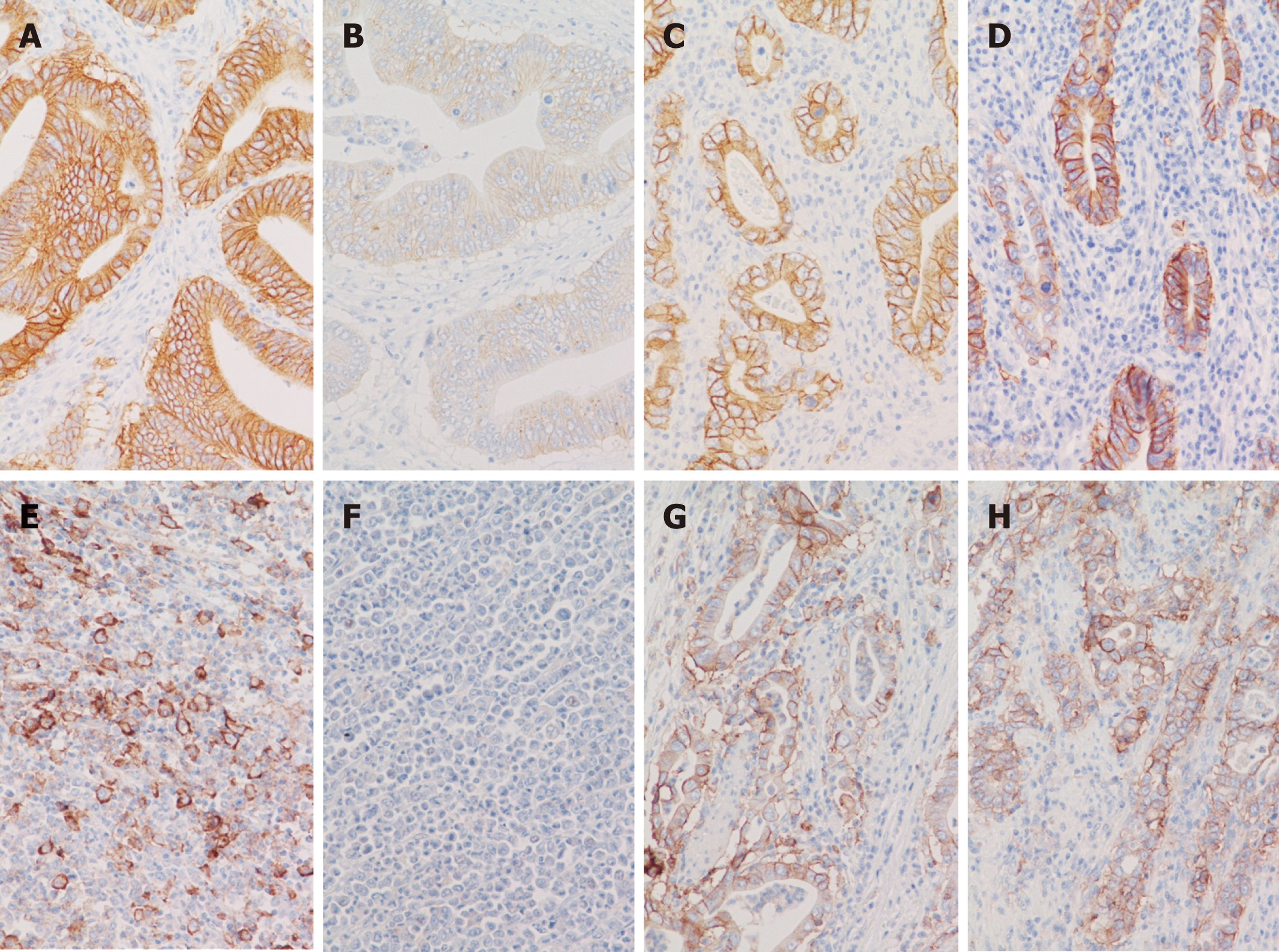
All three heterogeneous cases (cases 22, 26 and 27) completely lost HER-2 expression after long fixation by both 10% formalin and 10% NBF. Of note, the HER-2 expression of case 12, which diffusely and strongly expressed HER-2 (Figure 2A), was significantly weakened (assessed as 2+) after long fixation by 10% formalin (Figure 2B). In contrast, the HER-2 expression of case 31, which also diffusely and strongly expressed HER-2 (Figure 2C), maintaining strong expression (3+) even after long fixation by 10% NBF (Figure 2D).
In case 20, which expressed PD-L1 at the maximum 80% of cancer cells (Figure 2E), the PD-L1 expression was completely lost after long fixation by 10% formalin (Figure 2F). In contrast, the PD-L1 expression was maintained even after long fixation in cases 30 and 32, which were fixed by 10%NBF (Figure 2G and H).
In 2007, the ASCO/CAP guideline recommended the fixation duration of 6 h to 48 h using 10%NBF for HER-2 testing in breast cancer[20], and this recommendation was changed to 6 h-72 h in the updated 2013 ASCO/CAP guideline[3] based on the accumulated data and to conform with the ASCO/CAP estrogen receptor (ER)/progesterone receptor (PgR) testing guidelines[21].
Several studies reported that HER-2 expression shown by IHC was comparatively resistant to prolonged fixation and provided stable results, especially in positive (3+) cases[4-8]. Regarding fixatives, although the superiority of 10%NBF in IHC[22] and in the preservation of DNA[23] had been reported, Hashizume et al[7] reported that the HER-2 overexpression rate was not significantly different among 10% NBF, 15% NBF, and 20% formalin. In addition, Moatamed et al[8] analyzed the HER-2 amplification by IHC and ISH using mastectomy samples subjected to various fixation times with various fixatives, and they concluded that HER-2 testing results remain accurate beyond the ASCO/CAP recommendation.
Considering the results of breast cancer studies, the ASCO/CAP guideline regarding fixation duration seems too strict. However, that guideline was based on the concept of mitigating false-negative results of HER-2 testing and on the conclusions made in consensus meetings[24,25], and thus that guideline's recommendation is considered an ideal goal for HER-2 testing. The findings of previous studies regarding fixation durations may be good references for the interpretation of HER-2 testing beyond the ASCO/CAP recommendation in unavoidable circumstances.
It is well known that the positivity or heterogeneity of HER-2 expression in gastric cancer is quite different from that of breast cancer[12-14]. The HER-2 positivity in patients with gastric cancer varied widely (3.8% to 36.6%) in a study using a different method for HER-2 determination[26]. Many studies have demonstrated the heterogeneity of HER-2 expression in gastric cancer, and the percentages of heterogeneity in these studies varied from a minimum of 5% to a maximum of 69%, although the definition of heterogeneity was not universal in the studies[27-33].
As the HER-2 expression of gastric cancer is unique, the effects of the fixative conditions on the determination of HER-2 results in gastric cancer may differ from those of breast cancer. However, the amount of relevant evidence is insufficient. We could find only one study using a xenograft model of gastric cancer cell lines[15] and a clinical study of gastric cancer[16] that analysed the association between HER-2 test results and the formalin fixation status. To the best of our knowledge, no previous study has prospectively analyzed the effect of formalin fixation on HER-2 expression using the same gastrectomy specimens under different fixation times, as in the present study.
Our analyses did not confirm the phenomenon in which the expression was attenuated in proportion to the fixation duration (within 1 wk), in either our HER-2 or PD-L1 testing. Rather, there were cases that showed stronger expression in the specimen-pieces fixed for longer durations. We speculate that the reason for these results is based on the heterogenous expression of HER-2 and PD-L1 in gastric cancer. It may be difficult to overcome this problem of heterogeneity, because it is too expensive to perform HER-2 testing in multiple specimens.
Regarding fixatives, although we observed no significant difference between 10% formalin and 10% NBF within 1 wk of fixation, the superiority of 10% NBF was apparent in the very-long-term fixation.
Many studies have described the PD-L1 expression in gastric cancer, and a meta-analysis of 15 studies[34] concluded that (1) the expression of PD-L1 was associated with the overall survival of gastric cancer patients; and (2) Epstein-Barr virus infection and microsatellite instability cases are more likely to express PD-L1. Although the need for PD-L1 IHC in gastric cancer is expected to increase, the method of assessment for PD-L1 IHC is not yet standardized. The cut-off values of previous studies varied from 1% to 50% positivity of tumor cells[34].
We assessed the results of our PD-L1 IHC by using cut-off values of 1%, 10%, and 50% because the cut-off values of earlier studies varied widely. Like the results of our HER-2 IHC, the heterogeneous expression of PD-L1 influenced the results, and no significant influence of fixative duration on the assessment of PD-L1 IHC findings in gastric cancer was revealed within 1 wk of fixation. Although we also observed no significant difference between 10% formalin and 10% NBF within 1 wk of fixation in our assessment of PD-L1 expression, a significant difference was apparent in the long-term fixation, and the superiority of 10% NBF was reconfirmed.
The small number of cases (especially HER-2-positive cases) is a limitation of the present study. We could not perform the IHC/DISH of all of the whole-sectioned specimens, and we were not able to unify the durations of the very-long-term fixation. In addition, our results were probably affected by tumor heterogeneity. We therefore consider that our results were not conclusive. However, our results provide the interpretation of the cases beyond fixation duration of the ASCO / CAP recommendation that these cases are also worth HER-2 / PD-L1 examination. This is clinically very important.
In conclusion, we performed a prospective analysis to identify the effects of formalin fixation on the assessment of HER-2 and PD-L1 expression. Our analyses revealed that prolonged fixation did not show inferiority within 1 wk of duration. Although no significant difference was observed between 10% formalin and 10% NBF within a week of fixation, the superiority of 10% NBF was confirmed in the very-long-term (> 3 mo) fixation. To minimize the risk of false-negative results, it is important to comply with the ASCO/CAP recommendation as much as possible. Our present findings provide supporting information for the interpretation of HER-2 testing and PD-L1 testing for cases beyond the ASCO/CAP fixation recommendation due to unavoidable circumstances.
The needs for human epidermal growth factor receptor 2 (HER-2) and/or programmed death-ligand 1 (PD-L1) evaluations in gastric cancer are dramatically increasing. However, most of the evidences regarding the fixation duration or type of fixing solution are based on breast cancer.
As the HER-2 expression of gastric cancer is unique, we speculate that the effects of the fixative conditions on the determination of HER-2 results in gastric cancer may differ from those of breast cancer.
To investigate the real effects of fixation conditions on HER-2 testing or PD-L1 testing for gastric cancer using gastrectomy specimens.
Resected gastric specimens were each divided into four pieces and fixed in four strictly controlled different durations (6 h, 24 h, and 48 h, and 1 wk) by 10% formalin or 10% neutral buffered formalin (NBF). Immunohistochemistry (IHC) of HER-2 and PD-1 was performed, and a pathology examination was conducted.
Prolonged fixation did not show inferiority within the 1-wk period for the assessment of both HER-2 and PD-L1 expressions. The superiority of 10% NBF was confirmed in the long-term (> 3 mo) fixation.
In this pilot study, prolonged fixation within 1 wk showed no inferiority in HER-2 or PD-L1 testing. However, a large-numbered prospective study is needed to obtain conclusive results.
Our findings provide supporting information for the interpretation of HER-2 and/or PD-L1 testing for cases beyond the ASCO/CAP fixation recommendation due to unavoidable circumstances.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abadi ATB, Economescu M S- Editor: Dou Y L- Editor: A E- Editor: Tan WW
| 1. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5326] [Article Influence: 355.1] [Reference Citation Analysis (3)] |
| 2. | Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 433] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 3. | Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF; American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 854] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 4. | Bartley AN, Washington MK, Ventura CB, Ismaila N, Colasacco C, Benson AB, Carrato A, Gulley ML, Jain D, Kakar S, Mackay HJ, Streutker C, Tang L, Troxell M, Ajani JA. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Am J Clin Pathol. 2016;146:647-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Selvarajan S, Bay BH, Choo A, Chuah KL, Sivaswaren CR, Tien SL, Wong CY, Tan PH. Effect of fixation period on HER2/neu gene amplification detected by fluorescence in situ hybridization in invasive breast carcinoma. J Histochem Cytochem. 2002;50:1693-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Arber DA. Effect of prolonged formalin fixation on the immunohistochemical reactivity of breast markers. Appl Immunohistochem Mol Morphol. 2002;10:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Hashizume K, Hatanaka Y, Kamihara Y, Kato T, Hata S, Akashi S, Kato T, Koyatsu J, Tani Y, Tsujimoto M, Tsuda H. Interlaboratory comparison in HercepTest assessment of HER2 protein status in invasive breast carcinoma fixed with various formalin-based fixatives. Appl Immunohistochem Mol Morphol. 2003;11:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Moatamed NA, Nanjangud G, Pucci R, Lowe A, Shintaku IP, Shapourifar-Tehrani S, Rao N, Lu DY, Apple SK. Effect of ischemic time, fixation time, and fixative type on HER2/neu immunohistochemical and fluorescence in situ hybridization results in breast cancer. Am J Clin Pathol. 2011;136:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Ibarra JA, Rogers LW. Fixation time does not affect expression of HER2/neu: a pilot study. Am J Clin Pathol. 2010;134:594-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Tong LC, Nelson N, Tsourigiannis J, Mulligan AM. The effect of prolonged fixation on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast cancer: a prospective study. Am J Surg Pathol. 2011;35:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Kao KR, Hasan T, Baptista A, Truong T, Gai L, Smith AC, Li S, Gonzales P, Voisey K, Eriwvo P, Power J, Denic N. Effect of fixation time on breast biomarker expression: a controlled study using cell line-derived xenografted (CDX) tumours. J Clin Pathol. 2017;70:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Grillo F, Fassan M, Sarocchi F, Fiocca R, Mastracci L. HER2 heterogeneity in gastric/gastroesophageal cancers: From benchside to practice. World J Gastroenterol. 2016;22:5879-5887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Fusco N, Bosari S. HER2 aberrations and heterogeneity in cancers of the digestive system: Implications for pathologists and gastroenterologists. World J Gastroenterol. 2016;22:7926-7937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Wada R, Hirabayashi K, Ohike N, Morii E. New guidelines for HER2 pathological diagnostics in gastric cancer. Pathol Int. 2016;66:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Yamashita-Kashima Y, Shu S, Yorozu K, Hashizume K, Moriya Y, Fujimoto-Ouchi K, Harada N. Importance of formalin fixing conditions for HER2 testing in gastric cancer: immunohistochemical staining and fluorescence in situ hybridization. Gastric Cancer. 2014;17:638-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Matsusaka S, Nashimoto A, Nishikawa K, Miki A, Miwa H, Yamaguchi K, Yoshikawa T, Ochiai A, Morita S, Sano T, Kodera Y, Kakeji Y, Sakamoto J, Saji S, Yoshida K. Clinicopathological factors associated with HER2 status in gastric cancer: results from a prospective multicenter observational cohort study in a Japanese population (JFMC44-1101). Gastric Cancer. 2016;19:839-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5948] [Cited by in RCA: 7525] [Article Influence: 836.1] [Reference Citation Analysis (0)] |
| 18. | Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 911] [Article Influence: 101.2] [Reference Citation Analysis (1)] |
| 19. | Tran PN, Sarkissian S, Chao J, Klempner SJ. PD-1 and PD-L1 as emerging therapeutic targets in gastric cancer: current evidence. Gastrointest Cancer. 2017;7:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF; American Society of Clinical Oncology/College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 21. | Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907-922. [PubMed] |
| 22. | Arnold MM, Srivastava S, Fredenburgh J, Stockard CR, Myers RB, Grizzle WE. Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotech Histochem. 1996;71:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Baloglu G, Haholu A, Kucukodaci Z, Yilmaz I, Yildirim S, Baloglu H. The effects of tissue fixation alternatives on DNA content: a study on normal colon tissue. Appl Immunohistochem Mol Morphol. 2008;16:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Hammond ME, Barker P, Taube S, Gutman S. Standard reference material for Her2 testing: report of a National Institute of Standards and Technology-sponsored Consensus Workshop. Appl Immunohistochem Mol Morphol. 2003;11:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Yaziji H, Taylor CR, Goldstein NS, Dabbs DJ, Hammond EH, Hewlett B, Floyd AD, Barry TS, Martin AW, Badve S, Baehner F, Cartun RW, Eisen RN, Swanson PE, Hewitt SM, Vyberg M, Hicks DG; Members of the Standardization Ad-Hoc Consensus Committee. Consensus recommendations on estrogen receptor testing in breast cancer by immunohistochemistry. Appl Immunohistochem Mol Morphol. 2008;16:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Boku N. HER2-positive gastric cancer. Gastric Cancer. 2014;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 27. | Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 868] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 28. | Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 130] [Reference Citation Analysis (0)] |
| 29. | Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 428] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 31. | Lee HE, Park KU, Yoo SB, Nam SK, Park DJ, Kim HH, Lee HS. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur J Cancer. 2013;49:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Wang T, Hsieh ET, Henry P, Hanna W, Streutker CJ, Grin A. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum Pathol. 2014;45:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Ahn S, Ahn S, Van Vrancken M, Lee M, Ha SY, Lee H, Min BH, Lee JH, Kim JJ, Choi S, Jung SH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim S, Kim KM. Ideal number of biopsy tumor fragments for predicting HER2 status in gastric carcinoma resection specimens. Oncotarget. 2015;6:38372-38380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0182692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (1)] |