Published online Feb 6, 2019. doi: 10.12998/wjcc.v7.i3.347
Peer-review started: November 5, 2018
First decision: November 27, 2018
Revised: December 6, 2018
Accepted: December 14, 2018
Article in press: December 15, 2018
Published online: February 6, 2019
Processing time: 87 Days and 0.7 Hours
Intravenous leiomyomatosis (IVL) is a rare and complicated disease, which requires surgery by a multidisciplinary team. However, the optimal surgical approach has not been determined.
Here we report three cases of IVL treated with different surgical approaches. All patients presented with circulation symptoms. Two patients had lower extremity edema and the other had cardiopalmus. The diagnosis of IVL was confirmed based on the imagining examinations and pathological findings. All patients underwent surgical treatment and were discharged without any complications.
Preoperative examination is crucial for surgical planning and surgical approach is dependent on the patient’s condition and tumor involvement.
Core tip: Intravenous leiomyomatosis is a rare tumor that requires surgical treatment by a multidisciplinary team. We present three cases of IVL treated with different surgical approaches, including one-stage, two-stage, and minimally-invasive surgery. We describe our experience and difficulties in the surgery in order to guide fellow surgeons in treating this rare disease.
- Citation: He J, Chen ZB, Wang SM, Liu MB, Li ZG, Li HY, Zhao G. Intravenous leiomyomatosis with different surgical approaches: Three case reports. World J Clin Cases 2019; 7(3): 347-356
- URL: https://www.wjgnet.com/2307-8960/full/v7/i3/347.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i3.347
Intravenous leiomyomatosis (IVL) is a rare and benign tumor originating from female genitalia and extending into the extrauterine vein or even the right-sided cardiac chambers[1]. Approximately 300 cases[2] of IVL have been reported in the English literature since it was first reported by Hirshfield in 1896[3]. IVL comprised only 0.097% to 0.26%[4,5] of all female leiomyomatosis in different institutes. The average age of IVL presentation is 45 years[6]. Most patients are asymptomatic even with cardiac involvement. Surgical treatment is recommended for all patients. Due to massive extension of the tumor, multidisciplinary cooperation is required in the surgery. To date, there is still no universal consensus regarding the optimal surgical approach[1]. Here we present three cases of IVL and outline our experience in the surgical treatment and outcome.
The Ethics Committee of Guangdong General Hospital approved this study, and written consent was obtained from the patients.
Case 1: A 46-year-old nulliparous Chinese woman presented with chest distress and lower extremity edema for 1 mo.
Case 2: A 46-year-old multiparous Chinese women presented with cardiopalmus for 2 mo.
Case 3: A 54-year-old multiparous Chinese women presented with lower extremity edema for 1 year.
Case 1: The patient complained of chest distress and shortness of breath after activity for 2 mo. She had no chest pain, cough, or paroxysmal nocturnal dyspnea. The symptoms aggravated and combined with lower extremity edema. She came to our hospital for treatment. Her appetite, sleep, voiding, and stool were normal. There were no obvious weight or physical strength changes.
Case 2: The patient presented with cardiopalmus for 2 mo. The symptom could be relieved after resting for a few minutes. She had no chest distress or chest pain. The echocardiography at the local hospital detected multiple masses in the right heart chamber. She came to our hospital for further treatment. Her appetite, sleep, voiding, and stool were normal. There were no obvious weight or physical strength changes.
Case 3: The patient complained of lower extremity edema for 1 year. The symptom was associated with menstruation and was aggravated for 2 mo. Therefore, she came to our hospital for treatment. She also had abdominal distention but no abdominal pain or nausea. Her appetite, sleep, voiding, and stool were normal. There were no obvious weight or physical strength changes.
Case 1: The patient denies a history of hypertension, diabetes, and hepatitis. She denies any surgery or injury.
Case 2: The patient had a history of hysterectomy for uterine leiomyoma. She denies hypertension, diabetes, and valvar heart disease history. She denies any other surgery or injury.
Case 3: The patient had a history of hypertension, which had not been properly monitored or treated. She denies other diseases, surgeries, or injuries.
Case 1: The patient did not smoke or drink and had no exposure history to toxic substances and infected water. Her menstruation was normal.
Case 2: The patient never smoked and drank and had no exposure to toxic substances and infected water. She denies family history of tumor. She no longer menstruated.
Case 3: The patient did not smoke or drink and had no exposure history to toxic substances and infected water. There was no history of infective and hereditary diseases.
Case 1: Temperature: 36.5 °C; Pulse: 75 bpm; Blood pressure: 128/82 mmHg; Weight: 47.5 kg. No heart murmur was noted during auscultation. Breaths sounded clear, and no rale was noted. No peripheral vessel sign was noted. No lower extremity edema was detected.
Case 2: Temperature: 36.5 °C; Pulse: 85 bpm; Blood pressure: 110/70 mmHg; W: 57 kg. No heart murmur was noted during auscultation. Breaths sounded clear, and no rale was noted. No peripheral vessel sign was noted.
Case 3: Temperature: 37.1 °C; Pulse: 98 bpm; Blood pressure: 142/88 mmHg; W: 52 kg. No heart murmur was noted during auscultation. Breaths sounded clear, and no rale was noted. No peripheral vessel sign was noted. No lower extremity edema was detected.
Case 1: Blood test showed alanine aminotransferase 58.85 U/L, brain natriuretic peptide 140 pg/mL, estradiol 26 pg/mL, human epididymis peptide 4 78.46 pmol/L, and β-human chorionic gonadotropin 3.28 mIU/mL. Other laboratory results were within normal limits.
Case 2: Blood test showed bilirubin 20.9 μmol/L and brain natriuretic peptide 670.1 pg/mL. Other laboratory results were within normal limits.
Case 3: Blood test showed white blood count 11.34 × 109/L, hemoglobin 97.6 g/L, estradiol 195 pg/mL, β-human chorionic gonadotropin 3.28 mIU/mL, and brain natriuretic peptide 672.1 pg/mL. Other laboratory results were within normal limits.
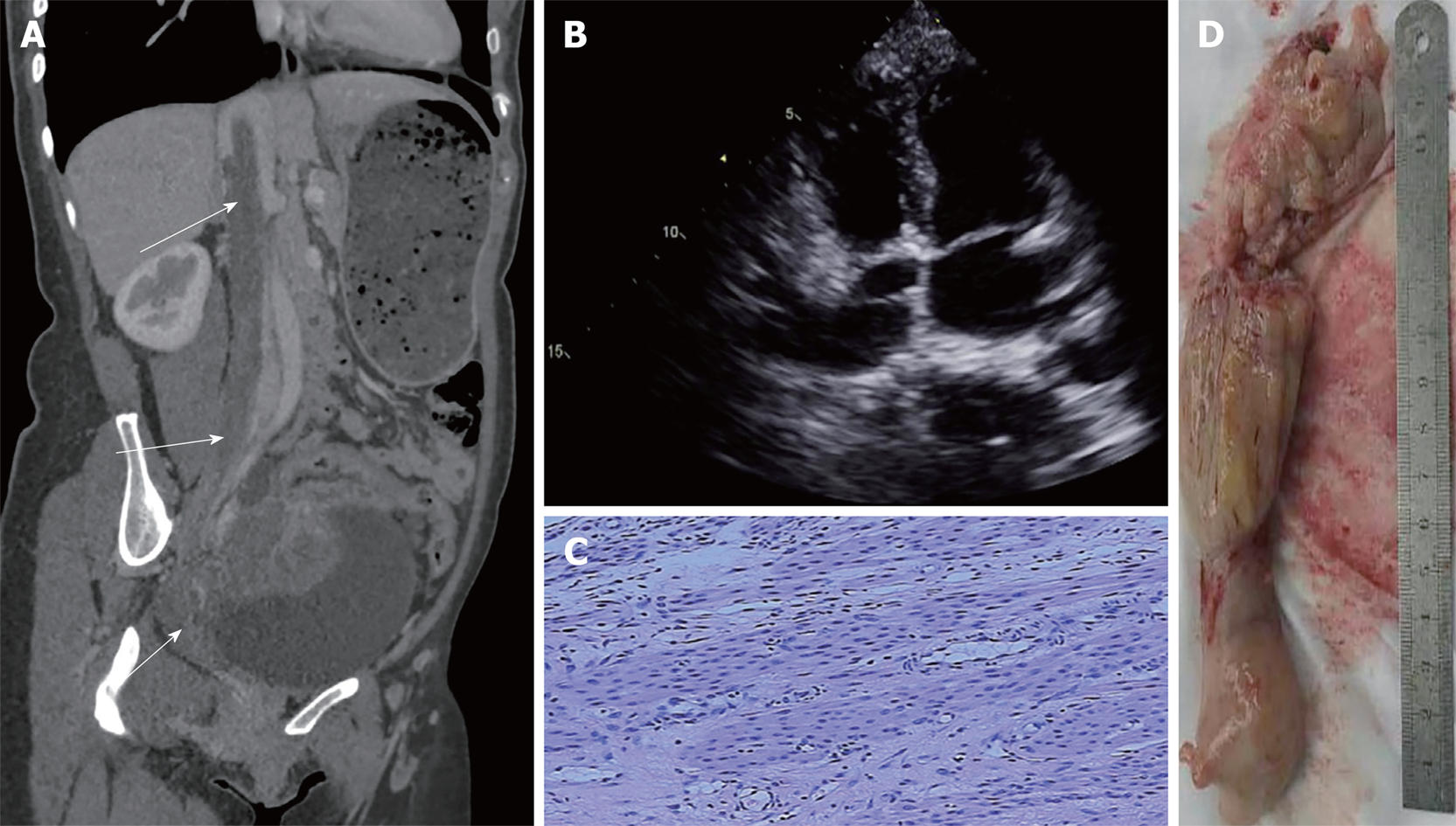
Case 1: Computed tomography (CT) showed a mass (Figure 1A) in the pelvis encroaching the uterus and involving the right internal iliac vein, the inferior cava vein (ICV) and the right atrium (RA). Echocardiography detected large immobile echogenic mass attached to the atrial septum (Figure 1B).
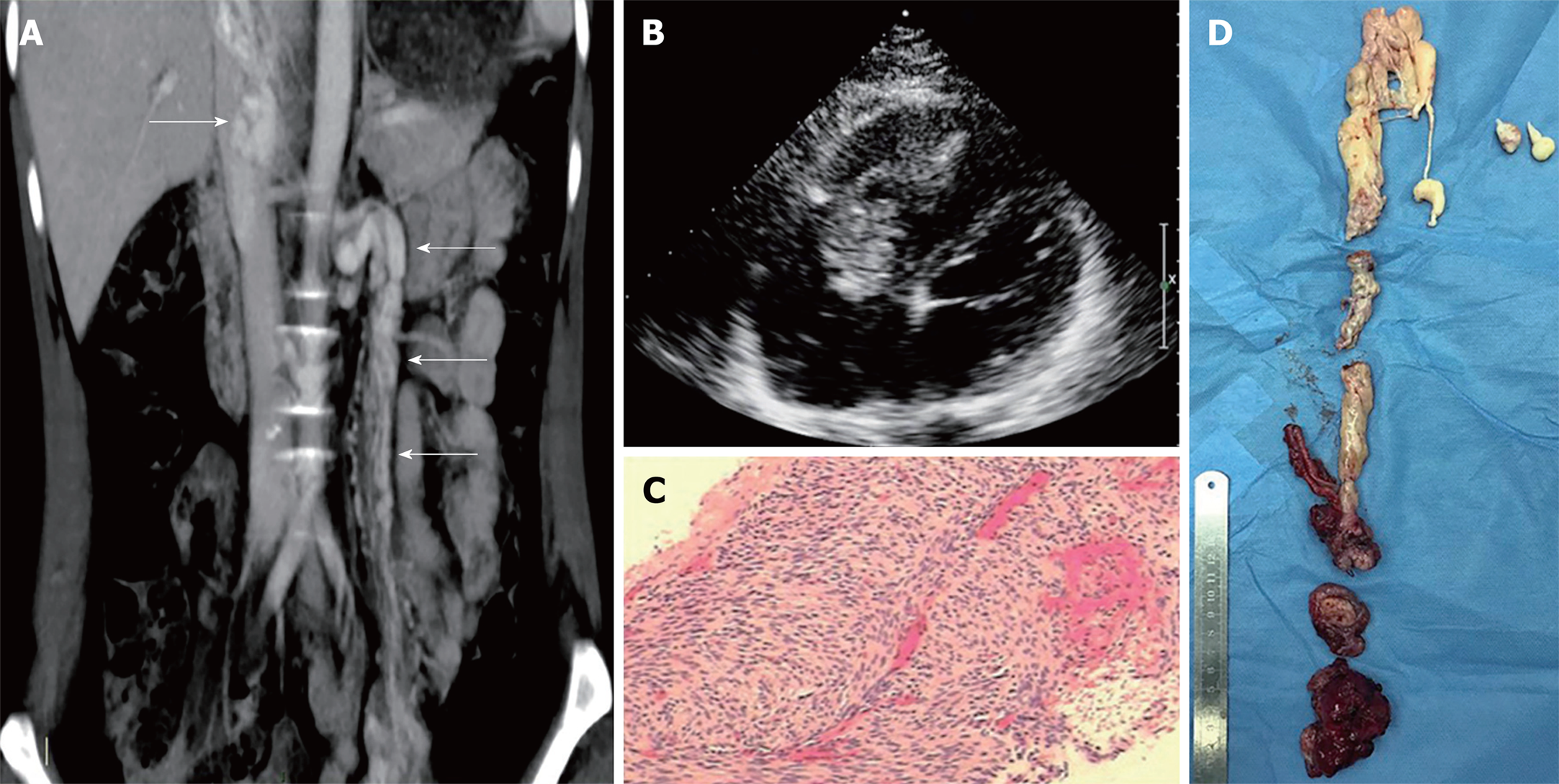
Case 2: CT showed soft tissue within the left uterine adnexa-left ovarian vein-left renal vein-ICV-RA-right ventricle (RV), and no involvement of the portal vein and its branches was detected (Figure 2A). Echocardiography revealed a massive continuous mass extending from the IVC through the RA and crossing into the RV (Figure 2B). Her vital sign was stable, and no specific change was found. A biopsy was performed to confirm the diagnosis of IVL.
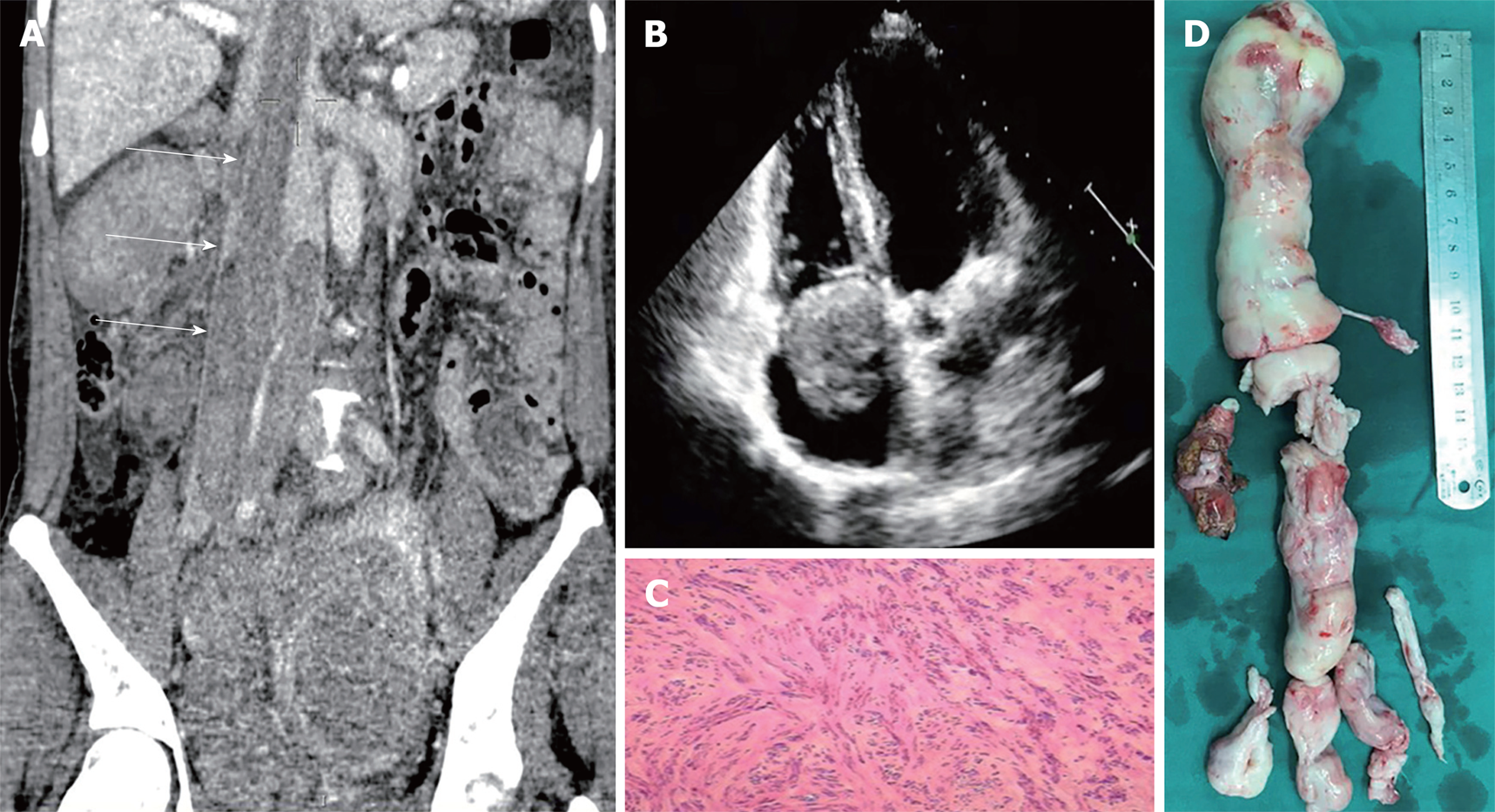
Case 3: CT revealed multiple masses located in the right lower quadrant and pelvis merging together with diameter of 13 cm. The tumor reached down to the right pelvic floor, surrounded the right ureter and extended to the RA through the right ovarian vein, right common iliac vein and ICV (Figure 3A). Echocardiography showed cord-like mass crossing into the RV through tricuspid valve (Figure 3B).
Guan-Di Chen, MD, PhD, Professor, Department of Gynecology: The patient’s main symptom was vein occlusion. Imagining findings indicated IVL that required surgical treatment. For women above 40 years old, we recommended hysterectomy and bilateral salpingo-oophorectomy.
San-Ming Wang, MD, PhD, Professor, Department of Vascular Surgery: There were multiple lesion from the right iliac vein to the RA. According to the imaging findings, IVL was the most likely diagnosis. Because the tumor invaded the RA, surgical treatment was necessary.
Cong Lu, MD, PhD, Chief Doctor, Professor, Department of Cardiovascular Surgery: I concurred with the above opinions. IVL was the most likely diagnosis. But the possibility of a malignant tumor still could not be ruled out. We recommended gynecologic surgery first, and when the IVL was confirmed after the pathological exam, a second operation would be scheduled.
Mu-Biao Liu, MD, PhD, Professor, Chief Doctor, Department of Gynecology: According to the patient history and preoperative exam, the diagnosis of IVL was confirmed. Bilateral salpingo-oophorectomy was recommended for this patient.
Gang Zhao, MD, PhD, Professor, Chief Doctor, Department of Vascular Surgery: The diagnosis of IVL was confirmed and surgical indication was clear. No attachment was detected and the invasion of the tumor was clear. There was a good chance to remove the tumor completely at once. In our experience, extraction from both the RA and the IVC might provide a better chance of complete resection and bleeding control.
Jin-Song Huang, MD, PhD, Chief Doctor, Professor, Department of Cardiovascular Surgery: I agreed with the above views. The patient’s general health condition was suitable for one-stage operation.
Gang Zhao, MD, PhD, Professor, Chief Doctor, Department of Vascular Surgery: According to the imaging findings, the most likely diagnosis is IVL. CT revealed the tumor in the pelvic area is huge, and the operation to completely resect the tumor could be time-consuming with a high chance for massive bleeding. Therefore, two-stage operation was recommended.
Huan-Lei Huang, MD, PhD, Chief Doctor, Professor, Department of Cardiovascular Surgery: The mass in the RA was large but mobile. No attachment to the RA was detected. There was a good chance to completely remove the mass.
Hai-Yan Ye, MD, PhD, Professor, Department of Gynecology: I agreed with the above opinions. The mass in the pelvic was very big with rich blood supply. It would be very difficult to completely resect the tumor. A two-stage operation might be beneficial.
The diagnosis of IVL in these three patients was confirmed based on the imagining examinations and pathological exam.
This patient was scheduled for two-stage operation. Due to the potentially malignant nature of the tumor, laparotomy was performed first. The uterus was slightly enlarged with uneven surface and a nodular mass arose from it. The right uterine vein was engorged. Total hysterectomy and bilateral salpingo-oophorectomy was performed. The operation went smoothly. Histologic examination of the tumor confirmed the diagnosis of leiomyoma. After 14-d of routine treatment, the patient recovered well and underwent a second-stage operation. The heart was exposed through a median sternotomy. Cardiopulmonary bypass (CPB) was instituted by ascending aorta and superior vena cava cannula and systemic cooling was commenced. Laparotomy was performed at the same time. The right common iliac vein and ICV were exposed after meticulous dissection. The right and left renal veins were controlled with vascular clamps. Myocardial protection was achieved by cold blood cardioplegia. Then venotomy was made on the ICV at the level of renal veins. In the meantime, right atriotomy was performed. The tumor exposed on the ICV incision was transected. The upper part of the tumor was extracted from the RA, and the rest of it was extracted from the ICV (Figure 1D). The whole extraction process was very smooth. The incision on the RA and ICV were closed separately. The operation time of these two surgeries were 165 min and 190, and the blood loss was 200 mL and 500 mL.
Postoperative pathological examination showed mass smooth muscle fibers proliferation with interstitial fibers edema and infiltration of a few lymphocytes. No cellular atypia was observed (Figure 1C). The rest of the surgery was uneventful, and postoperative treatment was uncomplicated.
The patient was scheduled for one-stage operation. After general anesthesia, simultaneous sternotomy and laparotomy was performed. A mass was seen in the right pelvis with diameter of 4 cm surrounding the left uterine adnexa. The left ovarian vein enlarged with a diameter of 2 cm containing a cord-like mass that extended to the IVC through the left renal vein. Total hysterectomy and bilateral salpingo-oophorectomy was performed, and the distal end of the left uterine vein was ligated. The vein containing the mass was isolated to the level of the left renal vein. The right posterior peritoneum was opened and the ICV was fully exposed. CPB was instituted by ascending aorta and superior vena cava cannula, and systemic cooling was commenced. Renal veins were controlled with vascular clamps, and incisions were made on the RA and IVC. Then the mass was transected through the ICV incision. The lower part of the mass was extracted successfully. When we tried to extract the upper part of the mass, there was obstruction in the RV and the ICV. Severe bleeding occurred in the ICV, and the blood pressure dropped dramatically. Systemic cooling to a lower temperature was commenced. After exploration, we found adhesion to the RV and part of the tumor extending into the portal vein causing the obstruction. We cut off the adhesion along with some of the tendon from the RV and sharply separated the tumor with the portal vein leaving part of the mass (Figure 2D). The incisions were closed quickly, and cannula on the IVC was placed to maintain the flow of CPB. The blood pressure went up gradually. No tricuspid regurgitation was detected by the transesophageal echocardiography during surgery. The rest of the surgery was uneventful. The whole operation took 600 min and the blood loss was 4000 mL.
Postoperative pathological examination showed a concentration of spindle cells with mass hyaline degeneration. Immunohistochemistry revealed smooth muscle actin (+++), Caldesmon (+++), estrogen receptor (+++), and progesterone receptor (+++) (Figure 2C).
The patient was scheduled for two-stage operation. During the first surgery, laparotomy was performed. A huge mass was seen in the pelvis reaching the sacrum on the top and attaching to the pelvic floor. The uterus, right oviduct, and right ovary were invaded by the tumor. The bladder and the right ureter were attached to the tumor. The tumor in the pelvis was excised completely, and both adnexa uteri were removed.
After a 2 wk recovery, the patient underwent the second operation. Laparotomy was performed. The abdominal adhesion was divided and the ICV was exposed. The CPB was instituted by femoral artery, femoral vein, and jugular vein cannula. Two small incisions were made on the right chest at the midclavicular line and maxillary line separately. The CPB was commenced at normal temperature. Atriotomy and venotomy were performed at the same time. The tumor was transected and extracted from the atrium and the ICV. While extracting the tumor on the bottom, there were some adhesions to the vein wall. We clamped the proximal end of the tumor and separated the tumor following the direction of the vein. We took out the tumor along with part of the vein intima causing a tear on the ICV incision (Figure 3D). The incisions were closed with 5-0 prolene, and the CPB was disconnected. No residual tumor was detected by the transesophageal echocardiography in the RA. The rest of the operation was uneventful. The time of these two operations were 395 min and 265 min, and the blood loss was 1200 mL and 2000 mL.
Postoperative pathological examination showed concentration of smooth muscle cells with local hyaline degeneration. Immunohistochemistry revealed P16 (-), P53 (-), Ki67 (10% +), Caldesmon (+++) and CD10 (±) (Figure 3C).
Postoperative treatment was uneventful. She was discharged 5 d after the second surgery. Follow-up CT did not detect any abnormality. She has remained asymptomatic.
Postoperative vasoactive agent and antibiotics were routinely used. She was discharged 21 d after the surgery without any complications. The follow-up CT 3 mo after discharge detected filling in the retrohepatic segment of the ICV.
Postoperative treatment was uneventful. She was discharged 11 d after the second surgery. Follow-up CT did not detect any residual tumor.
IVL is a rare and potentially fatal disease with a juxtaposition of benign histopathologic features and quasi-malignant clinical behavior. To date, approximately 300 cases of IVL have been reported[2]. Clinical symptoms are nonspecific and clinical diagnosis mostly relies on radiology and pathology examinations. Surgery is still the gold standard for treatment of IVL. Due to the extensive nature of the tumor and its involvement in multiple systems[7], multidisciplinary cooperation is required for its treatment. However, there are still no guidelines regarding the surgical approach for IVL. In this study, we reported three cases of IVL treated with different surgical approaches. We intended to share our experience and provide valuable guidance for fellow surgeons in treating this rare disease.
Surgery is currently the only effective treatment for IVL and complete tumor excision is a valid treatment for the prevention of recurrence[8]. The surgical difficulties in IVL are mainly associated with the adhesion between the tumor and the venous wall rather than the length of the tumor. Careful preoperative examination for evaluation of the adhesion, especially in the retrohepatic segment of the IVC and the RV is essential, and provides valuable information for surgical planning. Both one-stage and two-stage operations are viable treatment options depending on the patient’s general condition and invasion of the tumor. Recently, the medical community preferred an initial thoracic approach, which allows safe resection and reduces the risk of tumor dislodgement and pulmonary embolism. However, we have a different point of view. We believe that a one-stage operation using an abdominal approach could result in better control of the tumor and a better chance of complete resection.
In Case 1, preoperative examination did not rule out the possibility of a malignant tumor. Therefore, we chose a two-stage operation. However, this is certainly not a criterion for two-stage operation. The operation was very successful, and the tumor was completely resected.
In Case 2, we performed a one-stage operation as extensive adhesion was not detected on preoperative examination. However, when we tried to extract the tumor, occlusion in both the IVC and the RV was observed. Subsequent massive hemorrhage occurred in the IVC and the patient’s blood pressure dropped dramatically. We immediately commenced systemic cooling to provide better brain protection. The adhesion was then removed leaving part of the tumor in the IVC, the incisions were closed, and another cannula was placed in the IVC to maintain the circulation. The remainder of the procedure was uneventful. In cases where the mass is difficult to extract, severe bleeding may occur at the IVC incision or the RA incision, and the circulation is difficult to maintain. Therefore, proactive placement of a cannula in the femoral vein may be beneficial. The follow-up CT also confirmed adhesion in the IVC especially in the retrohepatic segment.
In Case 3, the tumor in the pelvic was huge and invaded the urinary system, which might lead to massive blood loss and trauma. Thus, we chose a two-stage operation. In the second operation, we used a minimally-invasive approach in the chest as there were fewer adhesions in the right cardio-chambers. The time of the open heart procedure was estimated to be short. We employed normothermic CPB with no cardiac arrest, which minimized trauma and disturbance in the circulation. This is the first time that minimally-invasive surgery has been performed in IVL. However, when we tried to extract the remainder of the tumor from the IVC incision, the tumor could not be removed. We separated the adhesion and removed the tumor along with part of the IVC intima, subsequently causing a tear in the IVC.
There are two theories regarding the origin of IVL[2]. One is that the IVL arises from the myometrium, invades the vein, and grows along with it. The other theory is that the IVL is a leiomyoma arising directly from the venous wall and is difficult to extract. The latter theory may explain the situation we encountered. Nonetheless, there is another explanation as we believe that ligation or sutures from the previous operation may have caused the obstruction. In such cases, we recommend proactively introducing two sutures on the venous wall to prevent future tearing. This will result in easier incision closure. It is also worth mentioning that the venous wall is very delicate and easily damaged. Extra care should be taken when trying to control the vein with a clamp. Pathological examination of tissue from all three cases confirmed the diagnosis of IVL and no other specific characteristics were found.
Minimally invasive surgery is a trend in cardiovascular surgery. It can save patients from sternotomy and reduce the surgical trauma. But there are still limitations in its application in cardiovascular surgery and this is the first attempt in IVL. Before the surgery, the mobility of the mass was assessed by the echocardiography and no attachment was found (especially in the hepatic vein). We made three small incisions in the chest and started the CPB at a normal temperature without cardiac arrest. We transected the tumor at the level of the IVL incision in the abdomen. The extraction process in the chest went smoothly and the CPB time was only 52 min, which is relatively shorter than another report[9]. During the surgery, we used transesophageal echocardiography to ensure that there was no residual tumor in the RA. The minimally invasive approach in the chest can reduce the surgical trauma, greatly preserve the cardiac function, and save some operation time.
Multidisciplinary approach, whether in one-stage or two-stage fashion, is considered to be safer and has become a common practice[10]. The primary indication for choosing the stage operation, in our opinion, is invasion of the tumor in the pelvis and the general condition of the patient. Because the whole operation for IVL can be time-consuming, the surgical trauma can be very severe. When the left ovarian vein is involved, we need to open the posterior peritoneum in both sides, which may result in extensive diffuse bleeding after the CPB or even deep hypothermic circulatory arrest. In such cases, we recommend two-stage operation.
As for the pros and cons of the one-stage and two-stage operation, one-stage operation can reduce the risk of a second anesthesia[11], the psychological burden of the patient, and the risk of tumor embolism. But it might increase the operation time and the trauma at one time. On the other hand, two-stage operations can decrease the surgical trauma. But the abdominal adhesion from the first operation might increase the difficulty of the second operation. We prefer the one-stage operation if the criteria is met.
In this series, the mean operation time was 571.7 min, which is consistent with another report[9]. The blood loss in the last two cases was considerably large due to the adhesion to the vein wall and subsequent mass hemorrhage. It is worth mentioning that the CPB time in Case 3 was relatively shorter than another report, which might indicate the effectiveness of the minimally invasive approach in IVL.
The pathological exam and the immunohistochemistry revealed the same characteristics as reported by Tang[2], which is classical leiomyomata with uniform spindle cells arranged in intersecting fascicles. Few mitotic figures were seen without cytologic atypia. The tumor was positive for smooth muscle actin and Caldesmon and negative for P16 and P53. Staining for the estrogen receptor and progesterone receptor was strongly positive.
Hormonal therapy for IVL patients is still controversial. The most common practice of hormonal therapies is used as a neoadjuvant for the patients with incomplete resection or who do not have surgery. The commonly used agents are gonadotropin-releasing hormone agonists, tamoxifen, and medroxyprogesterone[12]. The clinical outcomes are mixed. Four studies reported minimal or no response to the therapy[13-16], while some reported meaningful response[17-19]. Even in a comprehensive analysis by Li[10], hormonal therapy is considered invalid in preventing recurrence. In this series, there was residual tumor detected by follow-up CT in Case 2. Because the growth of the tumor is very slow, we decided to keep her in close follow-up without hormonal therapy. Recurrence of IVL is rare once the tumor was completely resected. There are patients remaining free of disease 11 years after the surgery[20]. Only one case of suspected pelvic recurrence has ever been reported[21].
In conclusion, we report three cases of IVL treated with different surgical approaches and highlight the outcomes of these surgical techniques. Surgery is the only effective option for IVL. With regard to the specific surgical approach for IVL, whether a one-stage or two-stage and minimally invasive or traditional approach is used will depend on the patient’s condition and tumor involvement. Preoperative examination is crucial for surgical planning.
Preoperative examination is crucial. It can show the involvement and adhesion of the tumor, which is important in surgical planning. The invasion of the tumor in the pelvic area is an important factor to decide one-stage or two-stage operation. Extraction of the tumor from the RA and IVC simultaneously might be beneficial as it can provide better bleeding control and complete excision. A minimally invasive approach in the chest might be a safe and effective procedure.
We thank the wonderful surgical team in the Guangdong General Hospital for their cooperation in the treatment of the three cases.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Park BK, Cianci P, Okumura K S- Editor: Dou Y L- Editor: Filipodia E- Editor: Song H
| 1. | Lam PM, Lo KW, Yu MY, Wong WS, Lau JY, Arifi AA, Cheung TH. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J Vasc Surg. 2004;39:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Tang L, Lu B. Intravenous leiomyomatosis of the uterus: A clinicopathologic analysis of 13 cases with an emphasis on histogenesis. Pathol Res Pract. 2018;214:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Hirschfeld B. Lehrbuch der pathologischen Anatomie. Dtsch med Wochenschr. 1883;9:97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Du J, Zhao X, Guo D, Li H, Sun B. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 18 cases, with emphasis on early diagnosis and appropriate treatment strategies. Hum Pathol. 2011;42:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 5. | Ma G, Miao Q, Liu X, Zhang C, Liu J, Zheng Y, Shao J, Cheng N, Du S, Hu Z, Ren Z, Sun L. Different surgical strategies of patients with intravenous leiomyomatosis. Medicine (Baltimore). 2016;95:e4902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Zhang G, Yu X, Shi H, Fan Q, Lang J, Liu B. Clinical characteristics and prognostic features of intravenous leiomyomatosis with inferior vena cava or intracardiac extension. J Vasc Surg Venous Lymphat Disord. 2017;5:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Guo X, Zhang C, Fang L, Guo L, Zhu W, Fang Q, Chen G, Miao Q, Sun J. Echocardiographic characteristics of intravenous leiomyomatosis with intracardiac extension: a single-institution experience. Echocardiography. 2011;28:934-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Maurer G, Nanda NC. Two-dimensional echocardiographic identification of intracardiac leiomyomatosis. Am Heart J. 1982;103:915-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Liu J, Liang M, Ma G, Liu X, Cheng N, Cao D, Yu C, Du S, Miao Q, Zhang C. Surgical treatment for intravenous-cardiac leiomyomatosis. Eur J Cardiothorac Surg. 2018;54:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Li B, Chen X, Chu YD, Li RY, Li WD, Ni YM. Intracardiac leiomyomatosis: a comprehensive analysis of 194 cases. Interact Cardiovasc Thorac Surg. 2013;17:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Liu B, Liu C, Guan H, Li Y, Song X, Shen K, Miao Q. Intravenous leiomyomatosis with inferior vena cava and heart extension. J Vasc Surg. 2009;50:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Marcus SG, Krauss T, Freedberg RS, Culliford AT, Weinreich DJ, Kronzon I. Pulmonary embolectomy for intravenous uterine leiomyomatosis. Am Heart J. 1994;127:1642-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Gehr NR, Lund O, Alstrup P, Nielsen JS, Villadsen AB, Bartholdy NJ. Recurrence of uterine intravenous leiomyomatosis with intracardiac extension. Diagnostic considerations and surgical removal. Scand Cardiovasc J. 1999;33:312-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Esmaeilzadeh M. Echocardiographic Evaluation of Intracardiac Masses. J Teh Univ Heart Ctr. 2008;3:59-66 Available from: URL: http://jthc.tums.ac.ir/index.php/jthc/article/view/85. |
| 15. | Morice P, Chapelier A, Dartevelle P, Castaigne D, Lhommé C. Late intracaval and intracardiac leiomyomatosis following hysterectomy for benign myomas treated by surgery and GnRH agonist. Gynecol Oncol. 2001;83:422-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Nishizawa J, Matsumoto M, Sugita T, Matsuyama K, Tokuda Y, Yoshida K, Matsuo T, Okayama S, Fujimoto S, Saito Y. Intravenous leiomyomatosis extending into the right ventricle associated with pulmonary metastasis and extensive arteriovenous fistula. J Am Coll Surg. 2004;198:842-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Khayata GM, Thwaini S, Aswad SG. Intravenous leiomyomatosis extending to the heart. Int J Gynaecol Obstet. 2003;80:59-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Mitsuhashi A, Nagai Y, Sugita M, Nakajima N, Sekiya S. GnRH agonist for intravenous leiomyomatosis with cardiac extension. A case report. J Reprod Med. 1999;44:883-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Hameleers JA, Zeebregts CJ, Hamerlijnck RP, Elbers JR, Hameeteman TM. Combined surgical and medical approach to intravenous leiomyomatosis with cardiac extension. Acta Chir Belg. 1999;99:92-94. [PubMed] |
| 20. | Itani Y, Otsuka Y, Deguchi F, Watanabe S, Masuda Y, Miyazaki A, Takahashi O. A case report of intravenous leiomyomatosis extending into the heart. Heart Vessels. 2000;15:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Ling FT, David TE, Merchant N, Yu E, Butany JW. Intracardiac extension of intravenous leiomyomatosis in a pregnant woman: A case report and review of the literature. Can J Cardiol. 2000;16:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |