Published online Oct 26, 2019. doi: 10.12998/wjcc.v7.i20.3364
Peer-review started: June 4, 2019
First decision: August 1, 2019
Revised: September 5, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 26, 2019
Processing time: 147 Days and 20.7 Hours
The diagnosis of multiple primary malignancies (MPMs) has increased due to the improvements and development of diagnostic techniques, in conjunction with extended life span. Notably however, reports of synchronous quadruple primary malignancies remain extremely rare.
Herein we describe the case of a 56-year-old woman who was diagnosed with synchronous quadruple multiple primary cancers, namely an endocervical adenocarcinoma admixed with neuroendocrine features, localized endometrial endometrioid adenocarcinoma, unilateral endometrioid ovarian carcinoma, and gastric adenocarcinoma. All four of these tumors were removed in one combined surgical procedure.
To our knowledge the above-described combination of multiple synchronous primary malignancies has not been previously reported. The nature of the association between them is unknown. Further research should focus on the etiology and mechanisms involved in MPMs.
Core tip: Multiple primary malignancies (MPMs) are rare and most involve two sites. Herein we report an exceptional case of quadruple primary malignancies in a single patient, including endocervical adenocarcinoma, endometrial endometrioid adenocarcinoma, endometrioid ovarian carcinoma, and gastric adenocarcinoma. The nature of MPMs remains unknown, and further research into the etiology and mechanisms of MPMs is warranted.
- Citation: Wang DD, Yang Q. Synchronous quadruple primary malignancies of the cervix, endometrium, ovary, and stomach in a single patient: A case report and review of literature. World J Clin Cases 2019; 7(20): 3364-3371
- URL: https://www.wjgnet.com/2307-8960/full/v7/i20/3364.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i20.3364
Multiple primary malignancy (MPM) is defined as two or more malignant tumors with distinct histology occurring at different locations. Depending on the time of diagnosis at each primary site, MPMs can be classified as either synchronous or metachronous[1,2]. In the literature, the prevalence of MPM is estimated to be in the range of 2%-17%[2]. It is rare, and most cases involve two sites. The occurrence of three or more primary tumors in a single patient has rarely been described. Herein we report an exceptional case of a 56-year-old woman who was successfully treated for endocervical adenocarcinoma, endometrial endometrioid adenocarcinoma, endometrioid ovarian carcinoma, and gastric adenocarcinoma via surgery at the Shengjing Hospital of China Medical University, in conjunction with a brief review of related literature.
A 56-year-old postmenopausal woman who was 160 cm in height and weighed 67.1 kg (body mass index 26.2) came to our institute with a 1-mo history of vaginal bleeding with no associated abdominal pain.
The patient has been treated for diabetes mellitus for the past 8 years. She had no history of hypertension and reported did not use tobacco or alcohol. She had no history of exposure to oral estrogen, and her family history was unremarkable.
Gynecologic examination revealed an enlarged smooth-faced cervix and decreased mobility of the uterus, but no gross lesion.
Serum carbohydrate antigen (CA)-199 was 85 U/mL (normal range 0-35 U/mL), and carcinoembryonic antigen (CEA), CA-125, and CA-724 were normal. Human papillomavirus (HPV) DNA testing was negative.
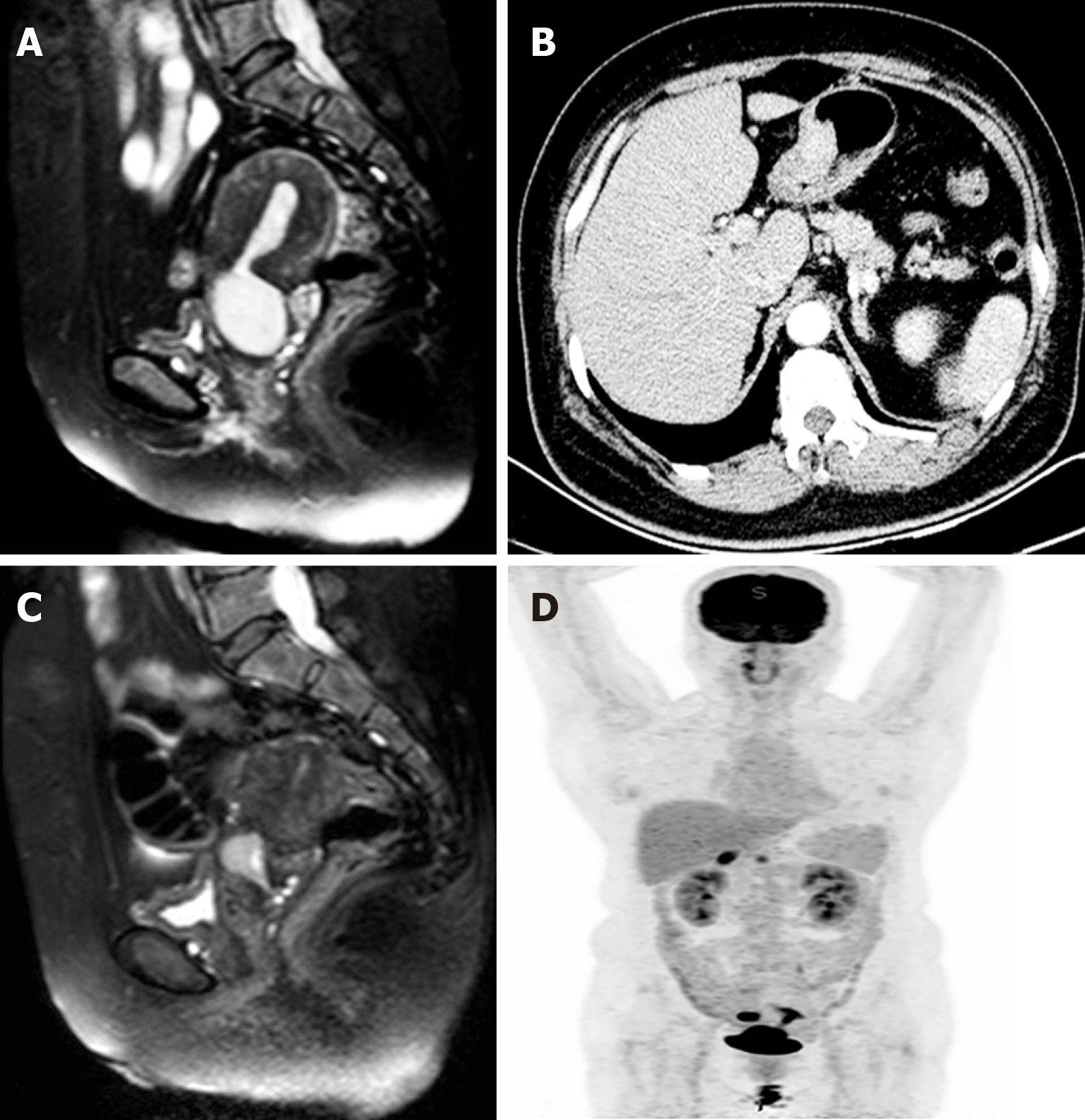
Biopsy of fractional curettage resulted in the diagnosis of endocervical poorly differentiated adenocarcinoma, and endometrial endometrioid adenocarcinoma, in conjunction with atypical hyperplasia. Pelvic magnetic resonance imaging (MRI) depicted a solid mass of 4.3 cm × 3.3 cm located in the cervical canal of the uterus that was indistinct from the anterior rectum wall, thickened and distorted endometrium and small cystic lesions of bilateral adnexa (left 1.6 cm × 0.8 cm, right 1.8 cm × 1.2 cm) (Figure 1A). Contrast computed tomography (CT) scanning depicted thickening of the wall of the greater curvature of the stomach with enlarged perigastric lymph nodes, and suspected malignancy (Figure 1B). Whole-body positron emission topography (PET)/CT with 18-fluorodeoxy-glucose (FDG) scanning revealed abnormal FDG-uptake in the cervix, uterine cavity, right adnexa, and stomach (Figure 1C). Further esophagogastroduodenoscopy examination revealed multiple ulcerative lesions in the gastric angle and antrum. Biopsy results revealed gastric intraepithelial neoplasia with focal intramucosal cancerization.
The patient was diagnosed with MPMs including endocervical adenocarcinoma, endometrial endometrioid adenocarcinoma, gastric carcinoma and suspected ovarian carcinoma.
Neoadjuvant chemotherapy was administered first, aimed at reducing the tumor load. After two courses of taxol (175 mg/m2)/oxaliplatin (130 mg/m2) chemotherapy, MRI was performed again and depicted a significantly decrescent cervical solid mass of approximately 1.7 cm × 2.2 cm (Figure 1D). After comprehensive multidisciplinary consultation and informing the patient of the challenges and uncertainties involved, a combined surgery was planned. For genital tract carcinoma transabdominal radical hysterectomy and bilateral oophorosalpingectomy were performed with pelvic and para-aortic lymph node dissection. For the gastric lesion radical distal gastrectomy, gastrojejunostomy and omentectomy were performed with perigastric lymph node dissection. During the exploratory laparotomy a solid mass was observed on the anterior wall of the rectus approximately 3 cm above the rectouterus reflexes peritoneum which was considered to be a metastasis of endocervical cancer. Partial rectectomy was synchronously performed. The entire operation lasted 8 h. There were no major complications during the operation.
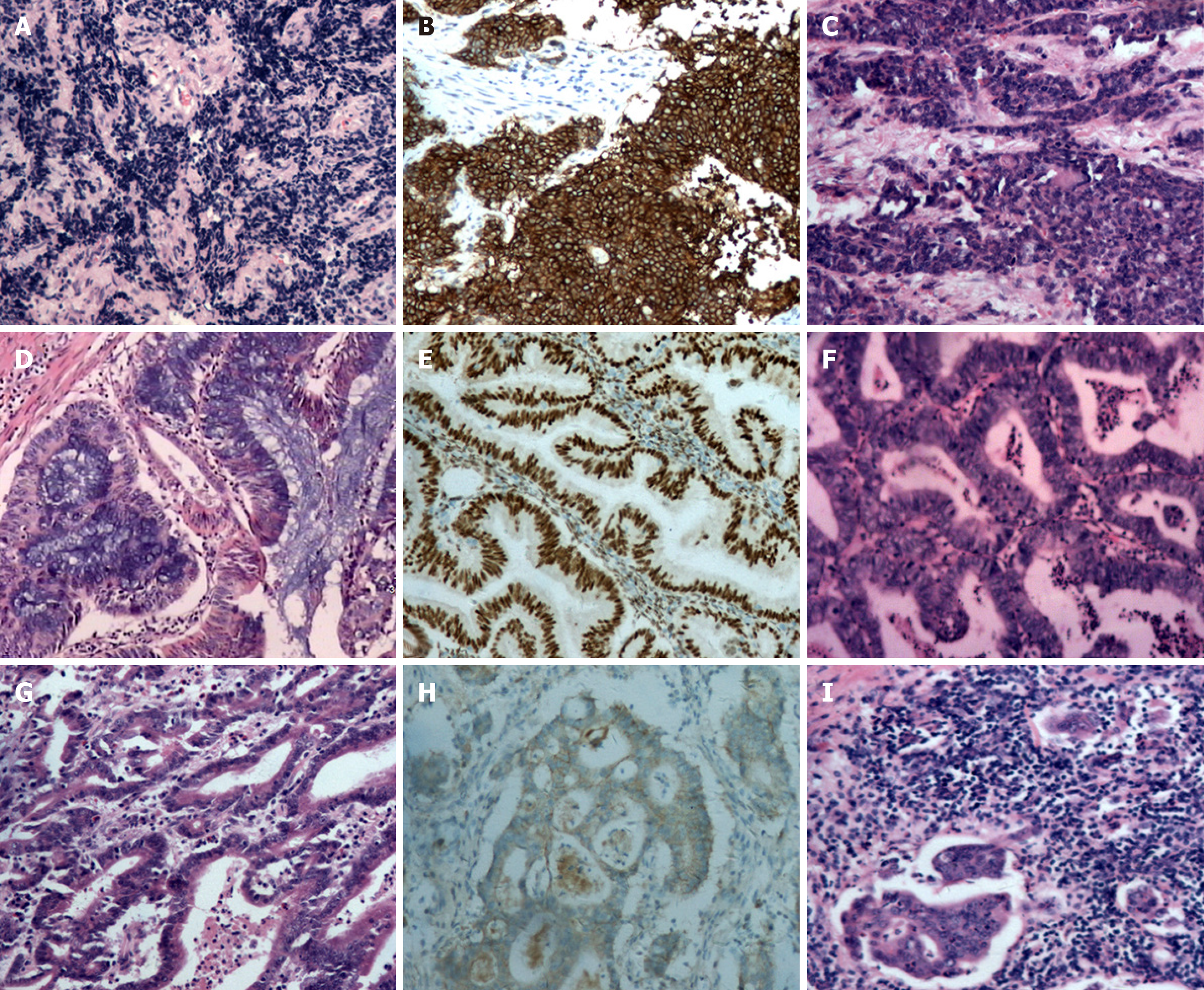
Histopathological examination of the surgical specimens with immunohistochemistry confirmed the diagnosis of MPMs, with observations including: (1) Poorly differentiated endocervical adenocarcinoma admixed with partial neuroendocrine changes, deep stromal invasion and rectal involvement, Ki67 and MOC-31 positively, partial positively for cytokeratin (CK), CK8/18, thyroid transcription factor-1and synaptophysin and negatively for vimentin, CEA, CD56, P63, P40, and chromogranin (Figure 2A, B and C); (2) Diffuse endometrial atypical hyperplasia combined with localized highly differentiated endometrioid adenocarcinoma without myometrial invasion, and tumor cells positive for estrogen receptor (ER) and progesterone receptor (PR) (Figure 2D and E); (3) Localized right ovarian endometrioid adenocarcinoma, and tumor cells positive for ER, PR, and CK7 but negative for CK20 (Figure 2F); and (4) Moderately to highly differentiated gastric adenocarcinoma with deep muscular infiltration and perigastric lymph node metastasis, tumor cells positive for human epidermal growth factor receptor 2 (Figure 2G, H and I).
The patient recovered smoothly but deep vein thrombosis (DVT) of the left lower leg was detected 15 d after surgery. For personal reason the patient declined thrombolytic therapy in our hospital and requested a referral to a local center. During the follow-up period she was cured of the DVT by approximately 2 mo after surgery at that local center. The patient declined subsequent adjuvant radio-chemotherapy and was lost to follow-up 1 year after surgery. Despite the potential informative value that it may have had, the expression of the genetic panel in this patient lacks of mean (data not displayed).
The most widely accepted criteria for the diagnosis of MPMs was proposed by Warren and Gates[1], and it requires that (1) each tumor is malignant; (2) each tumor has its own pathological features; (3) tumors occur in different parts of the organs, and are not continuous with each other; and (4) each tumor has its own metastatic pathway and the diagnosis of metastatic or recurrent tumors can be excluded. MPMs are known to be more commonly encountered in the gynecologic and gastrointestinal tracts most likely because they are derived from the same embryonic layer or tissue and in the case of gynecologic malignancies, responsive to the same hormones[3].
Notably there was some debate about the pathological diagnosis of primary ovarian cancer in the present case. Tumor cell morphology and immunohistochemistry markers suggested that the type of cancer in the right ovary was endometrioid adenocarcinoma which could easily have been mistaken for an endometrial cancer metastasis. Pathology results indicated that it was a focal highly differentiated endometrioid adenocarcinoma without myometrial or lympho-vascular space invasion, as well as a unilateral localized ovarian endometrioid cancer. Synchronous endometrial and ovarian cancer (SEOC) has been a matter of dispute in the past, because of the difficulties in differential diagnosis between two independent primary tumors and metastasis from one site to the other in this context, especially when the histologic types are concordant. Traditionally, the Ulbright and Roth criteria[4] followed by the Scully criteria[5] have been utilized to distinguish SEOC from metastatic endometrial or ovarian cancer. In endometrial tumors the criteria include the size of the tumor and depth of invasion, direct extension to the adnexa, lympho vascular space invasion, the presence of atypical hyperplasia in the surrounding endometrium, and grading. In ovarian tumors the criteria include the the presence of endometriosis, size and laterality of the tumor, surface implants, hilar location, lympho vascular space invasion, and multinodularity. SEOC is ordinarily more likely to be stage I disease with endometrioid histology[6,7]. In the present case we ultimately considered both to be primary carcinomas in the uterus corpus and ovary.
Factors contributing the increasing frequency of MPM diagnoses include improved living standards,advances in diagnostic testing modalities, the development of more sophisticated treatments, and improved cancer screening and surveillance procedures[2,8]. Metachronous MPMs are more common than synchronous malignancies with a ratio 2.7:1[3,9]. Most cases of MPMs involve two primary neoplasm, whereas triple, and quadruple primary neoplasms are exceedingly rare. The incidence of quadruple cancers has been reported to be less than 0.1%[10]. During the generation of this current report a PubMed-indexed English literature search yielded 9 reported cases of quadruple synchronous neoplasms[3,11-18] (Table 1). To our knowledge, to date the combination of triple synchronous neoplasms of the female genital system (cervix, endometrium, and ovary) in conjunction with one primary digestive tract cancer has never been reported.
| Reference | Year | Age (yr) | Presentation | Sites | Tumor histology | Treatment | Outcome | Follow-up (mo) |
| author | ||||||||
| Phupong et al[11] | 2007 | 50 | Menorrhagia | Ovary | Mucinous adenocarci-noma | RT | DOD | 3 |
| Ovary | Low malignant potential | |||||||
| Uterus corpus | Endometroid adenocarci-noma | |||||||
| Cervix | Endocervical adenosqua-mous carcinoma | |||||||
| Saglam et al[12] | 2008 | 63 | Postmenopau-sal bleeding Abdominal distention | Ovary | Mucinous adenocarcinoma | CT | NED | 12 |
| Fallopian tube | Early papillary adenocarcinoma | |||||||
| Uterus corpus | Endometroid adenocarcinoma | |||||||
| Cervix | Endocervical adenosquamous carcinoma | |||||||
| Kim et al[13] | 2013 | 73 | Dyspepsia | Thyroid | Papillary carcinoma | ET; CT | DOD | 8 |
| Breast | Invasive ductal adenocarcinoma | |||||||
| Pancreas | Adenocarcinoma | |||||||
| Stomach | gastrointestinal stromal tumor (GIST) | |||||||
| Grace et al[14] | 2015 | 70 | Aphasia Confusion | Brain | Glioblastoma | Surgery | NM | NM |
| Ileum | Neuroendocrine tumor | |||||||
| Inguinal region | Schwannoma | |||||||
| Appendix | Sessile serrated adenoma/ polyps | |||||||
| Klairmont et al[15] | 2015 | 74 | Right breast lesion | Breast | Invasive ductal carcinoma | Surgery; CT | NM | 18 |
| Esophagus | Adenocarcinoma | |||||||
| Colon | Adenocarcinoma | |||||||
| Lung | Squamous cell carcinoma | |||||||
| Maruyama et al[16] | 2015 | 69 | Tongue pain | Tongue | Squamous cell carcinoma | Surgery; RT; ET | DFS | 60 |
| Right Breast | Invasive ductal carcinoma | |||||||
| Left Breast | Intraductal carcinoma | |||||||
| Kidney | Chromophobe renal cell carcinoma | |||||||
| Meek et al[3] | 2016 | 95 | Nausea Vomiting Abdominal distention | Cecum | Adenocarci-noma | Surgery | NM | NM |
| Appendix | Sessile serrated adenoma | |||||||
| Appendix | Neuroendocrine tumor | |||||||
| Appendix | Schwann cell hamartoma | |||||||
| Nanashima et al[17] | 2017 | 67 | Epigastric pain | Stomach | Adenocarci-noma | Surgery; CT | DFS | 51 |
| Colon | Adenocarcinoma | |||||||
| Rectum | Neuroendocrine tumor | |||||||
| Pancreas | Papillary ductal adenocarci-noma | |||||||
| Fan et al[18] | 2017 | 53 | No discomfort | Stomach | Adenocarci-noma | Surgery; CT | DFS | 12 |
| Stomach | GIST | |||||||
| Esophagus | Squamous cell carcinoma in situ | |||||||
| Esophagus | Small cell carcinoma |
Although the underlying mechanisms responsible for the development of MPM are yet to be fully elucidated, frequently implicated factors can been collated into three broadly defined categories[2]. First, host factors include genetic susceptibility, immune status, hormonal usage and a history of chemo -and/or radiotherapy for the treatment of cancer. For example, Lynch syndrome patients are susceptible to colorectal cancers, endometrial cancers, and other malignancies[19]. Hereditary breast and ovarian cancer syndrome is a highly-penetrant, autosomal-dominant breast and ovarian cancer predisposition caused by germline mutations in the BRCA1 and BRCA2 genes[20]. Long-term non-resistant estrogen exposure is a major risk factor for endometrial cancer[21]. As well as congenital genetic mutations, somatically acquired genetic abnormalities such as punctiform mutations, loss of heterozygosity and microsatellite instability can also contribute to carcinogenesis[2]. Hájková et al[7] conducted comprehensive molecular analysis in 22 SEOC patients and reported that clonal origin was confirmed in all of them by way of at least one shared mutation in PTEN, AKT1, PIK3CA, KRAS, TP53, or ARID1A. Microsatellite instability phenotypes were detected in 5/22 (22.7%) SEOC of the patients. Secondly, lifestyle factors include such things as alcohol, and tobacco usage. A third is exposure to infectious environmental influences and occupational hazards. Helicobacter pylori and Epstein-Barr virus infection as well as behavioral factors such as alcohol consumption, and cigarette smoking are reportedly associated with a higher risk of developing gastric cancer[18]. HPV is an obligate component of most cervical cancers. In a multicenter epidemiological study, high-risk HPV DNA was detected in 94% of adenocarcinomas in situ, 85% of adenosquamous carcinomas, and 76% of adenocarcinomas[22]. The present patient had no family history of colon, gastric, breast or gynecological cancer, and no history of non-resistant estrogen usage, no alcohol consumption, or cigarette smoking. Genetic sequencing was performed but results lack of mean. It is unlikely that patients with synchronous primary cancers have hereditary cancer syndromes. Though a history of diabetes mellitus and being overweight may be relevant in the development of MPMs in the present patient, an unidentified mutation or other factors may exist.
Currently, several types of examinations can help to prevent overlooking synchronous MPMs, including contrast CT, MRI, and PET/CT, as well as various endoscopic examinations. In one retrospective study it was reported that PET/CT had higher sensitivity with regard to the detection of synchronous cancers in patients with head and neck squamous cell carcinoma than conventional work-up with CT, barium swallow esophagram and panendoscopy (88.2% vs 52.9%)[23]; however, PET/CT is an expensive examination and sometimes identifies false-positive lesions. Rapid development of endoscopic techniques is facilitating enhanced-visualization of lesion morphology and more accurate localization, particularly in the context of the diagnosis of cavitary organ lesions[24,25].
Currently there are no definitive guidelines for the management of MPMs involving separate organ. Synchronous MPMs should be discussed by a multidisciplinary team, and a treatment consensus is best devised via input from surgeons, oncologists, radiation oncologists, radiologists, pathologists, and the patient. In general, surgical interventions should initially aim to exclude the presence of metastatic disease. The present patient underwent combined radical resection of all tumors, which entailed a long operation under general anesthesia. Unfortunately she also suffered from postoperative DVT, which might could have been fatal[26]. In such cases, a balance must be met between providing effective treatment while preserving quality of life, and minimizing the morbidity of what is often a highly complex, protracted, and potentially toxic treatment course.
Synchronous primary quadruple malignancy is an extremely rare event. In this report, the clinical and pathologic details of the case of a 56-year-old female patient with synchronous with four synchronous primary tumors including poorly-differentiated endocervical adenocarcinoma, highly-differentiated endometrial endometrioid adenocarcinoma, endometrioid ovarian carcinoma, and moderately to highly differentiated gastric adenocarcinoma are presented for the first time. The etiology and mechanisms of MPM remain controversial, and further research is needed to explain these simultaneous cancers.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tan BK S-Editor: Dou Y L-Editor: Ma JY E-Editor: Qi LL
| 1. | Warren S, Gates O. Multiple primary malignant tumors: survey of the literature and a statistical study. Am J Cancer. 1932;16:1358-1414. |
| 2. | Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2:e000172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 369] [Article Influence: 46.1] [Reference Citation Analysis (1)] |
| 3. | Meeks MW, Grace S, Chen Y, Petterchak J, Bolesta E, Zhou Y, Lai JP. Synchronous Quadruple Primary Neoplasms: Colon Adenocarcinoma, Collision Tumor of Neuroendocrine Tumor and Schwann Cell Hamartoma and Sessile Serrated Adenoma of the Appendix. Anticancer Res. 2016;36:4307-4311. [PubMed] |
| 4. | Ulbright TM, Roth LM. Metastatic and independent cancers of the endometrium and ovary: a clinicopathologic study of 34 cases. Hum Pathol. 1985;16:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 170] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 5. | Scully RE, Young RH, Philip B. Tumors of the ovary, maldeveloped gonads, Fallopian tube and broad ligament: Atlas of tumor pahology (AFIP, Atlas of tumor pathology, No. 23). American registry of pathology, Washington, DC, 1999. . |
| 6. | Matsuo K, Machida H, Frimer M, Marcus JZ, Pejovic T, Roman LD, Wright JD. Prognosis of women with stage I endometrioid endometrial cancer and synchronous stage I endometrioid ovarian cancer. Gynecol Oncol. 2017;147:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Hájková N, Tichá I, Hojný J, Němejcová K, Bártů M, Michálková R, Zikán M, Cibula D, Laco J, Geryk T, Méhes G, Dundr P. Synchronous endometrioid endometrial and ovarian carcinomas are biologically related: A clinico-pathological and molecular (next generation sequencing) study of 22 cases. Oncol Lett. 2019;17:2207-2214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Wentzensen N, Arbyn M. HPV-based cervical cancer screening- facts, fiction, and misperceptions. Prev Med. 2017;98:33-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Kim SH, Park BS, Kim HS, Kim JH. Synchronous quintuple primary gastrointestinal tract malignancies: Case report. World J Gastroenterol. 2017;23:173-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Dehghani M, Jangjoo S, Monabati A, Masoomi Bandari D, Namdari N. An Unusual Case Report: Occurrence of Renal Cell Carcinoma, Basal Cell Carcinoma and Chronic Lymphocytic Leukemia in a Case of Papillary Thyroid Carcinoma Treated with Radioactive Iodine. Iran J Med Sci. 2018;43:659-663. [PubMed] |
| 11. | Phupong V, Khemapech N, Triratanachat S. Triple synchronous primary cervical, endometrial and ovarian cancer with four different histologic patterns. Arch Gynecol Obstet. 2007;276:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Saglam A, Bozdag G, Kuzey GM, Kuçukali T, Ayhan A. Four synchronous female genital malignancies: the ovary, cervix, endometrium and fallopian tube. Arch Gynecol Obstet. 2008;277:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Kim JS, Chung CY, Park HC, Myung DS, Cho SB, Lee WS, Min JJ, Joo YE. Synchronous quadruple primary tumors of thyroid, breast, pancreas, and stomach: a case report. Anticancer Res. 2013;33:2135-2138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Grace S, Muzaffar R, Veerapong J, Alkaade S, Poddar N, Phillips N, Guzman M, Batanian J, Vogler C, Lai JP. Synchronous quadruple primary neoplasms: glioblastoma, neuroendocrine tumor, schwannoma and sessile serrated adenoma in a patient with history of prostate cancer. Anticancer Res. 2015;35:2121-2127. [PubMed] |
| 15. | Klairmont M, Kopkash K, Favuzza J, Hill M, Rao R, Mahon B, Seder CW. Four Synchronous Primary Malignancies of the Breast, Lung, Colon and Esophagus. Anticancer Res. 2015;35:6159-6162. [PubMed] |
| 16. | Maruyama T, Nakasone T, Maruyama N, Matayoshi A, Arasaki A. Synchronous quadruple multiple primary cancers of the tongue, bilateral breasts, and kidney in a female patient with a disease-free survival time of more than 5 years: a case report. World J Surg Oncol. 2015;13:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Nanashima A, Tominaga T, Nonaka T, Wakata K, Kunizaki M, Tobinaga S, Sumida Y, Hidaka S, Kinoshita N, Sawai T, Nagayasu T. A case of multiple synchronous quadruple cancers of the stomach, sigmoid colon, rectum, and pancreas. Int J Surg Case Rep. 2017;35:4-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Fan H, Lu P, Xu L, Qin Y, Li J. Synchronous occurrence of hereditary gastric adenocarcinoma, gastrointestinal stromal tumor, and esophageal small cell and squamous carcinoma in situ: an extremely rare case report. BMC Cancer. 2017;17:720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Watson P, Riley B. The tumor spectrum in the Lynch syndrome. Fam Cancer. 2005;4:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Hoang LN, Gilks BC. Hereditary Breast and Ovarian Cancer Syndrome: Moving Beyond BRCA1 and BRCA2. Adv Anat Pathol. 2018;25:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | McDonald ME, Bender DP. Endometrial Cancer: Obesity, Genetics, and Targeted Agents. Obstet Gynecol Clin North Am. 2019;46:89-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Tjalma WA, Trinh XB, Rosenlund M, Makar AP, Kridelka F, Rosillon D, Van Dam PA, Collas De Souza S, Holl K, Simon P, Jenkins D. A cross-sectional, multicentre, epidemiological study on human papillomavirus (HPV) type distribution in adult women diagnosed with invasive cervical cancer in Belgium. Facts Views Vis Obgyn. 2015;7:101-108. [PubMed] |
| 23. | Chen SH, Chan SC, Chao YK, Yen TC. Detection of synchronous cancers by fluorodeoxyglucose positron emission tomography/computed tomography during primary staging workup for esophageal squamous cell carcinoma in Taiwan. PLoS One. 2013;8:e82812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Sumiyama K. Past and current trends in endoscopic diagnosis for early stage gastric cancer in Japan. Gastric Cancer. 2017;20:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Johary J, Xue M, Xu B, Xu D, Aili A. Use of hysteroscope for vaginoscopy or hysteroscopy in adolescents for the diagnosis and therapeutic management of gynecologic disorders: a systematic review. J Pediatr Adolesc Gynecol. 2015;28:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 580] [Article Influence: 64.4] [Reference Citation Analysis (0)] |