Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4075
Peer-review started: September 7, 2019
First decision: September 23, 2019
Revised: October 22, 2019
Accepted: November 14, 2019
Article in press: November 14, 2019
Published online: December 6, 2019
Processing time: 90 Days and 1.1 Hours
Non-Hodgkin’s lymphoma (NHL) can involve extralymphatic organs, resulting in diverse clinical manifestations, especially if the endocrine organs are affected. This type of involvement can often be difficult to detect accurately. Until now, no patients with NHL and concomitant bilateral adrenal and hypothalamic involvement have been reported. The purpose of this article is to discuss the diagnosis and treatment of lymphoma with bilateral adrenal gland and hypothalamic involvement so as to help physicians avoid misdiagnosis and missed diagnosis.
We describe a case of a 52-years-old male patient with bilateral adrenal masses, who presented with a fever of unknown origin on admission. Subsequently, hypopituitarism of the anterior pituitary followed by posterior pituitary developed. 18fluorine-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) showed lesions with a high metabolism in both adrenal glands, hypothalamus, left supraclavicular lymph nodes, and other organs. The etiological diagnosis was determined based on a left supraclavicular lymph node biopsy. The patient, who eventually present with panhypopituitarism, was finally diagnosed with diffuse large B cell lymphoma with bilateral adrenal gland and hypothalamic involvement. After immunochemotherapy, glucocorticoids administration and desmopressin acetate replacement therapy, the symptoms of fever and panhypopituitarism improved, and all the lesions reduced in size.
This report demonstrates that, although synchronous involvement of two endocrine organs is rare in NHL, extra caution should be taken when dysfunction occurs in multiple endocrine organs.
Core tip: The synchronous involvement of two endocrine organs is rare in Non-Hodgkin’s lymphoma (NHL). This is the first case of NHL with bilateral adrenal gland and hypothalamic involvement that caused adrenocortical insufficiency and panhypopituitarism. The presentation created a diagnostic dilemma, which was resolved by determination of various endocrine hormone levels, biopsy, and 18fluorine-fluorodeoxyglucose positron emission tomography/computed tomography. This article aims to discuss the treatment approaches available when a patient with NHL has multiple endocrine organs involved. This report can help physicians avoid misdiagnosis of this disease.
- Citation: An P, Chen K, Yang GQ, Dou JT, Chen YL, Jin XY, Wang XL, Mu YM, Wang QS. Diffuse large B cell lymphoma with bilateral adrenal and hypothalamic involvement: A case report and literature review. World J Clin Cases 2019; 7(23): 4075-4083
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4075.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4075
The clinical manifestations of non-Hodgkin’s lymphoma (NHL) are not specific. When other organs are involved, the clinical manifestations of NHL are complex and diverse; this makes accurate diagnosis difficult[1-3]. The synchronous involvement of two endocrine organs is rare in NHL. Until now, only four cases of NHL with bilateral adrenal and sellar involvement have been reported, and all the patients presented with pituitary involvement. Here, we describe a case of a middle-aged male patient with bilateral adrenal masses, who was finally diagnosed with diffuse large B cell lymphoma (DLBCL) with bilateral adrenal gland and hypothalamic involvement. We also discuss the diagnostic and treatment approaches available when a patient with NHL presents with multiple endocrine organ involvement. This report can help physicians avoid misdiagnosis of this disease.
The patient was a 52-year-old man who complained of intermittent fever, sweating, thirst, polyuria, anorexia, abdominal distension and dizziness for 20 d. Three days before he presented to us, and impaired liver function and bilateral adrenal masses were found in a local hospital.
His symptoms persisted for 20 d prior to his arrival at the local hospital. He subsequently underwent extensive examinations which indicated a low blood pressure of 90/60 mmHg. His blood biochemical tests demonstrated elevated aminotransferase levels, and the abdominal ultrasound revealed hypoechoic bilateral adrenal gland masses. Symptomatic treatment resulted in no relief.
The patient was diagnosed with hypertension a year ago, with the highest blood pressure of 135/105 mmHg, and he had never been treated systematically. Family history of the patient was negative.
On admission, he was hypovolemic, with a blood pressure of 88/68 mmHg, a pulse rate of 122 beats/min and a temperature of 37.6ºC. No superficial lymphadenopathy was noted. Although physical hypothermia was effective, intermittent fever persisted. After admission for a week, he developed rashes on his chest, back, and abdomen.
On admission, his blood biochemical tests were indicative of impaired liver function, with significant elevation of alanine aminotransferase, aspartate aminotransferase, glutamyl transferase, total bilirubin, direct bilirubin, and alkaline phosphatase levels. Routine blood tests showed a significantly decreased platelet count and slightly elevated red and white blood cell counts. The coagulation function test showed a marked elevation in plasma fibrinogen and D-dimer levels. Moreover, serum lactate dehydrogenase (LDH) and β2-microglobulin were elevated significantly. The results are shown in Table 1.
| Values | Reference range | |
| Alanine aminotransferase (U/L) | 232.8 | 0-40 |
| Aspartate aminotransferase (U/L) | 99.3 | 0-40 |
| Glutamyl transferase (U/L) | 1194.9 | 0-50 |
| Alkaline phosphatase (U/L) | 254.8 | 0-130 |
| Total bilirubin (μmol/L) | 28.6 | 0-21 |
| Direct bilirubin (μmol/L) | 14.2 | 0-8.6 |
| Platelet (109/L) | 48 | 100-300 |
| Red blood cell (1012/L) | 5.98 | 4.3-5.9 |
| White blood cell (109/L) | 11.4 | 3.5-10 |
| Fibrinogen (g/L) | 7.22 | 2.0-4.0 |
| D-dimer (μg/mL) | 0.92 | 0-0.5 |
| Lactate dehydrogenase (U/L) | 290.9 | 40-250 |
| β2-microglobulin (mg/dL) | 0.377 | 0.07-0.18 |
We also evaluated the function of the pituitary-target gland axes. The plasma adrenocorticotrophic hormone (ACTH) and cortisol values at 0 AM, 8 AM, and 4 PM, are shown in Table 2. Despite the cortisol level decline, the ACTH levels failed to increase via a feedback mechanism. In addition, the diurnal rhythm of cortisol and ACTH were disturbed. The results of the thyroid and gonadal function tests are shown in Table 3. The serum total thyroxine, free thyroxine, free triiodothyronine, and thyroid stimulating hormone values, were all lower than normal ranges. Hormones in the pituitary-gonadal axis, including testosterone, luteinizing hormone, and follicle stimulating hormone, were also markedly lower. The 24-h urinary catecholamine and vanillylmandelic acid levels were within the normal range.
| 0 AM | 8 AM | 4 PM | |
| Cortisol (nmol/L) | 109.9 | 116.17 | 98.05 |
| ACTH (pmol/L) | 8.88 | 4.31 | 8.15 |
| Values | Reference range | |
| Pituitary-thyroid axis function | ||
| Total thyroxine (nmol/L) | 41.1 | 55.34-160.88 |
| Triiodothyronine (nmol/L) | 1.20 | 1.01-2.95 |
| Free triiodothyronine (pmol/L) | 2.59 | 2.76-6.3 |
| Free thyroxine (pmol/L) | 8.41 | 10.42-24.32 |
| Thyroid stimulating hormone (mU/L) | 0.17 | 0.35-5.5 |
| Pituitary-gonadal axis function | ||
| Testosterone (nmol/L) | 0.75 | 14-25.4 |
| Luteinizing hormone (IU/L) | < 0.07 | 1.5-9.3 |
| Follicle stimulating hormone (IU/L) | 0.52 | 1.4-18.1 |
| Prolactin (nmol/L) | 19.12 | 2.1-17.7 |
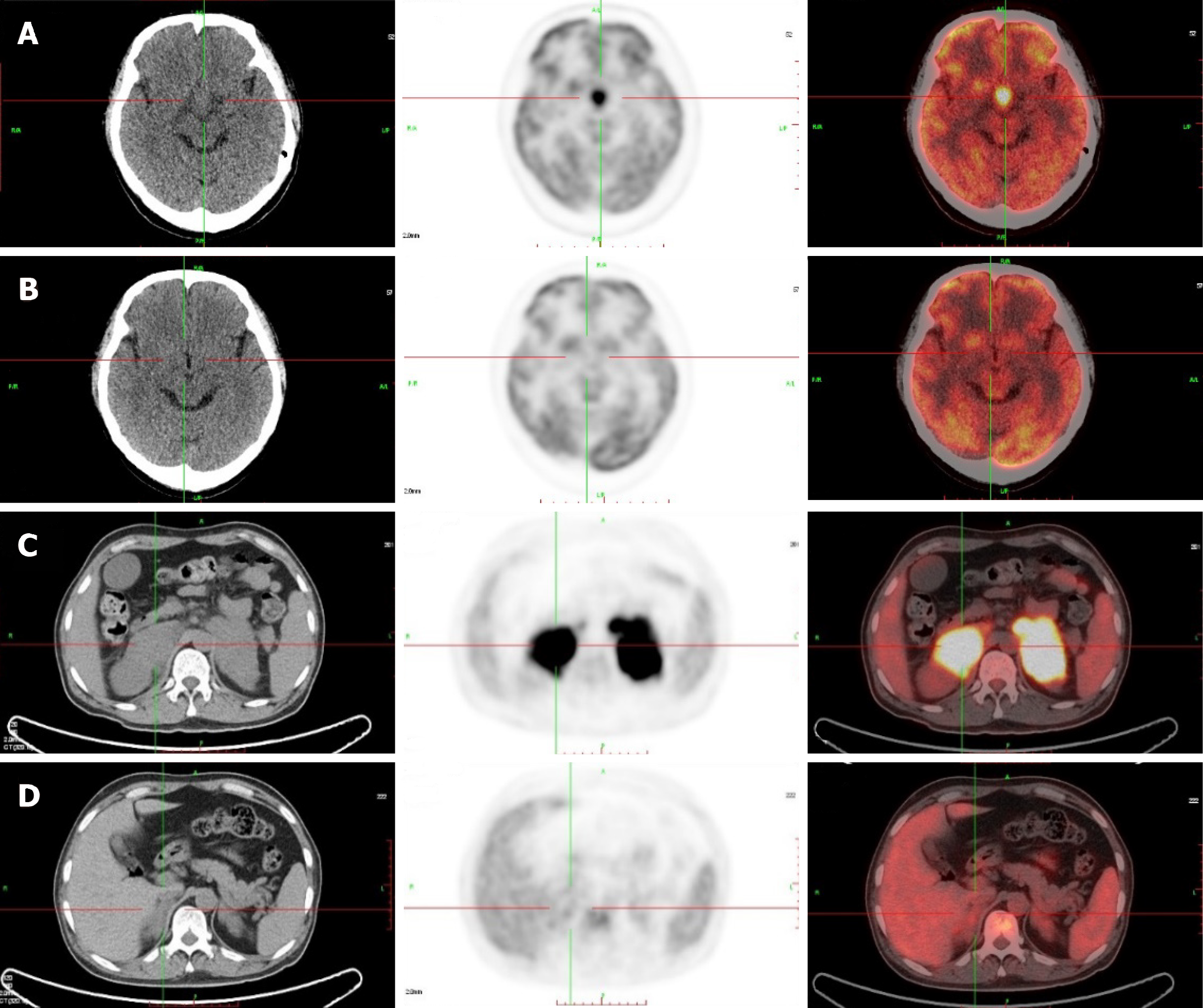
Computed tomography (CT) of the urinary system showed bilateral adrenal gland space occupying lesions with retroperitoneal lymphadenectases. Magnetic resonance imaging (MRI) of the pituitary gland revealed a space occupying lesion in the hypothalamus with an enlarged pituitary stalk. 18Fluorine-fluorodeoxyglucose positron emission tomography/CT (PET/CT) images showed lesions with high metabolism in both adrenal glands, the sellar area, left supraclavicular lymph nodes, retroperitoneal lymph nodes, left tonsil, and the left testis (Figure 1). Metaiodobenzylguanidine (MIBG) scintigraphy showed normal bilateral adrenal glands uptake.
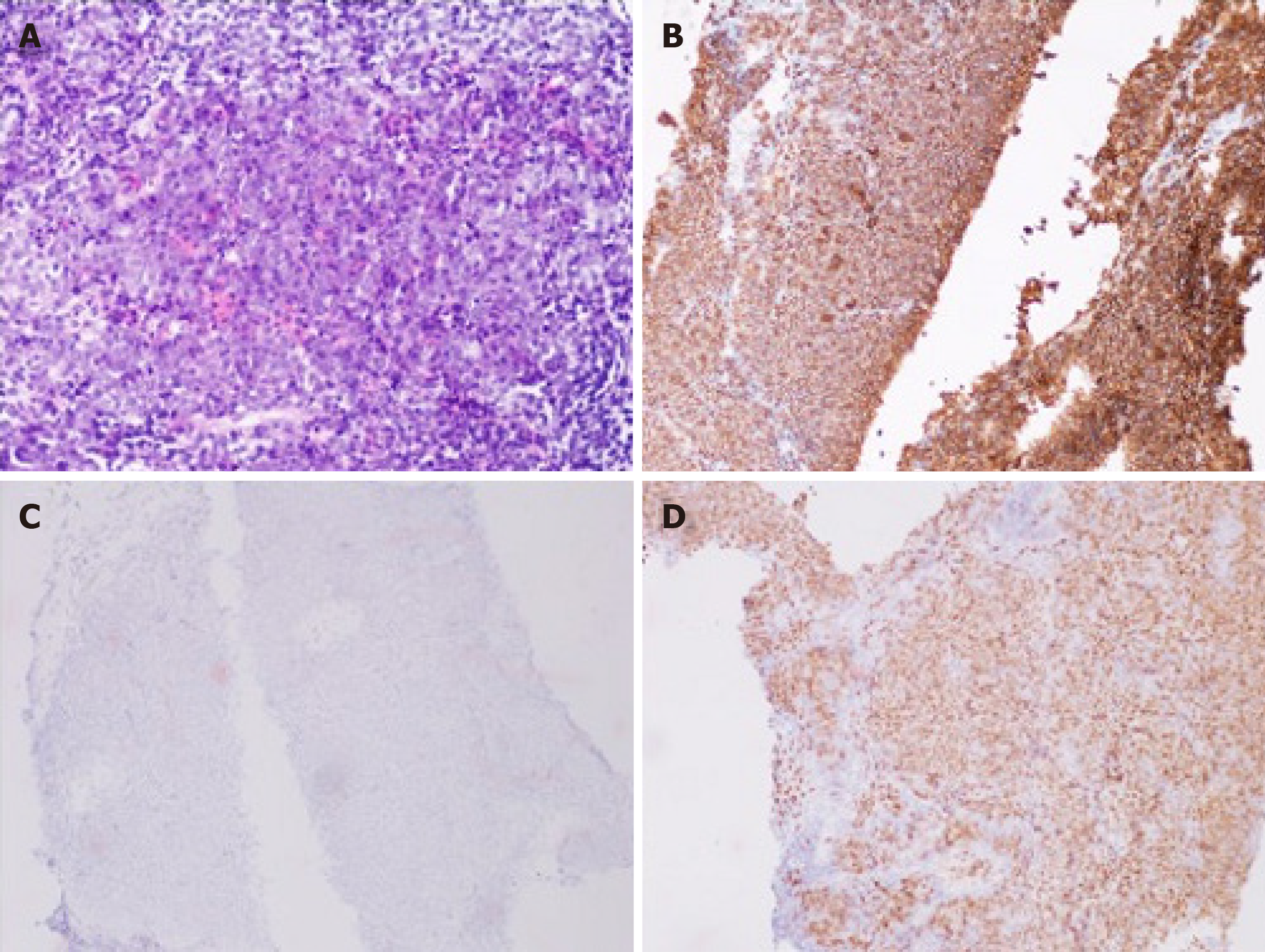
A biopsy of the left supraclavicular lymph nodes was carried out subsequently and the result showed lymphoid hyperplasia. Immunohistochemistry demonstrated lesion positivity for Bcl-2, Bcl-6, CD 20, PAX-5 and MUM-1, and negativity for CD10 (Figure 2); the levels of C-myc and Ki-67 were 70% and 80%, respectively.
According to the results of pituitary-target gland hormone measurement, the patient was diagnosed with anterior pituitary hypofunction. After hydrocortisone replacement therapy, posterior pituitary hypofuntion appeared.
The patient developed hypernatremia after hydrocortisone treatment for two possible reasons: first, a deficiency of antidiuretic hormone was prominent, leading to polyuria with a relative high blood sodium concentration; second, hydrocortisone played a partial role of a mineralocorticoid; therefore we decided to replace hydrocortisone by prednisone.
The patient developed acute-onset impaired liver function, a fever, a rash, and according to the biopsy and PET/CT results, bilateral adrenal and sellar occupying lesions were more likely to be NHL, causing panhypopituitarism. Therefore, the patient was treated in the endocrinology department with hormone replacement therapy for panhypopituitarism.
The diagnosis of NHL in this patient was clear, and therefore chemotherapy should be administered as soon as possible. In addition, in view of the multiple organs involved, an intrathecal injection was administered to prevent central neurological symptoms. Due to the multiple organ involvement of the tumor and poor prognosis according to the results of immunohistochemistry, an autologous stem cell transplantation was recommended.
The patient was finally diagnosed with DLBCL, Ann-Arbor stage IVB, a type of non-germinal center B-cell tumor, involving the bilateral adrenal glands, hypothalamus, left supraclavicular lymph nodes, retroperitoneal lymph nodes, left tonsil, and left testis. With hypothalamic and bilateral adrenal involvement, the condition also led to panhypopituitarism.
The patient was treated with the R-CHOP immunochemotherapy regimen (rituximab: 0.7 g on day 0, and cyclophosphamide: 1.3 g, doxorubicin: 60 mg, and vincristine: 4 mg on day 1, and methylprednisolone: 80 mg on days 1–5). He also received intrathecal dexamethasone and cytarabine at doses of 5 mg and 50 mg, respectively. A total of 6 courses of chemotherapy were delivered, and the interval between each course was 1 mo. After the 6 courses of chemotherapy, autologous stem cell transplantation was carried out.
For panhypopituitarism, the patient was prescribed desmopressin acetate, and hydrocortisone was replaced with prednisone acetate at a dose of 0.05 mg and 5 mg, twice a day, respectively.
A time line of this case is shown in Table 4. After immunochemotherapy, the patient recovered from his intermittent fever, the transaminase levels decreased significantly, and the platelet count returned to a normal range. After 4 courses of chemotherapy, PET/CT indicated that the lesions disappeared including the hypothalamus and adrenal gland lesions (Figure 1). After replacement therapy with prednisone acetate and desmopressin acetate twice a day at a dose of 5 mg and 0.05 mg, respectively, the serum sodium level declined to a normal range, and the symptoms relieved.
| Data | |
| 2017-08-03 | Admitted with bilateral adrenal occupying lesions and fever |
| 2017-08-04 | Laboratory examinations: Impaired liver function, decreased platelet count, and elevated LDH and β2-MG |
| 2017-08-07 | Decreased cortisol and ACTH |
| 2017-08-09 | Decreased TSH, FT3, FT4, LH, FSH, and testosterone |
| 2017-08-10 | MRI: A hypothalamic space occupying lesion; physical examination: rash and enlarged left supraclavicular lymph nodes; diagnosed with anterior pituitary hypofunction; treated with hydrocortisone |
| 2017-08-11 | Fever disappeared |
| 2017-08-14 | Low specific gravity urine and hypernatremia; diagnosed with panhypopituitrism; treated with desmopressin acetate and prednisone acetate |
| 2017-08-15 | Symptoms of polyuria and thirst were relieved; Biopsy: DLBCL |
| 2017-08-17 | PET/CT: high metabolism in bilateral adrenal glands, the sellar area, left supraclavicular lymph nodes, retroperitoneal lymph nodes, left tonsil, and left testis |
| 2017-08-22 | Started R-CHOP immunochemotherapy |
| 2017-12-15 | PET/CT: All lesions disappeared |
| 2018-05-17 | Treated with autologous stem cell transplantation |
We evaluated the risk for central nervous system (CNS) relapse by calculating the CNS-International Prognostic Index (CNS-IPI) score, which was 4. Therefore, the patient had a high risk for CNS relapse. We then recommended a pituitary MRI every year and endocrine hormone measurement every 3 mo.
Pheochromocytomas, lymphomas, and metastatic carcinomas are all common types of adrenal space occupying lesions[4-6]. In this case, the presence of pheochromocytoma and metastatic carcinoma were excluded by MIBG scintigraphy, CT scans, and laboratory examination. Lymphoma is the most common malignant cause of fevers of unknown origin[7]. The patient had intermittent fever for 20 d; infection and autoimmune diseases could therefore be excluded. A rash developed at a later stage and the results of the laboratory examination showed abnormal elevations in serum LDH, serum β2-microglobulin, and transaminases. All these symptoms and findings suggested a diagnosis of lymphoma, which was finally confirmed by biopsy and PET/CT. PET/CT also showed that the standardized uptake values (SUV) in bilateral adrenal gland and the hypothalamus were higher than those of the background; they were as high as those of the left supraclavicular lymph nodes. The findings indicated that DLBCL had bilateral adrenal gland and hypothalamic involvement in this case.
The diagnosis of panhypopituitarism was complicated. Since hormones levels of pituitary-target gland were all lower than the normal ranges, the patient was diagnosed with anterior pituitary hypofunction. He was accordingly received hydrocortisone replacement therapy. However, after treatment, the urine osmotic pressure and specific gravity declined to 151 mOsm/L and 1.005, respectively, while the serum sodium and chloride levels increased to 155.4 mmol/L and 117.3 mmol/L, respectively. In view of the hypovolemia, polyuria, and thirst on admission, his posterior pituitary function was also considered to be impaired, and he was diagnosed with panhypopituitarism.
Synchronous bilateral adrenal gland and hypothalamic–pituitary axis involvement has never been reported in patients with NHL. Literature review reveals 4 similar cases of NHL with bilateral adrenal gland and pituitary involvement. Two patients, 1 Chinese and 1 Japanese, presented with adrenal insufficiency and panhypopi-tuitarism[8,9]. A Chinese patient presented with adrenal insufficiency and only anterior pituitary hypofunction[10]. A British patient presented with adrenal insufficiency and mild diabetes insipidus[11].
The most common pathological type of bilateral adrenal lymphoma is DLBCL, which causes adrenal insufficiency to varying degrees[12-15]. However, this case was extraordinary in that the ACTH level was lowered instead of being raised due to the hypothalamic involvement. In previous literature, only a few patients with lymphoma presented with panhypopituitarism secondary to hypothalamic involvement[16]. Previous reports noted that DLBCL was the most common type of NHL involving the hypothalamic–pituitary axis[17,18]. In addition, cases of NHL with pituitary involvement often presented with variable clinical manifestations including anterior pituitary hypofunction, posterior pituitary hypofunction, visual disturbances, and acromegaly[19-24], and few cases presented with hyperprolactinemia[25,26]. In the current case, prolactin level also increased slightly in addition to developing panhypopituitarism. A study suggested that as the pituitary gland has a considerable reserve capacity, most cases of NHL with hypothalamus and pituitary involvement usually present with posterior pituitary hypofunction, and only a few patients present with anterior pituitary hypofunction[27]. A few previous reports have also shown that chemotherapy followed by autologous stem cell transplantation has a substantial effect on hypothalamic-pituitary lymphoma, which led to sustained remission and restoration of hormone levels, but the mechanism remains unclear[28,29]. However, this restoration of endocrine hormones has not been seen in this patient. Therefore, continued evaluation of endocrine hormones should be conducted during follow-up period.
CNS-IPI is an index used to evaluate the risk for CNS relapse in patients with DLBCL treated with R-CHOP. The index consists of 5 risk factors (age > 60; elevated LDH; Easter Cooperative Oncology Group status > 1; stage III/IV; extranodal site > 1) with additional kidney or adrenal involvement. The CNS-IPI score divides patients with DLBCL into 3 groups, low risk (0-1 factor), intermediate risk (2-3 factors) and high risk (4-5 factors) with a 2-year risk for CNS relapse of 0.6%, 3.4% and 10.2%, respectively[30]. The CNS-IPI score in this case was 4, which represents a high risk of CNS relapse. Therefore, prophylactic interventions are recommended to prevent CNS relapse. In addition, endocrine hormones measurement and pituitary MRI should be conducted regularly to identify CNS relapse.
The presented patient was diagnosed with DLBCL based on the left supraclavicular lymph nodes biopsy. Significantly increased SUVs were also noted in the left supraclavicular lymph nodes, bilateral adrenal area, sellar area, retroperitoneal lymph nodes, left tonsil, and left testis. Our experience with this case suggests that biopsy and PET/CT are effective for the diagnosis of NHL with multiple organ involvement[31].
To the best of our knowledge, this is the first case of DLBCL with bilateral adrenal hypothalamic involvement. When NHL involves endocrine organs, patients are predisposed to hypofunction of endocrine organs. Therefore, hormone replacement therapy is necessary. The case demonstrates the necessity of biopsies in combination with PET/CT in the diagnosis of NHL with involvement of extralymphatic organs.
The authors thank all of the treatment groups of the Chinese People’s Liberation Army General Hospital.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Paydas S, Teragawa H S-Editor: Wang JL L-Editor: MedE-Ma JY E-Editor: Wu YXJ
| 1. | Kumabe A, Kenzaka T, Nishimura Y, Aikawa M, Mori M, Matsumura M. A rare case of anasarca caused by infiltration of the pituitary gland by diffuse large B-cell lymphoma. BMC Endocr Disord. 2015;15:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Ravindra VM, Raheja A, Corn H, Driscoll M, Welt C, Simmons DL, Couldwell WT. Primary pituitary diffuse large B-cell lymphoma with somatotroph hyperplasia and acromegaly: case report. J Neurosurg. 2017;126:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Yasuda M, Akiyama N, Miyamoto S, Warabi M, Takahama Y, Kitamura M, Yakushiji F, Kinoshita H. Primary sellar lymphoma: intravascular large B-cell lymphoma diagnosed as a double cancer and improved with chemotherapy, and literature review of primary parasellar lymphoma. Pituitary. 2010;13:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Kopf D, Goretzki PE, Lehnert H. Clinical management of malignant adrenal tumors. J Cancer Res Clin Oncol. 2001;127:143-155. [PubMed] |
| 5. | Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95:4106-4113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Young WF. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 643] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 7. | Landman RE, Wardlaw SL, McConnell RJ, Khandji AG, Bruce JN, Freda PU. Pituitary lymphoma presenting as fever of unknown origin. J Clin Endocrinol Metab. 2001;86:1470-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Li JK, Chow CC, Yeung VT, Ko GT, Cockram CS. Adrenal and hypophyseal non-Hodgkin's lymphoma presenting with panhypopituitarism. Int J Clin Pract. 1998;52:513-514. [PubMed] |
| 9. | Nakashima Y, Shiratsuchi M, Abe I, Matsuda Y, Miyata N, Ohno H, Ikeda M, Matsushima T, Nomura M, Takayanagi R. Pituitary and adrenal involvement in diffuse large B-cell lymphoma, with recovery of their function after chemotherapy. BMC Endocr Disord. 2013;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Wang XL, Lü ZH, Mu YM, Dou JT, Lu JM, Zhong WW, Pan CY. Diffuse large cell non-Hodgkin lymphoma with pituitary and bilateral adrenal involvement. Intern Med J. 2012;42:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Megan Ogilvie C, Payne S, Evanson J, Lister TA, Grossman AB. Lymphoma metastasizing to the pituitary: an unusual presentation of a treatable disease. Pituitary. 2005;8:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Horiguchi K, Hashimoto K, Hashizume M, Masuo T, Suto M, Okajo J, Handa H, Kaneko Y, Yokoo H, Sasaki A, Okada S, Yamada M, Tsukamoto N, Nojima Y, Nakazato Y, Mori M. Primary bilateral adrenal diffuse large B-cell lymphoma demonstrating adrenal failure. Intern Med. 2010;49:2241-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Mozos A, Ye H, Chuang WY, Chu JS, Huang WT, Chen HK, Hsu YH, Bacon CM, Du MQ, Campo E, Chuang SS. Most primary adrenal lymphomas are diffuse large B-cell lymphomas with non-germinal center B-cell phenotype, BCL6 gene rearrangement and poor prognosis. Mod Pathol. 2009;22:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Ozimek A, Diebold J, Linke R, Heyn J, Hallfeldt K, Mussack T. Bilateral primary adrenal non-Hodgkin's lymphoma and primary adrenocortical carcinoma--review of the literature preoperative differentiation of adrenal tumors. Endocr J. 2008;55:625-638. [PubMed] |
| 15. | Rashidi A, Fisher SI. Primary adrenal lymphoma: a systematic review. Ann Hematol. 2013;92:1583-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Chan TW, Hoskins P. Panhypopituitarism secondary to hypothalamic involvement in a woman with diffuse large B-cell lymphoma. J Clin Oncol. 2010;28:e165-e166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Giustina A, Gola M, Doga M, Rosei EA. Clinical review 136: Primary lymphoma of the pituitary: an emerging clinical entity. J Clin Endocrinol Metab. 2001;86:4567-4575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Moshkin O, Muller P, Scheithauer BW, Juco J, Horvath E, Patterson BJ, Kamel-Reid S, Kovacs K. Primary pituitary lymphoma: a histological, immunohistochemical, and ultrastructural study with literature review. Endocr Pathol. 2009;20:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Büchler T, Ferra C, Virgili N, Montanya E, Grañena A. A relapsed non-Hodgkin lymphoma presenting as panhypopituitarism successfully treated by chemotherapy. J Neurooncol. 2002;59:35-38. [PubMed] |
| 20. | Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, Thalassinos NC. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004;89:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Liozon E, Soria P, Jaccard A, Boncoeur MP, Touati M, Nadalon S, Loustaud-Ratti V, Vidal E. [Diabetes insipidus revealing primary malignant non-Hodgkin's lymphoma of bone]. Rev Med Interne. 1998;19:830-834. [PubMed] |
| 22. | Merlo EM, Maiolo A, Brocchieri A, Tua A, Grignani G. Hypophyseal non-Hodgkin's lymphoma presenting with diabetes insipidus: a case report. J Neurooncol. 1999;42:69-72. [PubMed] |
| 23. | Scheinpflug K, Schalk E, Reschke K, Franke A, Mohren M. Diabetes insipidus due to herpes encephalitis in a patient with diffuse large cell lymphoma. A case report. Exp Clin Endocrinol Diabetes. 2006;114:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Yang J, Zhao N, Zhang G, Zheng W. Clinical features of patients with non-Hodgkin's lymphoma metastasizing to the pituitary glands. Oncol Lett. 2013;5:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Bolanowski M, Kuliszkiewicz-Janus M, Sokolska V. Diffuse malignant lymphoma type B with optic chiasm infiltration, visual disturbances, hypopituitarism, hyperprolactinaemia and diabetes insipidus. Case report and literature review. Endokrynol Pol. 2006;57:642-647. [PubMed] |
| 26. | Sumrall A, Herrin V. Recurrent, transformed non-Hodgkin's lymphoma presenting as chiasmal syndrome with hyperprolactinemia and hypopituitarism. J Miss State Med Assoc. 2010;51:35-36. [PubMed] |
| 27. | Valeros KA, Khoo E. Anterior panhypopituitarism in diffuse large B-cell stage IV lymphoma. J Clin Neurosci. 2014;21:1464-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Kenchaiah M, Hyer SL. Diffuse large B-cell non Hodgkin's lymphoma in a 65-year-old woman presenting with hypopituitarism and recovering after chemotherapy: a case report. J Med Case Rep. 2011;5:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Pekic S, Milicevic S, Colovic N, Colovic M, Popovic V. Intravascular large B-cell lymphoma as a cause of hypopituitarism: gradual and late reversal of hypopituitarism after long-term remission of lymphoma with immunochemotherapy. Endocrine. 2008;34:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Schmitz N, Zeynalova S, Nickelsen M, Kansara R, Villa D, Sehn LH, Glass B, Scott DW, Gascoyne RD, Connors JM, Ziepert M, Pfreundschuh M, Loeffler M, Savage KJ. CNS International Prognostic Index: A Risk Model for CNS Relapse in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP. J Clin Oncol. 2016;34:3150-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 313] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 31. | Toogood V, Milliken S, Morey A, Samaras K. Adrenal tumours: how to establish malignancy. BMJ Case Rep. 2014;2014:bcr2014203736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |