Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3098
Peer-review started: May 7, 2019
First decision: May 31, 2019
Revised: June 20, 2019
Accepted: July 3, 2019
Article in press: July 3, 2019
Published online: October 6, 2019
Processing time: 157 Days and 20.6 Hours
Synovial sarcoma, a rare mesenchymal tumor type with unclear histological origin and direction of differentiation, accounts for 6%–10% of soft tissue tumors. It is mainly located near the joints and tendons of the limbs, and occurs primarily in children or young adults. Primary renal synovial sarcoma (PRSS) is very rare, accounting for approximately 1% of synovial sarcomas. It is a spindle cell tumor type affecting mesenchymal tissue, and has morphological, genetic, and clinical characteristics, and a certain degree of epithelial differentiation. It is highly malignant and has the fourth highest incidence among soft tissue sarcomas. Here, we report a case of PRSS and share some valuable information about the disease.
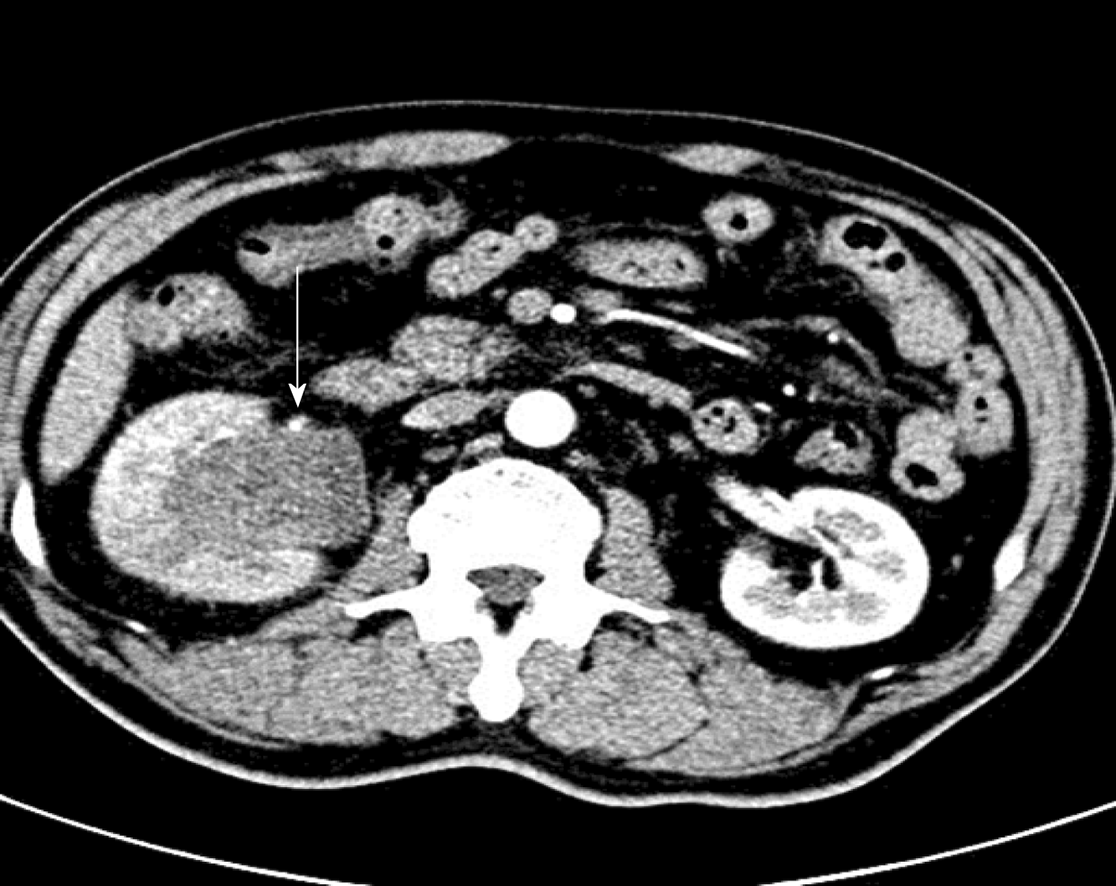
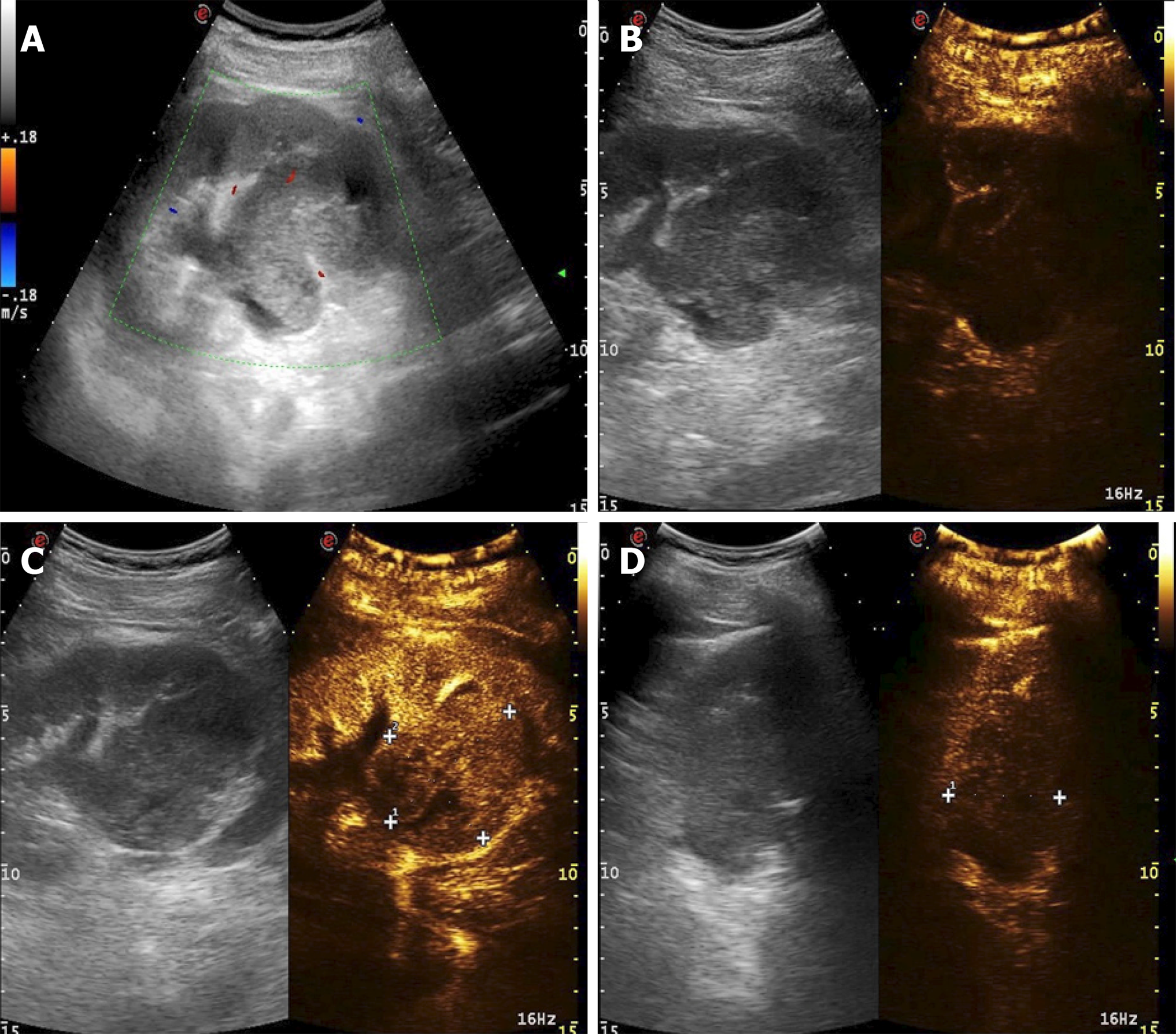
A 54-year-old male patient was admitted to the hospital for a space-occupying lesion in the right kidney for 2 d upon ultrasound examination. The patient had no cold or fever; no frequency, urgency or pain of urination; and no other discomfort. The results of a hemogram, blood biochemistry, and tumor markers were in the normal range. The patient was examined by computed tomography (CT), which indicated the presence of a soft tissue density shadow with a diameter of approximately 6.8 cm in the right renal pelvis area, showing uneven enhancement. Ultrasound indicated a cystic solid mass of approximately 6.8 cm × 6.5 cm in the right kidney, with an unclear boundary and irregular shape. Meanwhile, color Doppler flow imaging showed dotted blood flow signals in the periphery and interior. Contrast-enhanced ultrasound (CEUS) showed "slow in and fast out" hyperenhancement of the right renal mass after contrast agent injection. The postoperative pathological diagnosis was (right kidney) synovial sarcoma. Despite postoperative adjuvant chemotherapy, tumor recurrence was detected two years later.
PRSS is a rare malignant tumor. To date, no characteristic imaging findings have been observed. The diagnosis is confirmed primarily through postoperative pathological immunohistochemistry and SS18 (SYT) gene detection. In this case, CEUS was used preoperatively. We found that PRSS has the characteristic of "slow in and fast out" hyperenhancement, and its particular characteristics have diagnostic value. Postoperative adjuvant chemotherapy is not very effective.
Core tip: Synovial sarcomas are found mainly in the joints and tendons of the extremities, but rarely in the kidneys, and they are most common in children and young adults. Here, we report the imaging findings (notably contrast-enhanced ultrasound), histopathologic immunohistochemistry, and treatment of a case of primary renal synovial sarcoma. The purpose of this report is to help readers further understand this disease.
- Citation: Cai HJ, Cao N, Wang W, Kong FL, Sun XX, Huang B. Primary renal synovial sarcoma: A case report. World J Clin Cases 2019; 7(19): 3098-3103
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3098.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3098
Synovial sarcoma, a rare mesenchymal tumor type with unclear histological origin and direction of differentiation, accounts for 6%–10% of soft tissue tumors. It is mainly located near the joints and tendons of the limbs, and occurs primarily in children or young adults[1]. Primary renal synovial sarcoma (PRSS) is very rare, accounting for approximately 1% of synovial sarcomas. It is a spindle cell tumor type affecting mesenchymal tissue, and has morphological, genetic, and clinical characteristics, and a certain degree of epithelial differentiation. It is highly malignant and has the fourth highest incidence among soft tissue sarcomas[2]. PRSS is a rare malignant tumor with no specific clinical manifestations; it is mainly manifested as abdominal mass, abdominal pain, and hematuria, often with local invasion, as well as distant liver and lung metastases. The course of the disease develops rapidly[3]. The diagnosis is confirmed primarily through postoperative pathological immunohistochemistry and genetic SYT gene detection[4]. The prognosis of metastatic renal synovial sarcoma is poor, and the recurrence of non-metastatic renal synovial sarcoma is common. Therefore, multiple medical institutions are expected to cooperatively develop the best treatment and improve patient prognosis. Meanwhile, clinicians should consider the possibility of synovial sarcoma in patients with cystic solid renal lesions, as indicated through ultrasonography as multilocular cystic nephroma, to conduct early intervention, especially in young patients. Currently, approximately 70 cases of PRSS have been reported. Owing to the small number of cases, there is no unified standard for the imaging diagnosis and treatment of PRSS. Therefore, we report a case diagnosed by special contrast-enhanced ultrasound (CEUS) and describe the treatment course, which should provide a reference for future studies.
The patient, a 54-year-old man, was admitted to the hospital because of "a space-occupying lesion in the right kidney for 2 d upon ultrasound examination".
His past history was unremarkable.
His family history was unremarkable.
His physical examination on admission was unremarkable.
The results of a hemogram, blood biochemistry, and tumor markers were in the normal range.
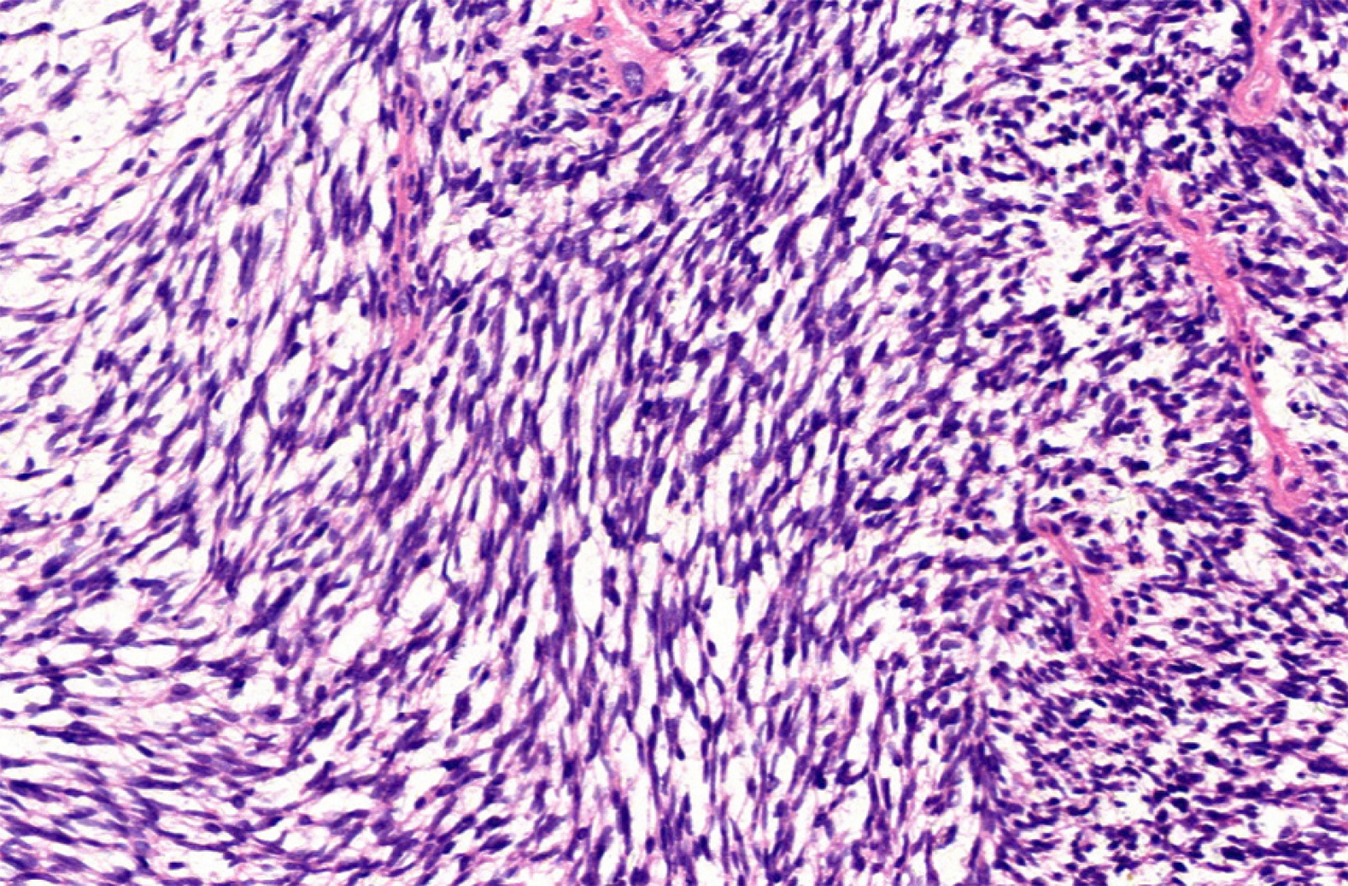
Computed tomography (CT) showed the mass as a soft tissue density shadow with a diameter of approximately 6.8 cm in the right renal pelvic area. The density was not uniform, and the boundary was not clear. It extended into the renal sinus and showed uneven enhancement (Figure 1), which manifested as partial deformation, a disappearance of the pelvis and calyces of the lower pole of the right kidney, and delayed enhancement of the left right renal parenchyma. No enlarged lymph nodes were observed behind the peritoneum. Ultrasound images showed a cystic solid mass of approximately 6.8 cm × 6.5 cm that was visible in the right kidney, which had an unclear boundary and irregular shape. Color Doppler flow imaging (CDFI) revealed dotted blood flow signals in the periphery and interior. CEUS revealed that, after the mass injection of the contrast agent, the right renal cortex began to enhance at 9 s, the renal mass began to enhance at 11 s, and the mass began to peak at 28 s. The mass subsided more rapidly than the renal cortex, and the right renal mass showed "slow in and fast out" hyperenhancement (Figure 2). The cystic solid masses were in the renal medulla, pelvis, and calyces, and they had a size of 6.8 cm × 5.5 cm × 5.5 cm. The abundant tumor cells were of spindle cell type, and a small number of tubules were observed among spindle cells through pathological examination. No tumor cells were found at the ureteral resection margin. Immunohistochemistry showed that the lesion was positive for SYT genome rearrangement, B-cell lymphoma-2 (Bcl-2), Vimentin, Pax-8, and CK7, and negative for smooth muscle actin (SMA), soluble protein-100, epithelial membrane antigen, CD99, and Pax-2. PRSS was diagnosed according to its imaging manifestations and pathological results (Figure 3).
Right renal synovial sarcoma.
Laparoscopic right radical nephrectomy was performed under general anesthesia. During the operation, the renal artery and renal vein were separated from the retroperitoneal peritoneal fascia, and cut off and ligated with forceps. The right adrenal gland was reserved. When the kidney was cut open, the mass was observed at the lower and middle pole protruding into the renal pelvis. There was no obvious invasion of the renal pelvis. The tumor had a yellowish jelly-like appearance. Chemotherapy with cyclophosphamide, adriamycin, and vincristine was administered for 4 wk postoperatively.
The right radical nephrectomy was followed by chemotherapy. CT indicated a recurrence of the original right renal region 2 years later.
Synovial sarcoma is a rare mesenchymal tumor with unclear histologic origin and direction of differentiation. It occurs mainly in the joints of the extremities and occasionally in the head and neck, heart, lungs, and prostate[5]. PRSS is very rare, accounting for less than 2% of renal neoplasms. It is a spindle cell tumor of mesenchymal tissue with a degree of epithelial differentiation and a morphologically and genetically unique clinical lesion. PRSS is highly malignant and was first reported by Faria et al[6] in 2000. Without specific clinical manifestations, PRSS is mainly observed as an abdominal mass with abdominal pain and hematuria, often with local invasion as well as distant liver and lung metastases, with a rapid disease course[7]. There were no obvious clinical symptoms in the patient, possibly because of the location and size of the mass and the effect of space occupation.
The literature on PRSS comprises mostly case reports. However, the performance of CEUS in PRSS diagnosis has not been described in any previous case reports. The ultrasound manifestation of PRSS in this case was a mixed solid and cystic mass, with lobulated edges, in the kidney. A multilocular cystic structure was observed in the mass, with a smooth and separated cyst wall, and some of the mass was accompanied by calcification. CDFI showed a dotted and linear blood flow signal in the parenchyma, in agreement with a report by Murphey et al[8]. Although magnetic resonance imaging (MRI) has a higher resolution for soft tissue, CEUS can display the blood flow distribution of microvessels in tumor tissue, and it allows for real-time comparison of the perfusion of normal tissue and lesion blood flow.
In this case, in the solid part of the mass enhanced by CEUS, the perfusion time of the contrast agent to the mass was slower than that for the normal parenchyma. After the mass was enhanced and reached the peak 28 s later, the time of regression was faster than that of the normal kidney tissue. The entire mass presented "slow in and fast out" hyperenhancement, and the enhancement pattern was extremely rare.
This disease should be distinguished from multilocular cystic renal cell carcinoma (MCRCC) and mixed renal carcinoma. MCRCC has a honeycomb pattern of enhanced signal in CEUS. The enhancement pattern is mainly synchronous with that of the surrounding renal parenchyma and decreases rapidly, and the peak intensity is higher than that of the renal cortex. As for mixed renal carcinoma, CEUS shows synchronous enhancement of the tumor and renal cortex, with a high peak value, uneven contrast agent perfusion, rapid regression, and annular high enhancement at the edge at the later stage of regression. Studies have reported that fine-needle biopsy cytology can be used for preoperative diagnosis, but may cause tumor cell metastasis through the blood[9]. In this case, both CT and CEUS were used to indicate malignancy, and right kidney surgery was used for eradication to obtain pathological tissue and avoid tumor metastasis.
At present, it is difficult to differentiate PRSS from other sarcomas in terms of morphology, which can be assessed by examining the expression of combinations of Vimentin, Bcl-2, CD56, CK, SMA, DES, Pax-8, and other markers[10]. In addition, TLE1 antibody can also be used as an important tissue factor to distinguish PRSS from other sarcomas. Studies by Mishra et al have shown that TLE1 is more specific and sensitive to synovial sarcomas than any immunohistochemical factor[11,12]. Molecular biology is also providing further confirmation that t(X;18) translocation can lead to syt-ssx fusion, and SYT rearrangement has become the gold standard for PRSS diagnosis[4]. A recent study has revealed a significant correlation between Pax-8 and t(X;18) translocations[13]. This case is consistent with the previously reported findings. The prognostic significance of syt-ssx fusion in synovial sarcoma has not been unified, but it has been shown to be an important prognostic indicator[4]. Because of the limited number of case reports, no guidelines for PRSS treatment have been established, but radical surgical resection is the preferred treatment. It has been reported that adjuvant chemotherapy is effective for primary tumors with a diameter of less than 5 cm[14]. Schaal et al[15] has reported a case of PRSS. After 4 wk of chemotherapy with isocyclofosfamide combined with doxorubicin, the tumor volume was reduced to 50% of its original size, and no recurrence was found during 1 year of follow-up. However, in this case, although chemotherapy with cyclophosphamide, adriamycin, and vincristine was administered for 4 wk after right kidney eradication, recurrence in the original right kidney area was observed 2 years later. The differences between these findings may be related to different tissue subtypes and individual differences in PRSS. Therefore, the clinical significance of postoperative adjuvant chemotherapy must be confirmed in a large number of cases.
In recent years, CEUS technology has developed rapidly, and it has the advantage of clearly showing the blood supply and perfusion within the tissue, thus revealing the internal structure of the lesion and allowing for comparison of the perfusion situation of the normal tissue and the blood flow of the lesion in real time. The CEUS of most renal malignancies presents as "fast in and fast out". But in this case, the "slow in and fast out" hyperenhancement was notable, which may be related to the type of PRSS. This feature may have value as a reference for the early diagnosis of PRSS in the future. Because PRSS is extremely rare, the number of reported cases is limited, and more cases must be confirmed in the future.
PRSS is extremely rare, and diagnosis relies on pathological immunohistochemistry and genetic testing. When cystic solid space in the kidney is found through ultrasound examination and CEUS shows a "slow in and fast out" pattern, the possibility of PRSS should be considered. There is no unified standard for postoperative adjuvant chemotherapy, and more cases must be confirmed.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Amin S, Ward J S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Ozkanli SS, Yildirim A, Zemheri E, Gucer Fİ, Aydin A, Caskurlu T. Primary synovial sarcoma of the kidney. Urol Int. 2014;92:369-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Grampurohit VU, Myageri A, Rao RV. Primary renal synovial sarcoma. Urol Ann. 2011;3:110-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Schoolmeester JK, Cheville JC, Folpe AL. Synovial sarcoma of the kidney: a clinicopathologic, immunohistochemical, and molecular genetic study of 16 cases. Am J Surg Pathol. 2014;38:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Pathrose G, John NT, Hariharan P. Renal Synovial Sarcoma in a Young Pregnant Lady: A Case Report and Clinico-Pathological Profile. J Clin Diagn Res. 2017;11:PD13-PD14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Huang Y, Liu D, Luo J, Chen W. Primary renal synovial sarcoma: A case report and literature review. J Cancer Res Ther. 2018;14:S267-S269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Argani P, Faria PA, Epstein JI, Reuter VE, Perlman EJ, Beckwith JB, Ladanyi M. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Iacovelli R, Altavilla A, Ciardi A, Urbano F, Manai C, Gentile V, Cortesi E. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. From the archives of the AFIP: Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26:1543-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | Vesoulis Z, Rahmeh T, Nelson R, Clarke R, Lu Y, Dankoff J. Fine needle aspiration biopsy of primary renal synovial sarcoma. A case report. Acta Cytol. 2003;47:668-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Chandrasekaran D, Narayanaswamy K, Sundersingh S, Senniappan K, Raja A. Primary Synovial Sarcoma of the Kidney with Inferior Vena Caval Thrombus. Indian J Surg Oncol. 2016;7:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | El Chediak A, Mukherji D, Temraz S, Nassif S, Sinno S, Mahfouz R, Shamseddine A. Primary synovial sarcoma of the kidney: a case report of complete pathological response at a Lebanese tertiary care center. BMC Urol. 2018;18:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Mishra S, Awasthi N, Hazra SP, Bera MK. Primary synovial sarcoma of the kidney. Saudi J Kidney Dis Transpl. 2015;26:996-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Rose L, Grignon D, Cheng L, Fan R, Zhang S, Alruwaii F, Chen S. Primary Renal Synovial Sarcomas: PAX 8 Immunostaining and Unusual Molecular Findings. Appl Immunohistochem Mol Morphol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | El Beaino M, Araujo DM, Lazar AJ, Lin PP. Synovial Sarcoma: Advances in Diagnosis and Treatment Identification of New Biologic Targets to Improve Multimodal Therapy. Ann Surg Oncol. 2017;24:2145-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Schaal CH, Navarro FC, Moraes Neto FA. Primary renal sarcoma with morphologic and immunohistochemical aspects compatible with synovial sarcoma. Int Braz J Urol. 2004;30:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |