Published online Nov 6, 2019. doi: 10.12998/wjcc.v7.i21.3505
Peer-review started: May 23, 2019
First decision: August 1, 2019
Revised: August 27, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: November 6, 2019
Processing time: 175 Days and 21.1 Hours
As a significantly important part of clinical practice, the professional nursing process can be advanced in many ways. Despite the fact that case reports are regarded to be of a lower quality grade in the hierarchy of evidence, one of the principles of evidence-based medicine is that decision-making should be based on a systematic summary of evidence. However, the evidence on the reporting characteristics of case reports in the nursing field is deficient.
To use the CARE guidelines to assess reporting quality and factors influencing the quality of case reports in the nursing field.
Nursing science citation indexed (SCI-indexed) journals were identified from the professional website. Each of the identified journals was searched on their website for articles published before December 2017. Twenty-one sub-items on the CARE checklist were recorded as “YES”, “PARTLY”, or “NO” according to information reported by the included studies. The responses were assigned corresponding scores of 1, 0.5, and 0, respectively. The overall score was the sum of the 21 sub-items and was defined as “high” (more than 15), “medium” (10.5 to 14.5), and “low” (less than 10). The means, standard deviations, odds ratios (OR), and the associated 95% confidence interval (CI) were determined using Stata 12.0 software.
Ultimately, 184 case reports from 16 SCI-indexed journals were identified, with overall scores ranging from 6.5 to 18 (mean = 13.6 ± 2.3). Of the included case reports, 10.3% were regarded low-quality, 52.7% were considered middle-quality, and 37% were regarded high-quality. There were statistical differences in the mean overall scores of the included case reports with funding versus those without funding (14.2 ± 1.7 vs 13.6 ± 2.4, respectively; P = 0.4456) and journal impact factor < 1.8 versus impact factor ≥ 1.8 (13.3 ± 2.3 vs 13.6 ± 2.4, respectively; P = 0.4977). Five items from the CARE guidelines, 5a (Patient), 6 (Clinical findings), 8c (Diagnostic reasoning), 9 (Therapeutic intervention), and 11d (The main take-away lessons) were well-reported (Reporting rate more than 90%) in most of the included case reports. However, only three items, 2 (Keywords, OR = 0.42, 95%CI: 0.19-0.92, P = 0.03), 4 (Introduction, OR = 0.35, 95%CI: 0.15-0.83, P = 0.017), and 11b (The relevant medical literature, OR = 0.19, 95%CI: 0.06-0.56, P = 0.003) were considered better-reported after the CARE guidelines published in 2013.
The reporting quality of case reports in the nursing field apparently has not improved since the publication of the CARE guidelines.
Core tip: This study brings attention to the reporting quality of case reports of nurses and researchers in the nursing field in order to help clinical nurses continue to accumulate knowledge of new methods and gain experience in the context of state-of-the-art nursing care.
- Citation: Yang KL, Lu CC, Sun Y, Cai YT, Wang B, Shang Y, Tian JH. How about the reporting quality of case reports in nursing field? World J Clin Cases 2019; 7(21): 3505-3516
- URL: https://www.wjgnet.com/2307-8960/full/v7/i21/3505.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i21.3505
Case reports are a common type of medical research article that details symptoms, signs, diagnosis, treatments, and follow-up of one or more patients[1]. They are becoming increasingly more common and comprise a significant proportion of the articles in many medical and nursing journals. The number of case reports produced over the past of decades has increased, and the PubMed database currently lists nearly 2 million records. Case reports have been acknowledged to be helpful in the identification of adverse and beneficial effects and in recognizing new diseases, unusual forms of common diseases, and the presentation of rare diseases[2,3].
As a significantly important part of clinical practice, the professional nursing process can be advanced in many ways[4]. Despite the fact that case reports are regarded to be of a lower quality grade in the hierarchy of evidence[5], one of the principles of evidence-based medicine is that decision-making should be based on a systematic summary of evidence[6]. There are various and complex forms of treatments in the nursing field, and only by reporting professional clinical work processes can new and effective nursing interventions be shared and implemented. In 2013, the CARE guidelines were published. These were designed to increase the accuracy, transparency, and usefulness of case reports[7]. Several case reports in different fields have been assessed via the CARE guidelines, including traditional Chinese medicine[8], anesthesia[9], PubMed-index Indian journals[10], and therapeutic massage and bodywork[11]. However, evidence on the reporting characteristics of case reports in the nursing field is deficient. Therefore, it is important to assess the reporting quality of case reports in the nursing field using the CARE guidelines.
The aim of this study was to identify factors influencing the quality of case reports and to explore the applicability of the CARE guidelines to nursing case reports by assessing the reporting quality case reports published in nursing science citation indexed (SCI-indexed) journals according to the CARE guidelines.
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Sixteen SCI-indexed journals in nursing (http://journal.medsci.cn/journal/index) were selected, and the websites of the selected journals were further searched to identify all published case reports.
The inclusion criterion was case reports, and the exclusion criteria were as follows: (1) Studies that reported over one case, because it was hard to record the scores; and (2) Studies not conducted on a disease.
The selected case reports were reviewed separately by two reviewers (Yang KL and Lu CC) and were cross-checked after reviewing. Differences in opinion were resolved through discussion or by consultation with a third party (Tian JH).
First, two reviewers separately pre-reviewed and screened the titles and abstracts of the selected case report studies, and then the full texts of the included case reports were read. Second, two reviewers extracted the relevant information and data from the included case reports. A well-designed extraction table was made in accordance with CARE guidelines to assess the reporting quality of the included case reports. The extraction table included: (1) General characteristics[12] [i.e. journal name, publication date, number of pages, number of authors, number of institutes, impact factor (IF), and funding source]; and (2) The CARE guidelines checklist, which contains 11 primary items (including two optional items) and 21 sub-items. Items 12 and 13 were not taken into consideration in this study because there is an ongoing debate regarding these two items[7].
The 21 sub-items could be answered by “YES” (clearly done), “PARTLY” (cannot answer or not applicable), or “NO” (clearly not done) according to detailed information contained in the included studies[13]. Differences of opinions were resolved through discussion or by consultation with a third party (Tian JH).
We exercised strict control over the quality of the study. Before selecting the included case reports, the reviewers were trained to distinguish case reports from irrelevant studies and reached an agreement on eligibility criteria. Before the data extraction, an extraction table was designed, and a pre-extraction was performed. From this process, the table was modified, and the process was repeated until a consensus regarding the extraction table was reached.
The corresponding scores of every “YES”, “PARTLY”, and “NO” recorded for each sub-item were assigned 1, 0.5, and 0, respectively. Finally, the scores of all sub-items were summed up to generate the overall scores. The maximum overall score was 21. Scores less than 10 indicated low-quality, which meant the study assessed had serious information flaws. Overall scores between 10.5 and 14.5 meant the study was regarded as medium-quality and had some information flaws. Studies with scores over 15 were regarded as high quality, meaning they had relatively complete information. The intraclass correlation coefficient (ICC) scores were calculated as well. When calculating the reporting number of each sub-item, “YES” was assigned to “a” and “PARTLY” and “NO” to “b”.
The reporting characteristics were analyzed according to different strata, which were defined by general characteristics that included the year of publication, countries, IF of the included journals, funding, and different periods. The data were summarized using descriptive statistics [frequencies, percentages, mean ± standard deviation (SD), the odds ratio (OR) and 95% confidence interval (CI)]. OR ≠ 1 and P < 0.05 represented differences between the two groups. The analyses were carried out using Stata 12.0.
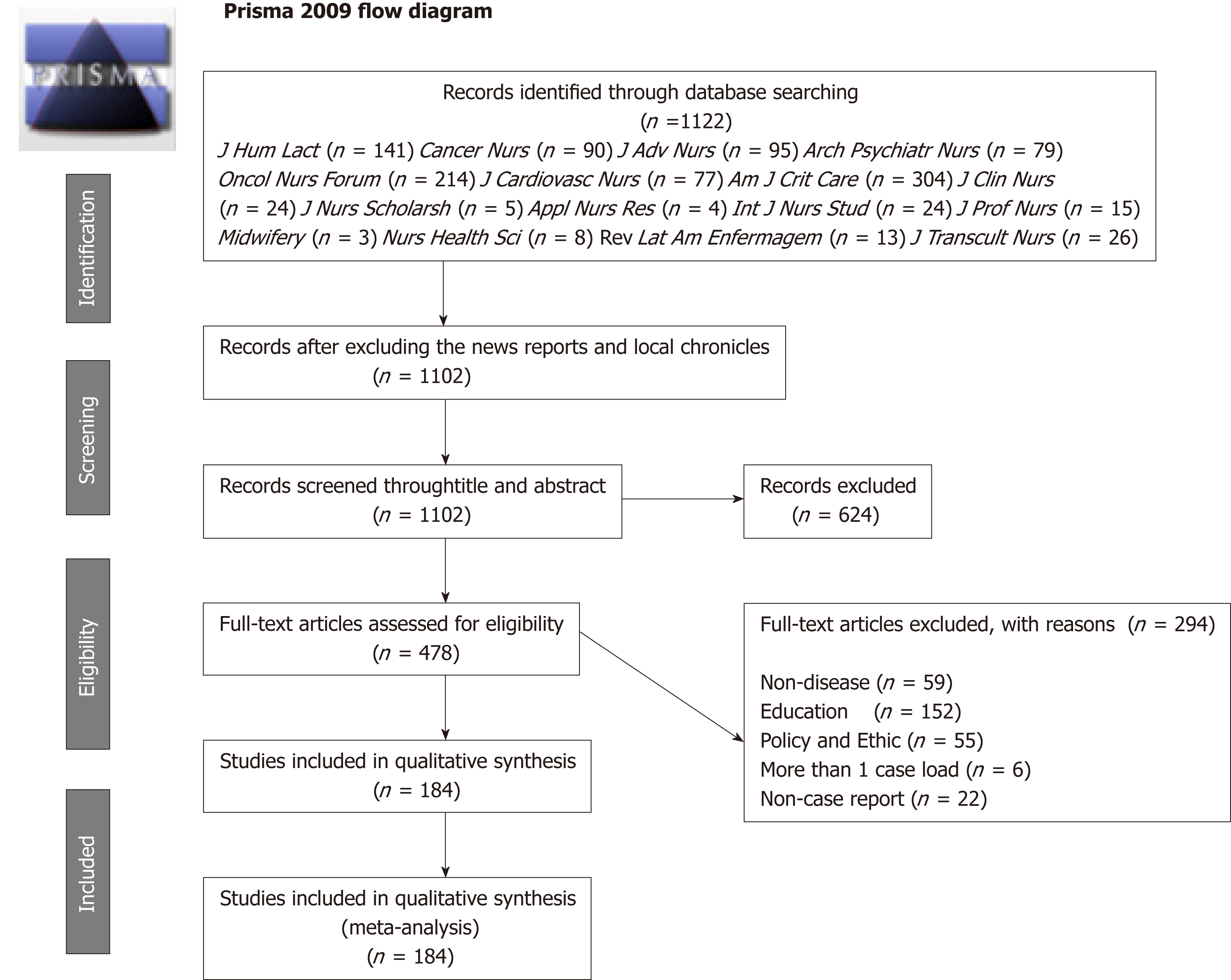
Originally, a total of 1122 case reports were searched from the websites of the selected journals. After reviewing them, 644 studies were excluded according to the exclusion criteria. Ultimately, there were 184 case reports included in this study. A flow chart of the study selection is shown in Figure 1. The basic information of the records identified in the 16 journals is listed in Supplementary File 1.
Table 1 shows the general characteristics of the included case reports. All 184 included case reports were published between 1984 and 2017. Of the included case reports, 81.5% (95%CI: 75.1%-86.9%) were published before the CARE guidelines were published. Furthermore, 85.9% (95%CI: 80.0%-90.6%) of the included case reports were published in journals with IFs no less than 1.8. The number of authors in the included case reports ranged from one to 11. Only one author was listed on 33.2% of the case reports (95%CI: 26.4%-40.5%), 29.3% (95%CI: 47.7%-62.2%) had two to four, and 12.0% (95%CI: 7.6%-17.5%) listed more than four authors. Most of publications (62.5%, 95%CI: 55.1%-69.5%) listed a single institution. Furthermore, only one included case report contained one page, and 47.3% (95%CI: 39.9%-54.8%) included case reports of two to four pages. The majority of the identified studies received no funding (94.5%, 95%CI: 90.2%-97.4%).
| Item | Number of case reports (%, 95%CI) |
| Year of publication | |
| Before CARE (1984.1-2013.7) | 150 (81.5, 75.1-86.9) |
| After CARE (2013.8-2017.12) | 34 (18.5, 13.1-24.9) |
| Journal’s IF | |
| IF < 1.8 | 26 (14.1, 9.4-20.0) |
| IF ≥ 1.8 | 158 (85.9, 80.0-90.6) |
| Different countries | |
| Developing countries | 14 (7.6, 4.2-12.4) |
| Developed countries | 170 (92.4, 87.6-95.8) |
| Funding sources | |
| With fundings | 10 (5.4, 2.6-9.8) |
| No fundings | 174 (94.6, 90.2-97.4) |
| Number of authors | |
| One author | 61 (33.2, 26.4-40.5) |
| Two to four authors | 101 (54.9, 47.4-62.2) |
| More than four authors | 22 (12.0, 7.6-17.5) |
| Number of author’s affiliation | |
| One affiliation | 115 (62.5, 55.1-69.5) |
| More than one affiliation | 69 (37.5, 30.5-44.9) |
| Number of pages | |
| One page | 1 (0.5, 0.0-3.0) |
| Two to four pages | 87 (47.3, 39.9-54.8) |
| More than four pages | 96 (52.2, 44.7-59.6) |
The mean score of all the included case reports was 15.6 ± 2.3. Of the included reports, 10.3% (95%CI: 6.3%-15.7%) were regarded as low-quality, 52.7% (95%CI: 45.2%-60.1%) were considered middle-quality, and 37% (95%CI: 30.0%-44.4%) were high-quality. There were statistical differences between the scores of case reports with funding versus without funding (mean ± SD: 14.2 ± 1.7 vs 13.6 ± 2.4, P = 0.4456) or according to the journal IF < 1.8 versus IF ≥ 1.8 (mean ± SD: 13.3 ± 2.3 vs 13.6 ± 2.4, P = 0.4977). These data are summarized in Table 2.
| Score | Yr of publication | Journal’s IF | Different countries | Funding sources | In total | ||||
| Before 2013, n = 150 | After 2013, n = 34 | IF < 1.8, n = 26 | IF ≥ 1.8, n = 158 | Developing countries, n = 14 | Developed countries, n = 170 | With funding, n = 10 | No reported, n = 174 | ||
| Mean± SD | 13.5 ± 2.4 | 14.2 ± 2.1 | 13.3 ± 2.3 | 13.6 ± 2.4 | 14.4 ± 2.5 | 13.5 ± 2.3 | 14.2 ± 1.7 | 13.6 ± 2.4 | 15.6 ± 2.3 |
| P value | P = 0.1114 | P = 0.4977 | P = 0.1687 | P = 0.4456 | |||||
| Range [No. (percentage,95%CI)] | |||||||||
| ≤ 10 | 18 (12.0, 7.3-18.3) | 1 (2.9, 0.1-15.3) | 2 (7.7, 0.9-25.1) | 17 (10.8, 6.4-16.7) | 1 (7.1, 0.2-33.9) | 18 (10.6, 6.4-16.2) | 0 (0.0, 0.0-30.8) | 19 (10.9, 6.7-16.5) | 19 (10.3, 6.3-15.7) |
| 10.5-14.5 | 80 (53.3, 45.0-61.5) | 17 (50.0, 32.4-67.6) | 17 (65.4, 44.3-82.8) | 80 (50.6, 42.6-58.7) | 5 (35.7, 12.8-64.9) | 92 (54.1, 46.3-61.8) | 6 (60.0, 26.2-87.8) | 91 (52.3, 44.6-59.9) | 97 (52.7, 45.2-60.1) |
| ≥ 15 | 52 (34.7, 27.1-42.9) | 16 (47.1, 29.8-64.9) | 7 (26.9, 11.6-47.8) | 61 (38.6, 31.0-46.7) | 8 (57.1,28.9-82.3) | 60 (35.3, 28.1-43.0) | 4 (40.0, 12.2-73.8) | 64 (36.8, 29.6-44.4) | 68 (37.0, 30.0-44.4) |
The ICC scores in the 21 sub-items were higher than 85% (Supplementary File 2). The items reported in the included case reports ranged from five to 18, and the mean was 13 records per case report. Among the included records, none of them reported all 21 sub-items. Five of the 21 sub-items were reported in more than 90% of the case reports: 5a (Demographic information), 6 (Clinical findings), 8c (Diagnostic reasoning), 9 (Therapeutic intervention), and 11d (The main “take-away” lessons of including case reports). However, the reporting rates of the other three items, 5c (Medical, family, and psychosocial history), 8d (Prognostic characteristics), and 10 (Follow-up and outcomes) were reported less than 20% in our selected studies. The reporting rate details are shown in Table 3.
| Items | Yr of publication | Journal’s IF | Different countries | Funding sources | In total | ||||
| Before 2013, n = 150 | After 2013, n = 34 | IF < 1.8, n = 26 | IF ≥ 1.8, n = 158 | Developing countries, n = 14 | Developed countries, n = 170 | With funding, n = 10 | No reported, n = 174 | ||
| 1 | 68 (45.3, 37.2-53.7) | 9 (26.5, 12.9-44.4) | 11 (42.3, 23.4-63.1) | 66 (41.8, 34.0-49.9) | 3 (21.4, 4.7-50.8) | 74 (43.5, 36.0-51.3) | 7 (70.0, 34.8-93.3) | 70 (40.2, 32.9-47.9) | 77 (41.8, 34.6-49.3) |
| 2 | 70 (46.7, 38.5-55.0) | 23 (67.6, 49.5-82.6) | 15 (57.7, 36.9-76.6) | 78 (49.4, 41.3-57.4) | 9 (64.3, 35.1-87.2) | 84 (49.4, 41.7-57.2) | 2 (20.0, 2.5-55.6) | 91 (52.3, 44.6-59.9) | 93 (50.5, 43.1-58.0) |
| 3a | 86 (57.3, 49.0-65.4) | 19 (55.9, 37.9-72.8) | 14 (53.8, 33.4-73.4) | 91 (57.6, 49.5-65.4) | 13 (92.9, 66.1-99.8) | 92 (54.1, 46.3-61.8) | 7 (70.0, 34.8-93.3) | 98 (56.3, 48.6-63.8) | 105 (57.1, 49.6-64.3) |
| 3b | 85 (56.7, 48.3-64.7) | 19 (55.9, 37.9-72.8) | 5 (19.2, 6.6-39.4) | 99 (62.7, 54.6-70.2) | 9 (64.3, 35.1-87.2) | 95 (55.9, 48.1-63.5) | 8 (80.0, 44.4-97.5) | 96 (55.2, 47.5-62.7) | 104 (56.5, 49.0-63.8) |
| 3c | 89 (59.3, 51.0-67.3) | 20 (58.8, 40.7-75.4) | 10 (38.5, 20.2-59.4) | 99 (62.7, 54.6-70.2) | 8 (57.1, 28.9-82.3) | 101 (59.4, 51.6-66.9) | 8 (80.0, 44.4-97.5) | 101 (58.0, 50.3-65.5) | 109 (59.2, 51.8-66.4) |
| 4 | 80 (53.3, 45.0-61.5) | 26 (76.5, 58.8-89.3) | 22 (84.6, 65.1-95.6) | 84 (53.2, 45.1-61.1) | 10 (71.4, 41.9-91.6) | 96 (56.5, 48.7-64.0) | 6 (60.0,26.2-87.8) | 100 (57.5, 49.8-64.9) | 106 (57.6, 50.1-64.8) |
| 5a | 137 (91.3, 85.6-95.3) | 33 (97.1, 84.7-99.9) | 25 (96.2, 80.4-99.9) | 142 (89.9, 84.1-94.1) | 14 (100.0, 76.8-100.0) | 153 (90.0, 84.5-94.1) | 9 (90.0, 55.5-99.7) | 158 (90.8, 85.5-94.7) | 167 (90.8, 85.6-94.5) |
| 5b | 100 (66.7, 58.5-74.1) | 26 (76.5, 58.8-89.3) | 20 (76.9, 56.4-91.0) | 106 (67.1, 59.2-74.3) | 9 (64.3, 35.1-87.2) | 117 (68.8, 61.3-75.7) | 7 (70.0, 34.8-93.3) | 119 (68.4, 60.9-75.2) | 126 (68.5, 61.2-75.1) |
| 5c | 10 (6.7, 3.2-11.9) | 3 (8.8, 1.9-23.7) | 3 (11.5, 2.4-30.2) | 10 (6.3, 3.1-11.3) | 1 (7.1, 0.2-33.9) | 12 (7.1, 3.7-12.0) | 1 (10.0, 0.3-44.5) | 12 (6.9, 3.6-11.7) | 13 (7.1, 3.8-11.8) |
| 6 | 140 (93.3, 88.1-96.8) | 31 (91.2, 76.3-98.1) | 24 (92.3, 74.9-99.1) | 147 (93.0, 87.9-96.5) | 12 (85.7, 57.2-98.2) | 159 (93.5, 88.7-96.7) | 9 (90.0, 55.5-99.7) | 162 (93.1, 88.3-96.4) | 171 (92.9, 88.2-96.2) |
| 7 | 105 (70.0, 62.0-77.2) | 20 (58.8, 40.7-75.4) | 13 (50.0, 29.9-70.1) | 112 (70.9, 63.1-77.8) | 9 (64.3, 35.1-87.2) | 116 (68.2, 60.7-75.2) | 7 (70.0, 34.8-93.3) | 118 (67.8, 60.3, 74.7) | 125 (67.9, 60.7-74.6) |
| 8a | 127 (84.7, 779-90.0) | 31 (91.2, 76.3-98.1) | 22 (84.6, 65.1-95.6) | 136 (86.1, 79.7-91.1) | 13 (92.9, 66.1-99.8) | 145 (85.3, 79.1-90.3) | 8 (80.0, 44.4-97.5) | 150 (86.2, 80.2-91.0) | 158 (85.9, 80.0-90.6) |
| 8b | 27 (18.0, 12.2-25.1) | 8 (23.5, 10.7-41.2) | 5 (19.2, 6.6-39.4) | 30 (19.0, 13.2-26.0) | 5 (35.7, 12.8-64.9) | 30 (17.6, 12.2-24.2) | 1 (10.0, 0.3-44.5) | 34 (19.5, 13.9-26.2) | 35 (19.0, 13.6-25.4) |
| 8c | 148 (98.7, 95.3-99.8) | 34 (100.0, 89.7-100.0) | 26 (100.0, 86.8-100.0) | 156 (98.1, 95.5-99.8) | 14 (100.0, 76.8-100.0) | 168 (98.8, 95.8-99.9) | 10 (100.0,69.2-100.0) | 172 (98.9, 95.9-99.9) | 182 (98.9, 96.1-99.9) |
| 8d | 1 (0.7, 0.0-3.7) | 2 (5.9, 0.7-19.7) | 1 (3.8, 0.1-19.6) | 2 (1.3, 0.2-4.5) | 0 (0.0, 0.0-23.2) | 3 (1.8, 0.4-5.1) | 0 (0.0,0.0-30.8) | 3 (1.7, 0.4-5.0) | 3 (1.6, 0.3-4.7) |
| 9 | 147 (98.0, 94.3-99.6) | 32 (94.1, 80.3-99.3) | 24 (92.3, 74.5-99.1) | 155 (98.1, 94.6-996) | 13 (92.9, 66.1-99.8) | 166 (97.6, 94.1-99.4) | 9 (90.0, 55.5-99.7) | 170 (97.7, 94.2-99.4) | 179 (97.3, 93.8-99.1) |
| 10 | 3 (2.0, 0.4-5.7) | 2 (5.9, 0.7-19.7) | 2 (7.7, 0.9-25.1) | 3 (1.9, 0.4-5.4) | 0 (0.0, 0.0-23.2) | 5 (2.9, 1.0-6.7) | 1 (10.0, 0.3-44.5) | 4 (2.3, 0.6-5.8) | 12 (6.5, 3.4-11.1) |
| 11a | 104 (69.3, 61.3-76.6) | 23 (67.6, 49.5-82.6) | 19 (73.1, 52.2-88.4) | 108 (68.4, 60.5-75.5) | 11 (78.6, 49.2-95.3) | 116 (68.2, 60.7-75.2) | 6 (60.0,26.2-87.8) | 121 (69.5, 62.1-76.3) | 127 (69.0, 61.8-75.6) |
| 11b | 88 (58.7, 50.3-66.6) | 30 (88.2, 72.5-96.7) | 18 (69.2, 48.2-85.7) | 100 (63.3, 55.3-70.8) | 9 (64.3, 35.1-87.2) | 109 (64.1, 56.4-71.3) | 9 (90.0, 55.5-99.7) | 109 (62.6, 55.0-69.8) | 118 (64.1, 56.7-71.1) |
| 11c | 113 (75.3, 67.6-82.0) | 17 (50.0, 32.4-67.6) | 15 (57.7, 36.9-76.6) | 115 (72.8, 65.1-79.6) | 11 (78.6, 49.2-95.3) | 119 (70.0, 62.5-76.8) | 7 (70.0, 34.8-93.3) | 123 (70.7, 63.3-77.3) | 130 (70.7, 63.5-77.1) |
| 11d | 138 (92.0, 86.4-95.8) | 33 (97.1, 84.7-99.9) | 26 (100.0, 86.8-100.0) | 145 (91.8, 86.3-95.5) | 13 (92.9, 66.1-99.8) | 158 (92.9, 88.0-96.3) | 10 (100.0,69.2-100.0) | 161 (92.5, 87.6-96.0) | 171 (92.9, 88.2-96.2) |
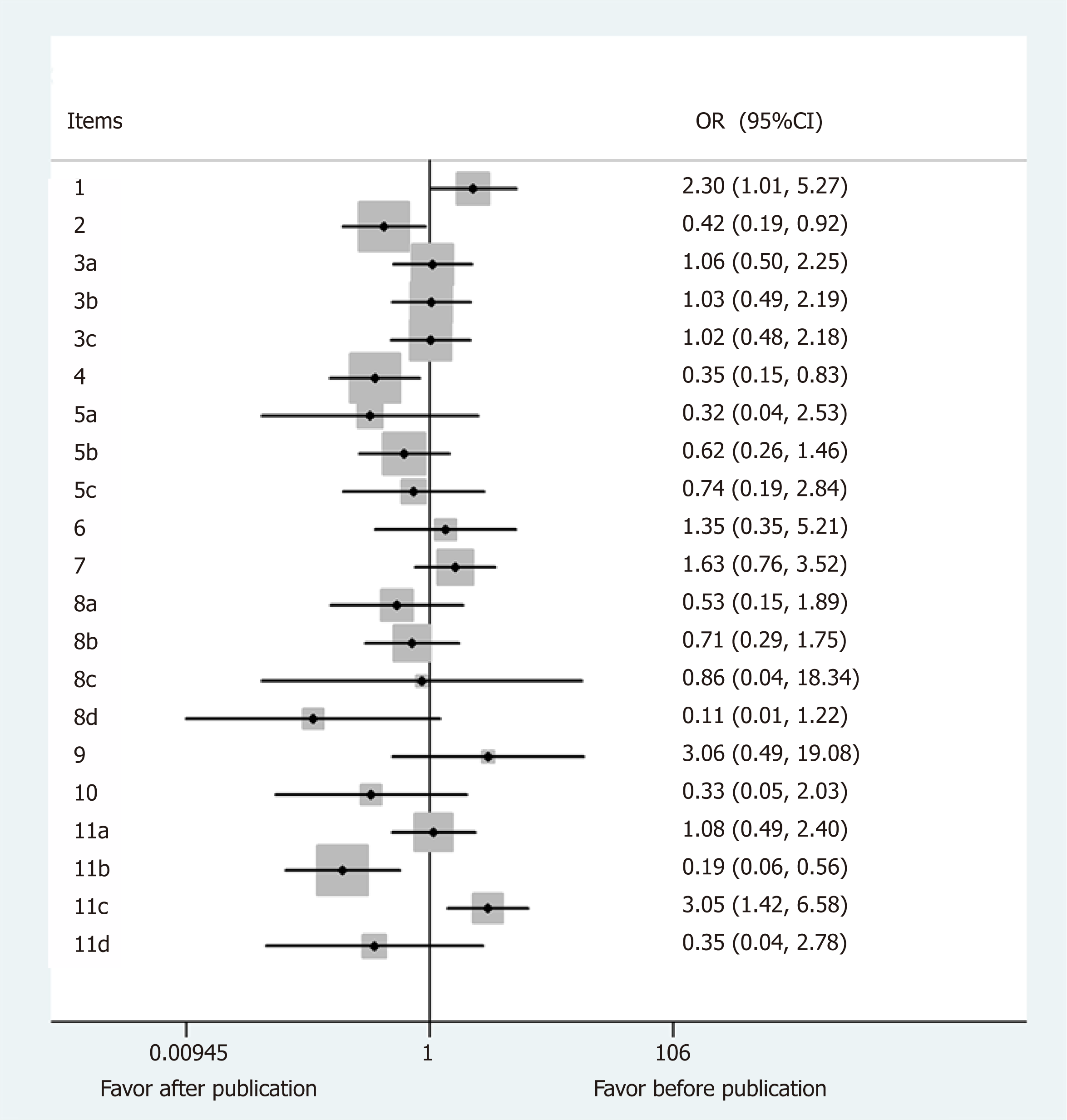
Comparison of the case reports before and after 2013 (Figure 2): Since the CARE guidelines were published in 2013, we intended to compare the reporting rates before and after publication. Three items, 2 (Keywords, OR = 0.42, 95%CI: 0.19-0.92, P = 0.03), 4 (Introduction, OR = 0.35, 95%CI: 0.15-0.83, P = 0.017), and 11b (The relevant medical literature, OR = 0.19, 95%CI: 0.06-0.56, P = 0.003) had better reporting rates after publication of the CARE guidelines. However, the reporting rate for items 1 (Title, OR = 2.30, 95%CI: 1.01-5.27, P = 0.048) and 11c (The rationale for conclusion, OR = 3.05, 95%CI: 1.42-6.58, P = 0.004) were not as high as that before publication of the guidelines.
Comparison of case reports with or without funding (Supplementary File 3): There was no statistically significant difference in the reporting of the 21 sub-items between reports with or without listed funding sources.
Comparison of the case reports in developed and developing countries (Supplementary File 4): The reporting rate for item 3a (Introduction) in journals from developing countries was better than that of developed countries (OR = 11.02, 95%CI: 1.41-86.15, P = 0.022). The rest of the items were not statistically different.
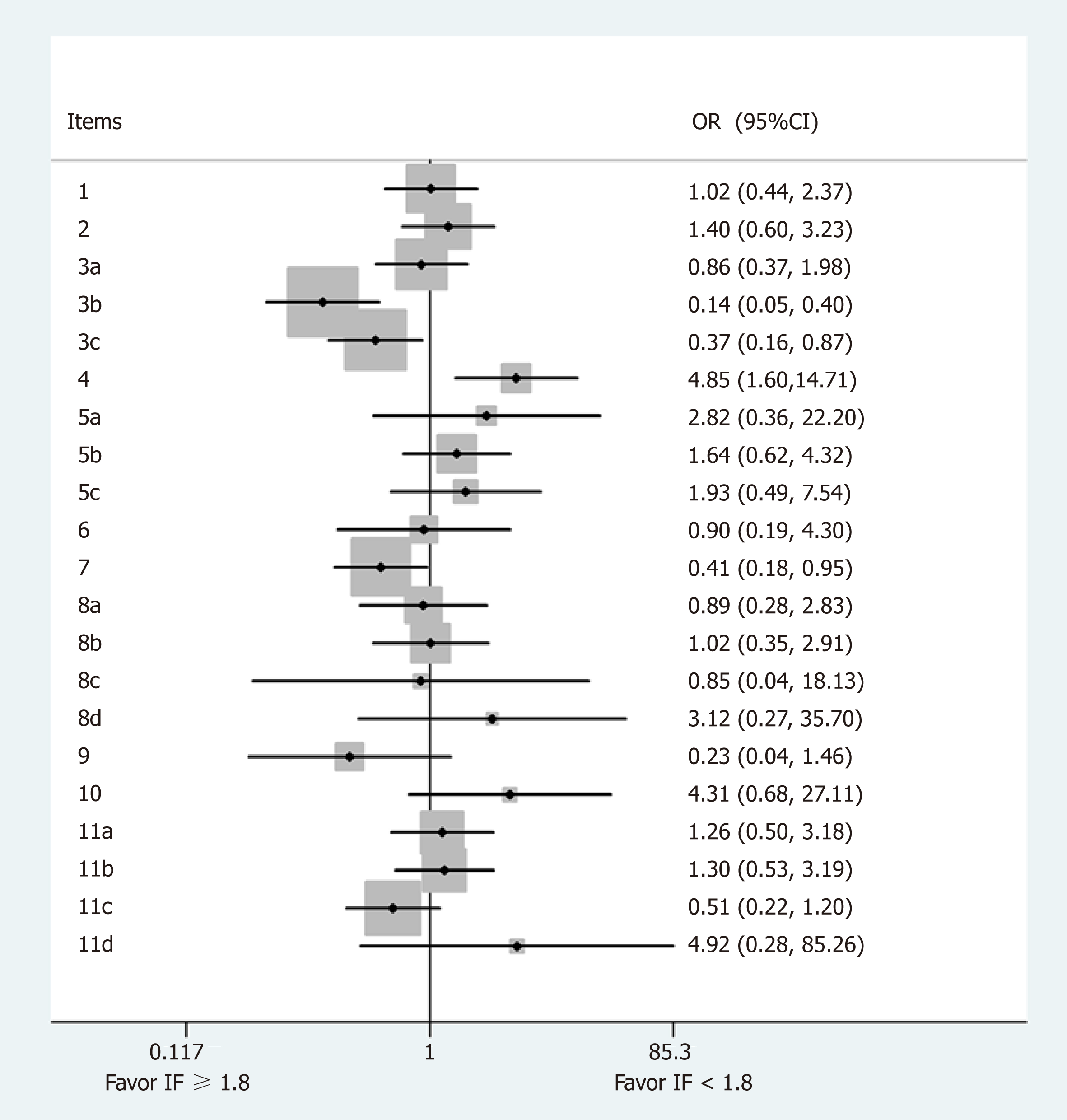
Comparison of case reports with IFs more or less than 1.8 points (Figure 3): The mean IF of the included journals was 1.8, so a comparison of the reporting rates was made between journals with an IF < 1.8 and IF ≥ 1.8. We found that journals with an IF ≥ 1.8 preferably reported items 3b (Case Presentation, OR = 0.14, 95%CI: 0.05-0.40, P = 0.000), 3c (Conclusion, OR = 0.37, 95%CI: 0.16-14.71, P = 0.023), and 7 (Timeline, OR = 0.41, 95%CI: 0.18-0.95, P = 0.038). In contrast, item 4 (Introduction, OR = 4.85, 95%CI: 1.60-14.71, P = 0.005) was reported more in journals with an IF < 1.8.
In this systematic review, 184 full-text case reports were investigated using the CARE guidelines checklist. The journal that contained the largest proportion of case reports was the Journal of Human Lactation, while eight journals contained only one case report. None of the included case reports from the 16 selected SCI-indexed journals contained all 21 sub-items. According to our assessment and the scores we assigned, the overall quality of the included case reports was middle-level, similar to the studies by Yuan et al[14] and Eldawlatly et al[9]. Nearly one-third of the included case reports listed only one author, and nearly 60% included case reports listing only one institution. Furthermore, 0.5% of the included case reports were a single page, and most of these were published in the 1990s. The diseases reported in the case reports were usually rare and unusual and were generally treated, managed, and discussed by more than two clinicians and nurses or even required multidisciplinary care. Hence, more attention should be paid to the authors listed because this reflects the degree of professional input into the case management and report.
The title and abstract of the case reports: Correct title and abstract formats and keywords generate appropriate and accurate record retrieval. The words “case report” (or “case study”) should be mentioned in the title, along with important symptoms of the disease and/or diagnosis and/or intervention, since there has been a lacking of high sensitivity retrieval strategy for case reports. The abstract provides a balanced and concise summary of a complete report, usually ranging from 100 to 250 words, depending on the journal, which is helpful for understanding the basic content of the article quickly. The structure and formats of abstracts, specifically, appear to be informative and may be useful to practitioners as a resource for guiding clinical decisions since they contain the main features of the described case, the main treatment measures and results, and a brief description of the clinical significance and experience[7,15-17]. In this study, the correct format was applied in less than half of the included case reports, and many of them failed to add “case report” or “case study” to the title and lacked adequate description in the abstract. These particular shortcomings need to be urgently improved.
The main body of the case reports: The included case reports that completely reported item 4 (Introduction) only accounted for 57.6%. Authors might give priority to the description and analysis of the case itself and thereby, give little attention to the introduction of the case presented. However, to acknowledge that a reported disease is rare and representative, the authors should describe the incidence of the disease and compare it with the published literature in the introduction. Although it does not require all the evidence that network meta-analyses and meta-analyses do, it still needed to identify the question and gap in the knowledge, which highlight the importance of the study and the uniqueness of the case [15,18,19].
Relevant demographic patient information, especially medical, family, and psychosocial history is important for nurses to evaluate a patient’s condition. The medical and family history in item 5c (Medical, family, and psychosocial history) was reported well, but the psychosocial history part was not satisfied. Some of the included case reports reported psychosocial history but without sufficient details or use of relevant scales to evaluate the patient’s mental and psychological condition. Compared with medical case reports that focus on the process and treatment of a disease, nursing is more concerned with holistic care[20], which emphasizes the role of mental evaluation. In order to have a more comprehensive and complete nursing evaluation and to make a more accurate nursing diagnosis, the appreciation of psychiatric evaluations in the nursing process assessment is indispensable[21].
Adding timeline in a chart or table has been suggested to display relevant events in the patient’s medical history in chronological order and to summarize briefly one or more key events in the case[22]. Over half of the included case reports listed important dates and times, but few of them presented them in the form of a table or figure. The other timeline styles, such as bold headings, definite dates, and italics, were considered to have the same effect as tables and figures. Therefore, the fixed timeline model was not compulsively required in our study.
Item 8d (Prognostic characteristics where applicable) was scarcely reported because some diseases and cases did not require a description and analysis of the factors influencing prognosis. For example, the Journal of Human Lactation is a journal about breastfeeding, and the case reports in this journal mostly pertain to lactation difficulties, which have no prognostic characteristics. The reporting rates for items 8a (Diagnostic methods, 85.9%) and 8c (Diagnostic reasoning, 98.9%) were much higher than items 8b (Diagnostic challenges, 19.0%) and 8d (Prognostic characteristics, 1.6%). The reason for this result might be that this part of the CARE guidelines does not pertain to the nursing field. From our perspective, case reports in the nursing field should focus on nursing evaluations and diagnoses. Most of the authors work as nurses or major in nursing, so they need to assess patients from a nursing discipline point and to make nursing diagnoses precisely[23]. Notably, the science of caring can make up for the lack of therapeutic science[24]. Hence, the applicability of these four items (8a-8d) in the nursing field needs further discussion.
In spite of the high reporting rate of item 9 (Therapeutic intervention), there were still some problems in the included case reports. The majority of the case reports in this study laid emphasis on medical treatments over nursing interventions. The interventions should be repeatable, and the intervention type, the implementation process of the interventions, and the reasons for changing interventions should be reported in detail in order to enhance the authenticity of the cases and the diagnosis and treatment process[3]. Although case reports cannot be considered systematic reviews, and thereby do not adhere to similar review, analysis, and reporting standards, specifying the research project protocol and providing sufficient details of the intervention and changes in the patients' conditions might increase the credibility of the cases[25]. Nursing interventions were not reported specifically in most of the included case reports, which did not provide the readers with many useful or referential experiences.
Longitudinal findings may help create a compelling case and clarify the relationship between outcomes and treatments. Therefore, other studies have recommended the reporting of objective and subjective findings throughout the course of care in order to track changes in the outcomes of interest[22,26]. However, item 10 (Follow-up and outcomes) was not comprehensively reported. Most of the case reports did not report adverse events or the adherence to or tolerability of the interventions. Follow-up is a significant intervention in transitional care[27]. All case reports should explicitly mention the presence or absence of adverse event[22]. In this study, only 6.5% of the included case reports stated whether there were any adverse and unanticipated events. One reason for the low report rate might be that in most cases, the authors tried to collect patient information retrospectively, which presented certain obstacle[28].
Since the publication of the CARE guidelines, more attention has been paid to the item 2 “Keywords”, item 4 “Introductions”, and item 11b “The relevant medical literature”, which increased the rationality of the articles and produced a more complete structure. Recommendations in the reports should be supported with published references. It is essential to discuss case report limitations transparently, since the results of individual cases may not be applicable to general patients[29]. Keywords that identify the focus of the case report should be selected using MeSH terminology (available at: http://www.pubmed.com) or Google Scholar. However, the keywords of the included case reports were not standardized. Although the reporting rate of the keywords was higher after the publication of the CARE guidelines, the standardization of keyword selection was unsatisfactory. For example, most of the included case reports in the Journal of Human Lactation listed “human milk”[30] and “breast milk”[31] as keywords but they were listed as “milk, human” in MeSH terminology[32]. Journals with IFs more than 1.8 had better reporting rates for items 3b (Case Presentation), item 3c (Conclusion), and item 7 (Timeline), which might indicate that the details in the abstract and the structure of the main text were addressed. Hence, publication of the CARE guidelines has enhanced reporting quality to a certain degree.
The comparison of funding sources and different countries did not show significant differences in reporting rates of the 21 sub-items. However, the scores of the included case reports with funding were higher than those without funding. To some extent, the funding source was a possible reason for reporting quality improvement.
First, only SCI nursing journals were included in this study. Thus, our results may not be generalizable to case reports published in other nursing journals. Additionally, the inclusion criteria may have been too restrictive, leading to the inclusion of fewer articles in this study. Furthermore, some of the journals only included one case report, which might influence the outcomes of the calculations.
In conclusion, sufficient reporting of case reports is the responsibility of all medical professionals. When writing a case report, the authors should report in the form prescribed according to the CARE guidelines. In this study, the nursing care reports were assessed to be middle-quality, but many details remain to be resolved and improved. Thus, the quality of case reports in the nursing field still needs improvement. Moreover, there are some differences between nursing care and medicine, so more research regarding the nursing-adapted CARE guidelines should be conducted in the future.
We would like to thank the library of Lanzhou University for database accessing and acquiring full texts, and Xi-Ping Shen from the Department of Epidemiology and Biostatistics of School of Public Health of Lanzhou University for providing the help of biostatistics service.
As a significantly important part of clinical practice, the professional nursing process can be advanced in many ways. Although case reports are regarded as lower grade in the hierarchy of evidence, they are still important in the nursing field. However, the evidence on reporting characteristics of case reports in nursing field is deficient.
Information provided in the higher quality case reports could assist nurses with the opportunity to deal with intractable cases or rare diseases. Clinical nursing practice must continue to accumulate knowledge of new methods and experience in the context of state-of-the-art nursing care to improve the well-being of patients.
The aim of this study was to identify factors influencing the quality of case reports and to explore the applicability of the CARE guidelines to nursing case reports by assessing the reporting quality of case reports published in nursing science citation indexed journals according to the CARE guidelines.
Twenty-one sub-items on the CARE checklist were recorded as “YES”, “PARTLY”, or “NO” according to information reported by the included studies. The responses were assigned corresponding scores of 1, 0.5, and 0, respectively. The overall scores were the sum of the 21 sub-items and were defined as “high” (more than 15), “medium” (10.5 to 14.5), and “low” (less than 10). The means, standard deviations, and odds ratios and the associated 95% confidence intervals were determined using Stata 12.0 software.
The overall quality of nursing case reports is not high. Of the 21 items, only five items (11d, 9, 8c, 6, 5a) were reported in more than 90% of the included case reports, and three items (5c, 8d, 10) were reported less than 10%.
The reporting quality of case reports in the nursing field apparently has not improved since the publication of the CARE guidelines.
There are some differences between nursing and medicine. Therefore, more research on the extended version of the CARE guidelines suited for the nursing field can be conducted in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nag DS S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Iles RL. Guidebook to better medical writing. Iles Publications; 2003. . |
| 2. | Hauben M, Aronson JK. Gold standards in pharmacovigilance: the use of definitive anecdotal reports of adverse drug reactions as pure gold and high-grade ore. Drug Saf. 2007;30:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Albrecht J, Werth VP, Bigby M. The role of case reports in evidence-based practice, with suggestions for improving their reporting. J Am Acad Dermatol. 2009;60:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Benedet SA, Padilha MI, Gelbke FL, Bellaguarda MLDR. The model professionalism in the implementation of the Nursing Process (1979-2004). Rev Bras Enferm. 2018;71:1907-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Aronson JK. Anecdotes as evidence. BMJ. 2003;326:1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Yao L, Sun R, Chen YL, Wang Q, Wei D, Wang X, Yang K. The quality of evidence in Chinese meta-analyses needs to be improved. J Clin Epidemiol. 2016;74:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group. The CARE guidelines: consensus-based clinical case report guideline development. J Diet Suppl. 2013;10:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | An GH, Tang XT, Chen YL, Zhao Y. Reporting characteristics of case reports of acupuncture therapy with CARE guidelines. Chin J Integr Med. 2018;24:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Eldawlatly A, Alsultan D, Al Dammas F, Ahmed A, Al Andas R, Zahoor B. Adaptation of CARE (CAse REport) guidelines on published case reports in the Saudi Journal of Anesthesia. Saudi J Anaesth. 2018;12:446-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Ravi R, Mulkalwar A, Thatte UM, Gogtay NJ. Medical case reports published in PubMed-indexed Indian journals in 2015: Adherence to 2013 CARE guidelines. Indian J Med Ethics. 2018;3:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Munk N, Shue S, Freeland E, Ralston R, Boulanger KT. Identifying Inconsistencies and Reporting Deficits in Therapeutic Massage and Bodywork (TMB) Case Reports Authored by TMB Practitioners: a TMB-Adapted CAse REport (CARE) Guidelines Audit Through 2014. Int J Ther Massage Bodywork. 2016;9:3-14. [PubMed] |
| 12. | Samaan Z, Mbuagbaw L, Kosa D, Borg Debono V, Dillenburg R, Zhang S, Fruci V, Dennis B, Bawor M, Thabane L. A systematic scoping review of adherence to reporting guidelines in health care literature. J Multidiscip Healthc. 2013;6:169-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Xiu-xia L, Ya Z, Yao-long C, Ke-hu Y, Zong-jiu Z. The reporting characteristics and methodological quality of Cochrane reviews about health policy research. Health Policy. 2015;119:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Yuan T, Pan YX, Gu WJ, Wang MX, Zhang JY, Wei D, Tian JH, Yang KH. Reporting quality assessment of the case reports published in the journals of Pediatrics in Chinese Science Citation Database. Zhongguo Xunzheng Erke Zazhi. 2017;12:27-32. [DOI] [Full Text] |
| 15. | Cohen H. How to write a patient case report. Am J Health Syst Pharm. 2006;63:1888-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Fontelo P, Gavino A, Sarmiento RF. Comparing data accuracy between structured abstracts and full-text journal articles: implications in their use for informing clinical decisions. Evid Based Med. 2013;18:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hua F, Walsh T, Glenny AM, Worthington H. Structure formats of randomised controlled trial abstracts: a cross-sectional analysis of their current usage and association with methodology reporting. BMC Med Res Methodol. 2018;18:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Green BN, Johnson CD. How to write a case report for publication. J Chiropr Med. 2006;5:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Li L, Tian J, Tian H, Moher D, Liang F, Jiang T, Yao L, Yang K. Network meta-analyses could be improved by searching more sources and by involving a librarian. J Clin Epidemiol. 2014;67:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 20. | Mariano C. Holistic nursing as a specialty: holistic nursing - scope and standards of practice. Nurs Clin North Am. 2007;42:165-188, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Caldeira S, Timmins F, de Carvalho EC, Vieira M. Spiritual Well-Being and Spiritual Distress in Cancer Patients Undergoing Chemotherapy: Utilizing the SWBQ as Component of Holistic Nursing Diagnosis. J Relig Health. 2017;56:1489-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 1022] [Article Influence: 127.8] [Reference Citation Analysis (0)] |
| 23. | Nanda International. Nursing Diagnoses: Definitions and Classification 2015-2017. New Jersey: Wiley-Blackwell Ltd 2014; . |
| 24. | Watson J. Caring theory as an ethical guide to administrative and clinical practices. JONAS Healthc Law Ethics Regul. 2006;8:87-93. [PubMed] |
| 25. | Ge L, Tian JH, Li YN, Pan JX, Li G, Wei D, Xing X, Pan B, Chen YL, Song FJ, Yang KH. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol. 2018;93:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 26. | Knottnerus JA, Tugwell P. How to write a research paper. J Clin Epidemiol. 2013;66:353-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Olson DM, Bettger JP, Alexander KP, Kendrick AS, Irvine JR, Wing L, Coeytaux RR, Dolor RJ, Duncan PW, Graffagnino C. Transition of care for acute stroke and myocardial infarction patients: from hospitalization to rehabilitation, recovery, and secondary prevention. Evid Rep Technol Assess (Full Rep). 2011;1-197. [PubMed] |
| 28. | Kaszkin-Bettag M, Hildebrandt W. Case reports on cancer therapies: the urgent need to improve the reporting quality. Glob Adv Health Med. 2012;1:8-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Cooper ID. How to write an original research paper (and get it published). J Med Libr Assoc. 2015;103:67-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Cohen RS. Fentanyl transdermal analgesia during pregnancy and lactation. J Hum Lact. 2009;25:359-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Davanzo R, De Cunto A, Travan L, Bacolla G, Creti R, Demarini S. To feed or not to feed? Case presentation and best practice guidance for human milk feeding and group B streptococcus in developed countries. J Hum Lact. 2013;29:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Cornish L. The use of prophylactic dressings in the prevention of pressure ulcers: a literature review. Br J Community Nurs. 2017;22:S26-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |