Published online Aug 6, 2019. doi: 10.12998/wjcc.v7.i15.2022
Peer-review started: March 4, 2019
First decision: May 31, 2019
Revised: June 25, 2019
Accepted: July 3, 2019
Article in press: July 3, 2019
Published online: August 6, 2019
Processing time: 156 Days and 23.5 Hours
Noninvasive biomarkers have been developed to predict hepatitis B virus (HBV) related fibrosis owing to the significant limitations of liver biopsy. Both serum biomarkers and imaging techniques have shown promising results and may improve the evaluation of liver fibrosis. However, most of the previous studies focused on the diagnostic effects of various imaging techniques on fibrosis in all chronic liver diseases.
To compare the performance of common imaging methods and serum biomarkers for prediction of significant fibrosis caused only by HBV infection.
A systematic review was conducted on the records available in PubMed, EMBASE, and the Cochrane Library electronic databases until December 2018. We systematically assessed the effectiveness of two serum biomarkers and three imagine techniques in predicting significant fibrosis solely caused by HBV infection. The serum biomarkers included aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis index based on the 4 factors (FIB-4). The three imaging techniques included acoustic radiation force impulse (ARFI), FibroScan, and magnetic resonance elastography (MRE). Three parameters, the area under the summary receiver operating characteristic curve (AUSROC), the summary diagnostic odds ratio, and the summary sensitivity and specificity, were used to examine the accuracy of all tests for liver fibrosis.
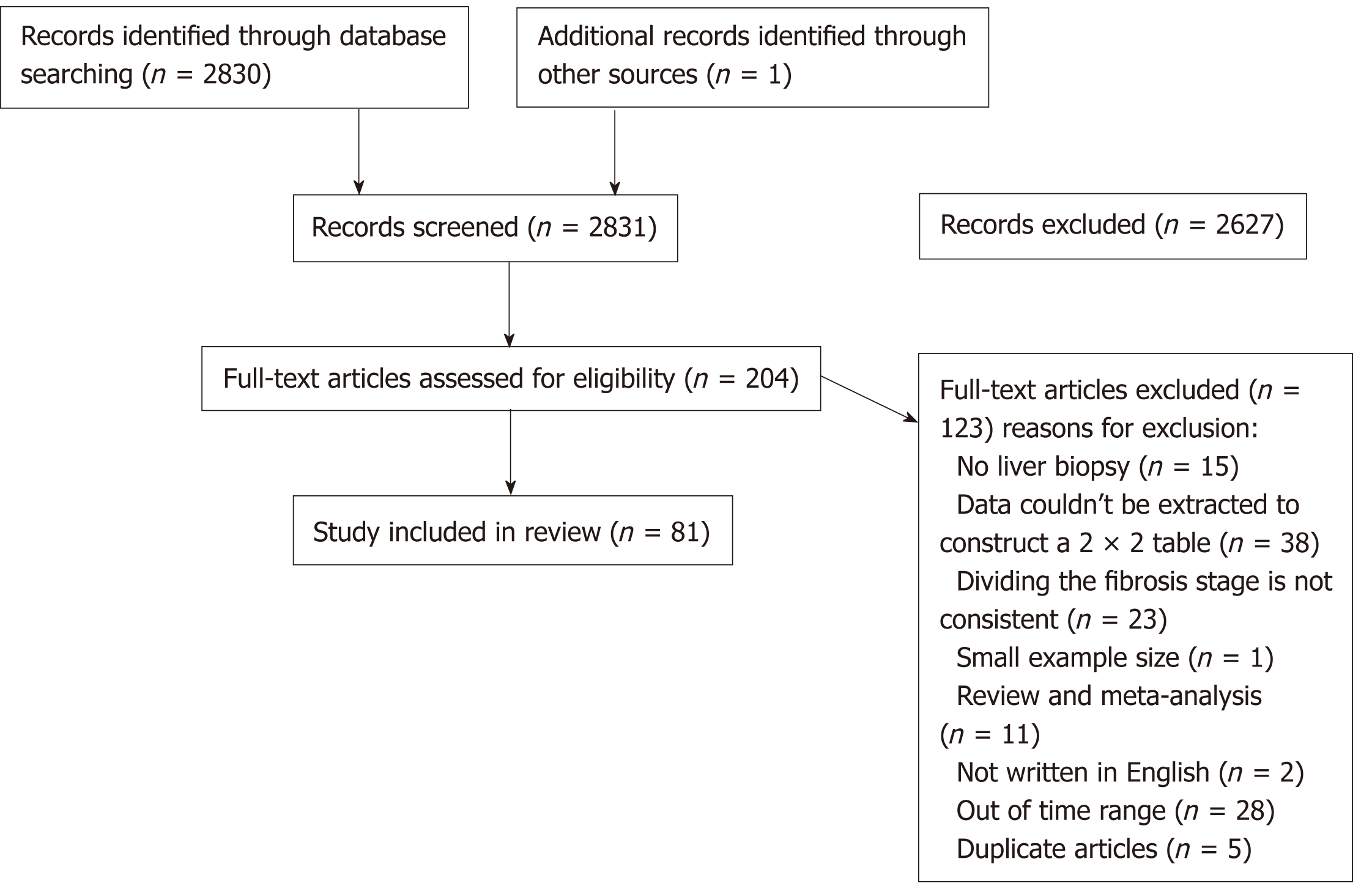
Out of 2831 articles evaluated for eligibility, 204 satisfied the predetermined inclusion criteria for this current meta-analysis. Eventually, our final data contained 81 studies. The AUSROCs of serum biomarkers of APRI and FIB-4 were both 0.75. For imaging techniques (ARFI, FibroScan, and MRE), the areas were 0.89, 0.83, and 0.97, respectively. The heterogeneities of ARFI and FibroScan were statistically significant (I2 > 50%). The publication bias was not observed in any of the serum biomarkers or imaging methods.
These five methods have attained an acceptable level of diagnostic accuracy. Imaging techniques, MRE in particular, demonstrate significant advantages in accurately predicting HBV-related significant fibrosis, while serum biomarkers are admissible methods.
Core tip: Many researchers compared the diagnostic effects for liver fibrosis by new techniques within the domain of imaging techniques separately or focused on fibrosis in all chronic liver diseases. We perform a meta-analysis to compare the effectiveness of both some common imaging methods and serum biomarkers for prediction of significant fibrosis among hepatitis B virus (HBV)-monoinfected patients. The results reveal that imaging techniques have significant advantages in prediction of HBV-related significant fibrosis.
- Citation: Xu XY, Wang WS, Zhang QM, Li JL, Sun JB, Qin TT, Liu HB. Performance of common imaging techniques vs serum biomarkers in assessing fibrosis in patients with chronic hepatitis B: A systematic review and meta-analysis. World J Clin Cases 2019; 7(15): 2022-2037
- URL: https://www.wjgnet.com/2307-8960/full/v7/i15/2022.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i15.2022
Liver fibrosis is a consequence of the accumulation of extracellular matrix compone-nts, caused by persistent liver damage and consequent wound healing reaction[1,2]. It can develop to cirrhosis, even hepatic failure and hepatocellular carcinoma[3]. Approximately one-third of cirrhosis cases worldwide are caused by hepatitis B virus (HBV) infection[2]. It is estimated that about 450 million people are chronically infected with HBV around the world[1], resulting in 600000 to 1000000 deaths annually[4]. Therefore, the accurate diagnosis of the degree of liver fibrosis is essential for the management of chronic hepatitis B (CHB) patients and making clinical decision for doctors.
Liver biopsy is the current reference standard for the evaluation and staging of fibrosis, which is an invasive technique. However, it has several disadvantages, such as patients’ reluctance, pain, hemoperitoneum[5], intra- and interobserver variations, and sampling errors[6]. Therefore, several noninvasive methods, including serum biomarkers combining indices/scores and imaging techniques like magnetic resonance imaging (MRI), have been investigated for their potential in assessment of liver fibrosis.
There has been a dramatic increase in developing various noninvasive tests since 1991[7]. There are direct serum noninvasive tests such as hepascore and hyaluronic acid, indirect serum markers which consist of the combination of routine biochemical or haematological tests such as fibrosis index based on the 4 factors (FIB-4), aspartate aminotransferase-to-platelet ratio index (APRI), or Forns index and Fibrotest combined direct and indirect tests[8]. Among all of these tests, APRI and FIB-4, being extensively investigated in numerous studies, demonstrated the greatest potential.
On the other side, with the development of imaging technology, several imaging devices have been developed for the diagnosis and staging of liver fibrosis, which are truly noninvasive methods when compared with serum biomarkers. Historically, radiologists and clinicians have relied on the assessment of morphological changes associated with liver fibrosis. Other imaging methods depend on changes in physical properties that can be assessed by quantification techniques. These include mechanical properties, texture, T1ρ lengthening, perfusion, diffusion, and hepatocellular function[9]. Ultrasound (US)-based elastography techniques are portable, relatively inexpensive, fast to acquire, and do not require postprocessing[10]. In particular, 1D transient elastography, commercialized as FibroScan (Echosens, Paris, France), has been widely validated in clinical trials, adopted clinically, and used by clinicians at point of service[9]. Moreover, focal point shear-wave elastography, commercialized as acoustic radiation force impulse (ARFI) (Siemens Medical Solutions, Mountain View, CA), is mapped in 2D using US tracking pulses, and the resulting tissue displacement is measured[11]. It is integrated in a conventional US machine that can be performed with conventional US probes during an abdominal US scan[12,13]. In addition, many MRI techniques for imaging of liver fibrosis are being developed. They typically cover a larger liver volume than US elastography techni-ques, which may reduce sampling variability and technical failure rate.
These methods have exhibited promising results and may improve the manage-ment of liver fibrosis. However, most previous studies compared the diagnostic effects of these new imaging techniques alone and focused on fibrosis in all chronic liver diseases, which might underestimate or overestimate the role of the biomarkers. Therefore, we performed a meta-analysis to compare the pooled performance of some common imaging methods and serum biomarkers for prediction of significant fibrosis among HBV-monoinfected patients.
Online database search was performed on EMBASE, PubMed, and the Cochrane Library (01/2008-12/2018) for the following terms: Diagnosis test, liver stiffness measurement, LSM, transient elastography, FibroScan, MR elastography, MRE, ARFI, acoustic radiation force impulse, point shear-wave elastography, imaging techniques, hepatitis B virus, HBV, chronic hepatitis B, CHB, and fibrosis. Other studies were identified by a manual search for referenced studies or review articles. EndNote X9 software was used to manage the references.
Studies should be included if they conformed all of the following five criteria: (a) The study evaluated the performance of the APRI and/or ARFI and/or FIB-4 and/or FibroScan and/or MRE for the prediction of fibrosis in HBV infected patients. Studies on patients with other etiologies of liver diseases were also included if data for HBV-infected patients could be independently extracted. Special populations of HBV-infected patients [e.g., HBV/HIV, HBV/ hepatitis C virus (HCV), or HBV/ hepatitis D virus (HDV) coinfection] were excluded; (b) Liver biopsy was used to diagnose liver fibrosis as a golden standard; (c) Data could be extracted to construct at least one 2 × 2 table of test performance, based on some cutoff points of the five biomarkers for the significant fibrosis stage; (d) The study assessed the diagnostic accuracy for fibrosis stage F ≥ 2 according to METAVIR or a comparable staging system; and (e) The study included at least 50 patients. Studies of smaller sample sizes were excluded due to concerns on their applicability.
Two reviewers (WWS and XYX) evaluated study eligibility, extracted data from the study, and graded the study quality independently. Any disagreements between the reviewers were resolved by detailed discussions together with a third reviewer (LHB). The parameters in our literature search included author, year of publication, region, age, patient gender , body mass index (BMI), histological scoring system, number of patients, average length of liver specimen, time interval between biopsy and other diagnostic tests, diagnostic method, prevalence of the fibrosis stage, as well as cutoff values to identify the fibrosis stage[14].
XYX and ZQM independently appraised the quality of included studies using the quality assessment of diagnostic accuracy studies questionnaire (QUADAS-2)[15]. It could estimate the internal and external validity of diagnostic accuracy of studies used in systematic reviews.
We extracted and tabulated the data in a series of 2 × 2 tables that included sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) at each threshold value. The primary target was to identify significant fibrosis, defined by METAVIR[16], Batts and Ludwig[17], Scheuer[18], and Chinese Hospital system[19], for stages F2 to F4, and Ishak[20] for stages F3 to F6. This gauge was chosen because significant fibrosis is often considered a threshold for the initiation of antiviral therapy[21]. The method of diagnostic test accuracy was examined in order to provide clinically meaningful results. We examined the area under the summary receiver operating characteristic curve (AUSROC) curve, the summary diagnostic odds ratio (DOR), and the summary sensitivity and specificity to further examine the accuracy of all tests for liver fibrosis.
The heterogeneity of the results between studies was assessed statistically using the Cochran-Q and the quantity I2. I2 value can describe the percentage of total variation across studies that is attributable to heterogeneity rather than chance[22]. If there was significant heterogeneity, a meta-regression was conducted to explore the covariates that may induce heterogeneity, according to the following predefined characteristics: (1) Study design (prospective or retrospective); (2) Length of liver specimen (≥ 10 mm, ≥ 15 mm, ≥ 20 mm, or not); (3) Liver biopsy scoring system; (4) Number of centers (single or multicenter); (5) Sample size; (6) Publication year (2014-2018 or 2008-2013); (7) Time interval between biopsy and tests (same day or not); (8) Prevalence of signifi-cant fibrosis; (9) BMI; and (10) Location of study.
The potential publication bias was assessed using the Deeks funnel plots[23]. All analyses were performed with Meta-Disc software (v. 1.4) and Stata15.0.
The study selection process is shown in Figure 1. A total of 2831 studies were searched by the described search strategies, of which 2627 were excluded following title and abstract screening. Of those, 81 papers were included in the review following full-text screening (Text S5); 47 studies were related to the APRI[24-70], 11 to the ARFI[29,42,69-77], 32 to the FIB-4[24,25,27,30,35,36,39,40,43-46,48-55,57,58,60,62,63,65-69,78-79], 29 to the FibroScan[28,38,42,52,53,58,60,61,71,73,80-98], and 6 to the MRE[99-104].
In the 47 studies of APRI, there were 13725 patients (median age, 37 years; 68.9% of males). The total prevalence of significant fibrosis was 55.9% (range: 16.7%-83.7%). Most studies were conducted in Asia (42 studies). Three studies were from Europe, one from North America, and another from Africa. We did not restrict study participants to any age, but only one study was conducted on children and adolescents exclusively[59]. Twenty-nine studies used the METAVIR score, ten used the Scheuer score, six utilized the Ishak score, and two used the Chinese Hospital System. There were 11179 patients (median age, 37 years; 68.7% of males) in 32 studies evaluating the performance of FIB-4. The total prevalence of significant fibrosis was 57.5% (range: 16.7%-83.7%). Twenty-nine studies were conducted in Asia, two in Europe, and one in North America. All studies included adults only. Seventeen studies used the METAVIR score, nine used the Scheuer score, four utilized the Ishak score, and two used the Chinese Hospital System.
A total of 1527 patients (median age, 36 years; 67.5% of males) were contained in 11 studies on ARFI. The total prevalence of F2 was 54.2% (range: 23.9%-74.3%). Ten studies were conducted in Asia and one in Germany. All the subjects were adults. Six studies used the METAVIR score, two used the Chinese Hospital System, and one used each of the Scheuer score, the Ishak score, and the Batts and Ludwig score.
In the 29 FibroScan studies, a total of 5035 patients (median age, 39 years; 71.4% of males) were included. The overall prevalence of significant fibrosis was 49.4% (range: 14.8%-85%). Twenty-one studies were from Asia, six from Europe, one from Africa, and another from North America. Twenty studies used the METAVIR score, five utilized the Batts and Ludwig score, three utilized the Scheuer score, and one used the Chinese Hospital System.
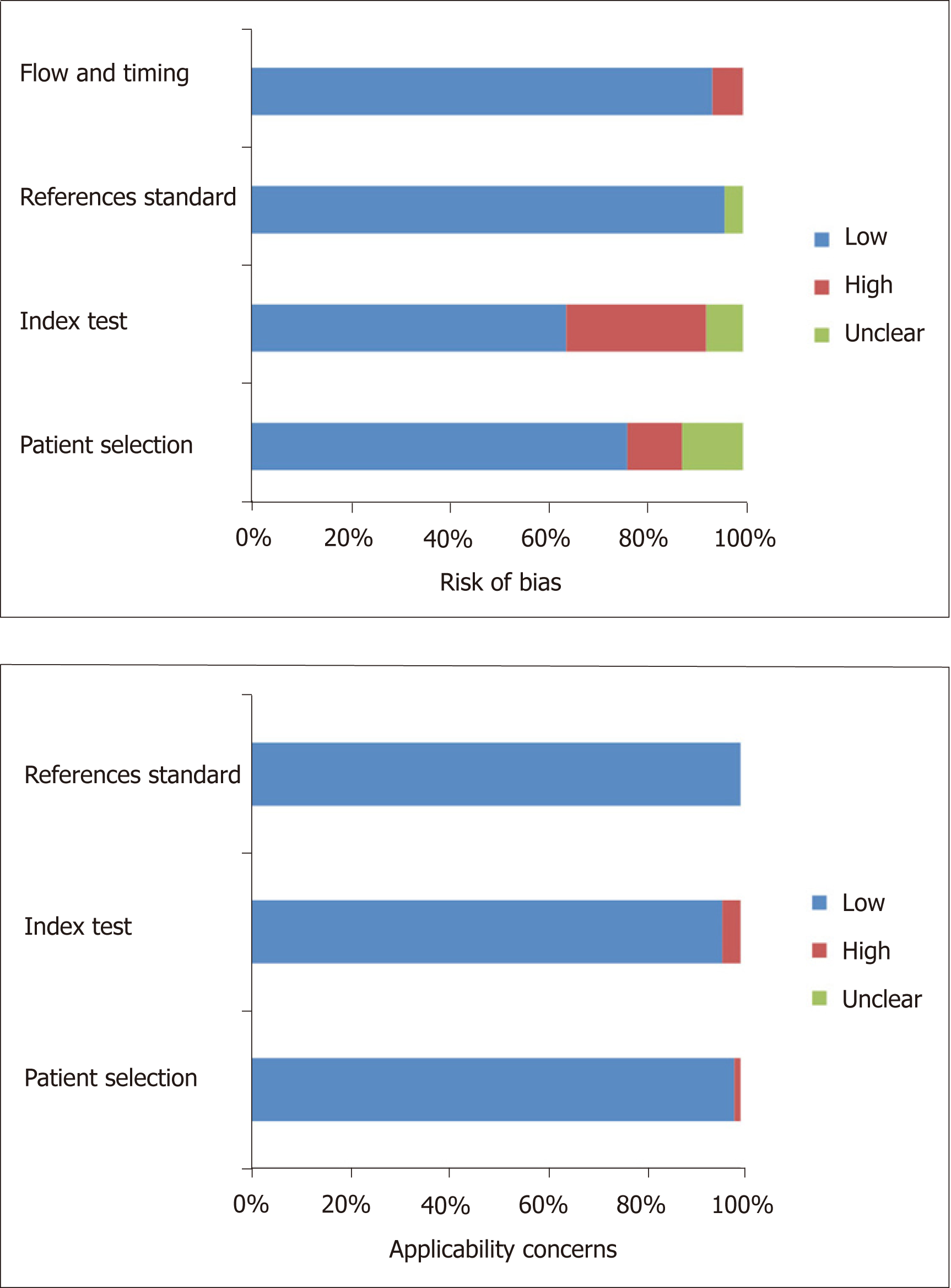
There were 1228 patients (median age, 49 years; 71.0% of males) used to evaluate the performance of MRE in six studies. The total prevalence of significant fibrosis was 61.4% (range: 45.2%-77.4%). One MRE study was from America and the other five were conducted in Asia. The results of methodological quality assessment according to the QUADAS-2 scale are described for all studies (Figure 2, Text S5).
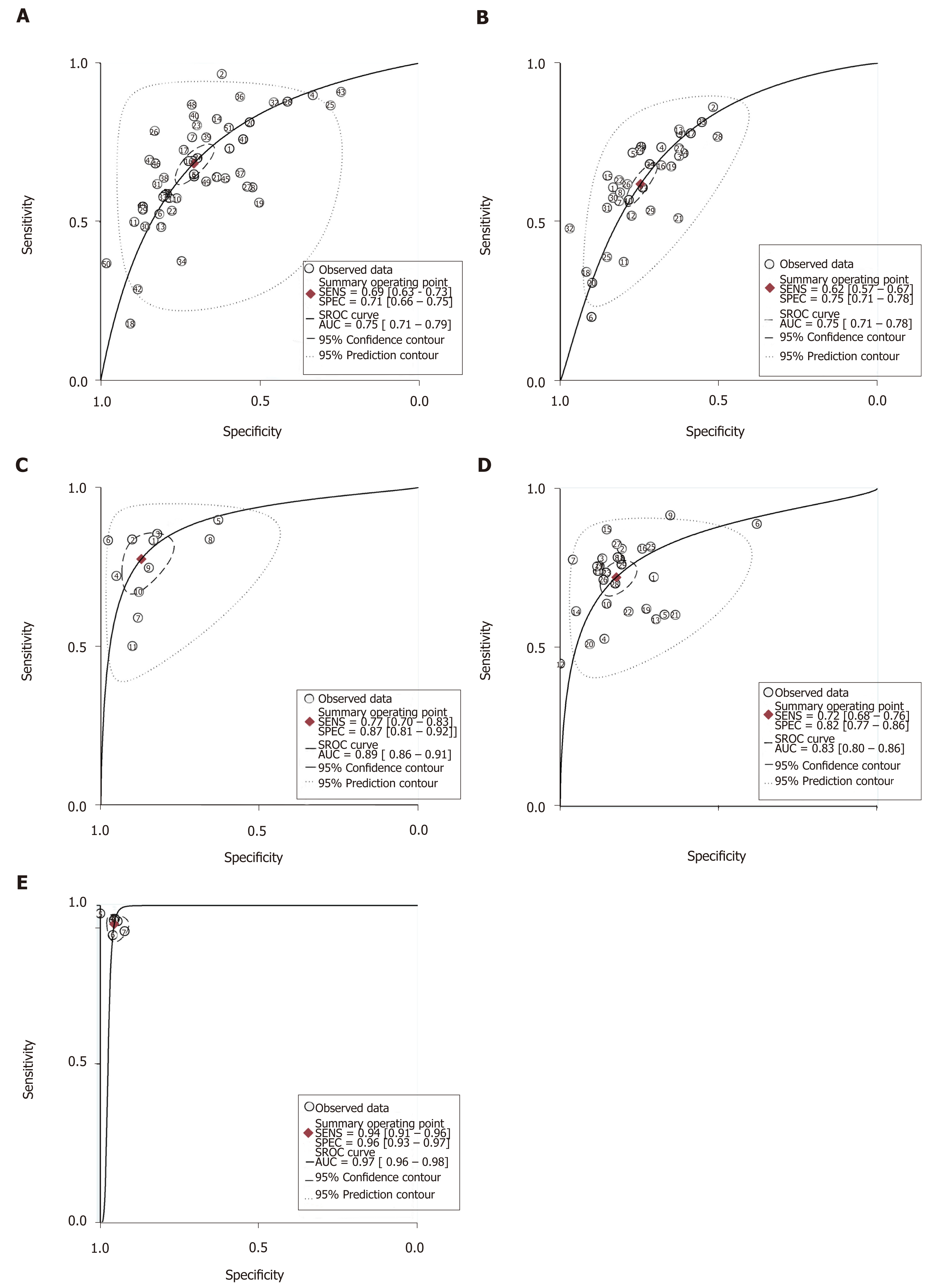
In the 47 studies evaluating the APRI, the area under the ROC curve (AUROC) ranged from 0.54 to 0.87. The AUSROC and the pooled sensitivity, specificity, and DOR are presented in Table 1 and Figure 3. The Cochran-Q was 1199.4 and I2 > 50%, which indicated significant heterogeneity across the included studies (P < 0.001). Meta-regression analysis was used to explore the heterogeneity of the APRI accuracy for predicting significant fibrosis, which was affected by BMI and prevalence of signifi-cant fibrosis with regard to sensitivity and only prevalence of significant fibrosis with regard to specificity (Figure S1). In the 32 studies evaluating the FIB-4 for the prediction of fibrosis stage of F2, the AUROC ranged from 0.56 to 0.85. The AUSROC was 0.75 (0.71-0.78) (Figure 3). The summary sensitivity, specificity, and DOR are shown in Table 1. The score of Cochran-Q was 488.9 (P < 0.05) and heterogeneity was statistically significant. The heterogeneity of FIB-4 accuracy was mainly affected by prevalence of significant fibrosis with regard to sensitivity, and prevalence of significant fibrosis and study design with regard to specificity according to the meta-regression analysis (Figure S2). The summary sensitivity and specificity of serum indicators by BMI and prevalence of significant fibrosis are listed in Tables 2 and 3.
| SEN (95%CI) | SPE (95%CI) | DOR (95%CI) | |
| APRI | 0.69 (0.63-0.73) | 0.71 (0.66-0.75) | 5 (4-6) |
| FIB-4 | 0.62 (0.57-0.67) | 0.75 (0.71-0.78) | 5 (4-5) |
| ARFI | 0.77 (0.70-0.83) | 0.87 (0.81-0.92) | 23 (15-38) |
| FibroScan | 0.72 (0.68-0.76) | 0.82 (0.77-0.86) | 12 (9-16) |
| MRE | 0.94 (0.91-0.96) | 0.96 (0.93-0.97) | 348 (185-656) |
| Diagnostic test | BMI | Number of studies (n) | AUSROC (95%CI) | SEN (95%CI) | SPE (95%CI) |
| APRI | Overweight | 7 (985) | 0.78 (0.74-0.81) | 0.78 (0.59-0.90) | 0.71 (0.61-0.79) |
| Normal | 13 (2598) | 0.75 (0.71-0.79) | 0.63 (0.55-0.71) | 0.77 (0.68-0.84) | |
| NA | 27 (10142) | 0.74 (0.70-0.77) | 0.69 (0.62-0.74) | 0.68 (0.61-0.74) | |
| FIB-4 | Overweight | 5 (717) | 0.76 (0.72-0.79) | 0.58 (0.47-0.70) | 0.80 (0.67-0.89) |
| Normal | 6 (1367) | 0.70 (0.66-0.74) | 0.58 (0.46-0.69) | 0.75 (0.60-0.85) | |
| NA | 21 (9095) | 0.75 (0.71-0.79) | 0.64 (0.57-0.70) | 0.74 (0.69-0.78) | |
| ARFI | Overweight | 5 (679) | 0.85 (0.82-0.88) | 0.76 (0.64-0.85) | 0.86 (0.80-0.91) |
| Normala | 3 (481) | 0.91 (0.88-0.95) | 0.84 (0.80-0.88) | 0.76 (0.64-0.85) | |
| NAa | 3 (367) | 0.93 (0.85 – 0.99) | 0.72 (0.63-0.80) | 0.95 (0.91-0.97) | |
| Fibroscan | Overweight | 12 (1927) | 0.81 (0.77-0.84) | 0.68 (0.62-0.74) | 0.85 (0.76-0.91) |
| Normal | 11 (2103) | 0.83 (0.79-0.86) | 0.73 (0.66-0.79) | 0.82 (0.73-0.88) | |
| NA | 6 (1005) | 0.85 (0.81-0.88) | 0.79 (0.69-0.87) | 0.78 (0.72-0.83) |
| Diagnostic test | F2% | Number of studies (n) | AUSROC (95%CI) | SEN (95%CI) | SPE (95%CI) |
| APRI | F2% < 50 | 16 (4566) | 0.72 (0.68-0.76) | 0.68 (0.59-0.76) | 0.66 (0.57-0.75) |
| F2% ≥ 50 | 31 (9159) | 0.77 (0.73-0.81) | 0.69 (0.62-0.75) | 0.73 (0.68-0.78) | |
| FIB-4 | F2% < 50 | 9 (3234) | 0.75 (0.71-0.79) | 0.60 (0.47-0.72) | 0.76 (0.68-0.83) |
| F2% ≥ 50 | 23 (7945) | 0.75 (0.71-0.78) | 0.63 (0.57-0.68) | 0.74 (0.69-0.78) | |
| ARFI | F2% < 50 | 4 (554) | 0.89 (0.85-0.91) | 0.73 (0.62-0.82) | 0.93 (0.68-0.97) |
| F2% ≥ 50 | 7 (973) | 0.88 (0.85-0.91) | 0.80 (0.72-0.87) | 0.82 (0.74-0.88) | |
| Fibroscan | F2% < 50 | 12 (2272) | 0.85 (0.81-0.88) | 0.77 (0.70-0.83) | 0.80 (0.71-0.86) |
| F2% ≥ 50 | 17 (2763) | 0.82 (0.78-0.85) | 0.69 (0.64-0.74) | 0.84 (0.78-0.88) |
The AUROC ranged from 0.72 to 0.96 in 11 studies evaluating the ARFI. When combined, the AUSROC was 0.89 (0.86-0.91). The summary DOR was 23 (95%CI: 15-38), and the score of Cochran-Q was 27.8 and I2 > 50%, indicating significant heterogeneity across the included studies (P < 0.05). In meta-regression analysis, we found that the causes of the heterogeneity were publishing year and sample size for sensitivity, and prevalence of significant fibrosis and BMI for specificity (Figure S3). In the 29 studies evaluating the FibroScan, the AUROC ranged from 0.61 to 0.94. The AUSROC and the pooled sensitivity, specificity, and DOR are also shown in Table 1 and Figure 3. There was significant heterogeneity across the included studies (I2 > 50%, P < 0.001). The heterogeneity of the FibroScan was mainly affected by prevalence of significant fibrosis and BMI with regard to sensitivity or specificity (Figure S4). In the six studies evaluating the MRE for the prediction of significant fibrosis, the AUROC ranged from 0.98 to 0.99. The AUSROC was 0.97 (0.96-0.98) (Figure 3). The summary sensitivity, specificity, and DOR are listed in Table 1, and the Cochran-Q and I2 of this measure were 0.44 (P > 0.05) and 0, respectively. The result of the heterogeneity was statistically insignificant. The subgroup analysis of imaging methods by BMI and prevalence of significant fibrosis are listed in Tables 2 and 3, respectively.
Funnel plots of these markers are illustrated in Figure S5. Symmetry was noted in the funnel plots and publication bias was not observed in any of the serum biomarkers or the imaging methods.
Liver fibrosis progression is common in people infected with HBV. Patients with significant fibrosis should be considered for clinical therapy, which can potentially reduce and even reverse complications[105]. Considering the risks and limitations of biopsy, researchers should make persistent efforts in researching some noninvasive methods to more conveniently and securely identify patients with significant fibrosis. FIB-4 and APRI are such noninvasive serum biomarkers that they have been gaining increasing acceptance in clinical practice. FibroScan, ARFI, and MRE gradually reveal an advantage with the development of US-based or MRI elastography techniques. These methods may reduce the demand for liver biopsy and also help to monitor the efficacy of treatments[62].
Many scholars have researched the diagnostic effect of APRI and FIB-4 and usually compared them with other new methods. In addition, the World Health Organization (WHO) recommends APRI as the preferred noninvasive test to assess significant fibrosis or cirrhosis and FIB-4 to detect fibrosis stages ≥ F3, considering lower cost, routinely available methods, and untrained staff[106]. Therefore, among all serum biomarkers, only APRI and FIB-4 were singled out in our analysis. Based on the results from an increasing number of studies, the difference between APRI and FIB-4 is not significant in relative diagnostic outcomes, although some articles described that FIB-4 had a higher diagnostic accuracy for cirrhosis when compared with APRI [107]. If only APRI and FIB-4 are available, APRI based on AST, ULN, and platelet count is suggested to diagnose liver significant fibrosis considering resource-limited settings.
With the development of imaging technology, US imaging and MRI devices have been explored to diagnose and stage liver fibrosis, which are truly noninvasive methods. In our meta-analysis, the AUSROCs of imaging techniques are generally bigger than those of APRI and FIB-4. Of these tests, transient elastography performed with FibroScan (Echosens, Paris) has been evaluated widely and has a good performance in predicting cirrhosis, which is corroborated by the Guidelines Development Group. They considered it the most useful test for the assessment of cirrhosis in middle-income countries[106]. In addition, FibroScan acquires information from a much larger portion of the liver tissue compared to biopsy, and therefore, the risk of sampling error is significantly lowered. However, its performance is mediocre for diagnosing moderate liver fibrosis, and the AUSROC ranked in the middle of the five methods. Morbid obesity (BMI > 30), narrow intercostal spaces, and food intake may diminish the accuracy of FibroScan[107]. Because of the limitation of US technology, some researchers advocated that the accuracy of ARFI is similar if not superior to FibroScan for the diagnosis of liver fibrosis[108,109]. Our meta-analysis reveals that ARFI has a higher diagnostic accuracy than FibroScan in identifying HBV-related significant fibrosis. In contrast to FibroScan that has a fixed region of interest box at a fixed insertion depth, ARFI could be performed at variable depths. Furthermore, it can also be performed in obese patients and patients with ascites and integrated in a conventional US system[13]. MRE, the only MRI index, had the best result in prediction of significant fibrosis. The AUSROC of MRE reaches the standard of “best” and even closes to “1”[110]. And the summary sensitivity and specificity have reached 94% and 96%, respectively. It typically covers larger liver tissue than US elastography techniques, which may reduce sampling variability. While MRE has been incorporated into MRI devices, it might be more expensive and require more operator training and expertise. Therefore, it is not widely applied at present, and the number of research articles is limited.
Meta-regression method is a convenient and reliable way for heterogeneity screening. In our study, BMI, prevalence of significant fibrosis, sample size, publishing year, and study design provide heterogeneity in summarizing test results. This is consistent with a range of previous studies[38,111,112]. Some researchers suggested that BMI may reduce the accuracy of ultrasonic technique for detecting significant fibrosis[9,38]. Our results reveal that BMI may affect predicting outcomes of either serum biomarkers or imaging techniques. Imaging technology shows a better predictive effect in the normal weight group, while serum indicators reflect their superiorities in overweight HBV patients (Table 2). The high proportion of fibrosis stage ≥ 2 will reduce the accuracy of evaluating fibrosis by all methods (Table 3). Furthermore, publishing year and sample size are the main contributors to the heterogeneity of ARFI. The AUSROC of ARFI was 0.89 when we excluded the only one article before 2014, the AUSROC of which was 0.75. Therefore, the technology of ARFI, a new diagnostic method, is progressing gradually, and the evaluation effect is increasingly improved. Besides, the AUSROCs in subgroups of sample size between 100 and 300 were much more than others in most cases (Text S6). Although other methods showed no significant differences in the AUSROC among subgroups of sample size, there was a general pattern that the areas were larger in the middle while smaller on both sides (Text S6). This phenomenon is more prominent if the group was changed into three subgroups (n < 100, 100 ≤ n ≤ 300, and n > 300) (Table 4). A few years ago, our team observed that when the sample size was larger than 150, the diagnostic accuracy of predicting fibrosis was noticeably enhanced[111]. Therefore, the sample size of 150-300 will be beneficial to the accuracy of diagnostic tests.
| Diagnostic test | Sample size | Number of studies (N) | AUSROC (95%CI) | SEN (95%CI) | SPE (95%CI) |
| APRI | n < 100 | 8 (588) | 0.73 (0.69-0.77) | 0.67 (0.60-0.74) | 0.68 (0.61-0.75) |
| 100 ≤ n < 300 | 27 (5286) | 0.77 (0.73-0.80) | 0.69 (0.62-0.76) | 0.72 (0.65-0.78) | |
| n ≥ 300 | 12 (7851) | 0.73 (0.69-0.77) | 0.67 (0.57-0.76) | 0.69 (0.58-0.78) | |
| FIB-4 | n < 100 | 6 (411) | 0.77 (0.73-0.80) | 0.64 (0.54-0.73) | 0.77 (0.67-0.85) |
| 100 ≤ n < 300 | 14 (2917) | 0.76 (0.72-0.80) | 0.62 (0.53-0.71) | 0.77 (0.69-0.83) | |
| n ≥ 300 | 12 (7851) | 0.74 (0.70-0.77) | 0.62 (0.53-0.69) | 0.74 (0.67-0.79) | |
| ARFI | n < 100 | 2 (173) | 0.72/0.75 | 0.83/0.50 | 0.65/0.90 |
| 100 ≤ n < 300 | 9 (1354) | 0.90 (0.87-0.92) | 0.78 (0.71-0.84) | 0.88 (0.82-0.93) | |
| n ≥ 300 | 0 | - | - | - | |
| Fibroscan | n < 100 | 6 (445) | 0.80 (0.76-0.83) | 0.70 (0.57-0.80) | 0.76 (0.68-0.83) |
| 100 ≤ n < 300 | 20 (3659) | 0.84 (0.81-0.87) | 0.74 (0.69-0.79) | 0.83 (0.77-0.87) | |
| n ≥ 300a | 3 (931) | 0.82 (0.69-0.95) | 0.62 (0.57-0.66) | 0.88 (0.84-0.90) |
However, there are several limitations in our systematic review. First of all, the cut-off value was not considered due to the absence of threshold effect in all tests (Text S7). Second, the ALT levels were not considered because only a limited number of studies calculated the results by the subgroup of ALT. Third, only the studies published in English and Chinese languages were included. Lastly, other methods, though we have retrieved them, were not considered in this meta-analysis, because the number of articles was not adequate.
In conclusion, these five methods have attained an acceptable level of diagnostic accuracy in our meta-analysis. Imaging techniques are generally better than serum biomarkers in prediction of HBV-related liver significant fibrosis. MRE is a promising indicator and will surprise us with the development of global economy and popu-larization of technology. If condition allows, ARFI could be a better choice, than FibroScan, for assessment of liver fibrosis in HBV-monoinfected patients with obesity or ascites. The prediction effects of serum markers are generally admissible. Their low cost and easiness in operation make them attractive especially in areas with limited resources and access to imaging technology.
Liver fibrosis can develop to cirrhosis and even hepatic failure and hepatocellular carcinoma. Approximately one-third of cirrhosis cases worldwide are caused by HBV infection. Therefore, the accurate diagnosis of the extent of liver fibrosis for chronic hepatitis B patients is essential. Many serum biomarkers combining indices/scores and imaging or magnetic resonance imaging techniques have been undergoing dramatic development because of several drawbacks of liver biopsy. These methods have promising results and may improve the management of liver fibrosis. However, most of the previous studies compared the diagnostic effects of these new techniques within the domain of imaging techniques or focused on fibrosis in all chronic liver diseases, which might misestimate the role of these biomarkers. Therefore, we performed a meta-analysis to compare the pooled performance of some common imaging methods with serum biomarkers for prediction of significant fibrosis among HBV-monoinfected patients.
We aimed to assess the accuracy of diagnostic tests for predicting significant fibrosis among patients monoinfected with HBV. Most studies are centered on the domain of imaging techniques and serum biomarkers separately or focused on fibrosis in all chronic liver diseases. Therefore, the key points are that data for HBV infected patients could be extracted independently and we want to integrate and compare the performance of methods of different fields. With the development of medicine and technology, more innovative methods could be invented in the near future. It will provide a boost to precision medicine that chooses a more appropriate and effective method to evaluate liver fibrosis for different populations of patients.
We aimed to compare the pooled performance of some common imaging methods with serum biomarkers for prediction of significant fibrosis among HBV-monoinfected patients. Some serum biomarkers have been calculated and imaging techniques have been developed for the diagnosis of liver fibrosis, respectively. Integrating and comparing the performance of methods of different fields could provide a basis for future research and clinical application and a boost to precision medicine.
We examined the areas under the summary receiver operating characteristic curves, the summary diagnostic odds ratios, as well as the summary sensitivities and specificities to further examine the accuracy of all tests for liver fibrosis. Then, we assessed the heterogeneity between studies using the Cochran-Q and I2. And the Deeks funnel plots were used to assess publication bias. Meta-regression was conducted to further accurately explore the covariates that may induce heterogeneity.
Our meta-analysis revealed that ARFI showed a higher diagnostic accuracy than FibroScan in identifying HBV-related significant fibrosis. Furthermore, it can also be performed in obese patients and in patients with ascites and be integrated in a conventional ultrasound system. MRE, the only MRI index, had the best result in prediction of significant fibrosis. The area under the SROC curve of MRE reaches the standard of “best” and even closes to “1”. The performances of APRI and FIB-4 are poorer than imaging techniques. However, the cut-off value should be considered in future studies.
Imaging techniques are better than serum biomarkers in prediction of HBV-related liver significant fibrosis in general. MRE is a promising indicator and other serum biomarkers are general.
We thank Prof. Li-Jin Xu for comments that greatly improved the manuscript. And we also thank all participants for their contribution of time to this study.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tsuchiya A, El-Bendary M, Tai DI S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Wang J
| 1. | Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 485] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 2. | Kawada N. Evolution of hepatic fibrosis research. Hepatol Res. 2011;41:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191-211, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 605] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 4. | Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004;38:S158-S168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 403] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 5. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1735] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 6. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 596] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 7. | Poynard T, Aubert A, Bedossa P, Abella A, Naveau S, Paraf F, Chaput JC. A simple biological index for detection of alcoholic liver disease in drinkers. Gastroenterology. 1991;100:1397-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Crossan C, Tsochatzis EA, Longworth L, Gurusamy K, Davidson B, Rodríguez-Perálvarez M, Mantzoukis K, O'Brien J, Thalassinos E, Papastergiou V, Burroughs A. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: systematic review and economic evaluation. Health Technol Assess. 2015;19:1-409, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 9. | Petitclerc L, Sebastiani G, Gilbert G, Cloutier G, Tang A. Liver fibrosis: Review of current imaging and MRI quantification techniques. J Magn Reson Imaging. 2017;45:1276-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Zhang E, Wartelle-Bladou C, Lepanto L, Lachaine J, Cloutier G, Tang A. Cost-utility analysis of nonalcoholic steatohepatitis screening. Eur Radiol. 2015;25:3282-3294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 646] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 12. | Wang L, Wang M, Zhao W, Shi Y, Sun Y, Wu X, You H, Jia J. [Key points of 2015 EASL-ALEH clinical practice guidelines: non invasive tests for evaluation of liver severity and prognosis]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:488-492. [PubMed] |
| 13. | Frulio N, Trillaud H, Perez P, Asselineau J, Vandenhende M, Hessamfar M, Bonnet F, Maire F, Delaune J, De Ledinghen V, Morlat P. Acoustic Radiation Force Impulse (ARFI) and Transient Elastography (TE) for evaluation of liver fibrosis in HIV-HCV co-infected patients. BMC Infect Dis. 2014;14:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Adebajo CO, Talwalkar JA, Poterucha JJ, Kim WR, Charlton MR. Ultrasound-based transient elastography for the detection of hepatic fibrosis in patients with recurrent hepatitis C virus after liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2012;18:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9546] [Article Influence: 681.9] [Reference Citation Analysis (0)] |
| 16. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1414] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 17. | Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 847] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 18. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1197] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 19. | Diseases CSoHaCSoI. Chinese medical association: Chinese program of prevention and cure for viral hepatitis. Chin J Intern Med. 2001;40:62-68. |
| 20. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3780] [Article Influence: 126.0] [Reference Citation Analysis (1)] |
| 21. | Strader DB, Wright T, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1232] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 22. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46389] [Article Influence: 2108.6] [Reference Citation Analysis (3)] |
| 23. | Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2223] [Article Influence: 111.2] [Reference Citation Analysis (1)] |
| 24. | Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, Wu C, Liu C, Huang L, Jiang T, Meng F, Lu Y, Ai H, Xie XY, Yin LP, Liang P, Tian J, Zheng R. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2019;68:729-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 350] [Article Influence: 58.3] [Reference Citation Analysis (1)] |
| 25. | Chen YP, Hu XM, Liang XE, Huang LW, Zhu YF, Hou JL. Stepwise application of fibrosis index based on four factors, red cell distribution width-platelet ratio, and aspartate aminotransferase-platelet ratio for compensated hepatitis B fibrosis detection. J Gastroenterol Hepatol. 2018;33:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Celikbilek M, Dogan S, Gursoy S, Zararsız G, Yurci A, Ozbakır O, Guven K, Yucesoy M. Noninvasive assessment of liver damage in chronic hepatitis B. World J Hepatol. 2013;5:439-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Gümüşay O, Ozenirler S, Atak A, Sönmez C, Ozkan S, Tuncel AF, Yılmaz G, Akyol G. Diagnostic potential of serum direct markers and non-invasive fibrosis models in patients with chronic hepatitis B. Hepatol Res. 2013;43:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Li Q, Chen L, Zhou Y. Diagnostic accuracy of liver stiffness measurement in chronic hepatitis B patients with normal or mildly elevated alanine transaminase levels. Sci Rep. 2018;8:5224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Chen X, Wen H, Zhang X, Dong C, Lin H, Guo Y, Shan L, Yao S, Yang M, Le X, Liu Y. Development of a Simple Noninvasive Model to Predict Significant Fibrosis in Patients with Chronic Hepatitis B: Combination of Ultrasound Elastography, Serum Biomarkers, and Individual Characteristics. Clin Transl Gastroenterol. 2017;8:e84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Zhang Z, Wang G, Kang K, Wu G, Wang P. The Diagnostic Accuracy and Clinical Utility of Three Noninvasive Models for Predicting Liver Fibrosis in Patients with HBV Infection. PLoS One. 2016;11:e0152757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Liang B, Li Y, Zhao A, Xie F, Guo Z. Clinical utility of serum matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 concentrations in the assessment of liver fibrosis due to chronic hepatitis B. J Int Med Res. 2012;40:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Zhou K, Gao CF, Zhao YP, Liu HL, Zheng RD, Xian JC, Xu HT, Mao YM, Zeng MD, Lu LG. Simpler score of routine laboratory tests predicts liver fibrosis in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2010;25:1569-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Güzelbulut F, Sezıklı M, Akkan-Çetınkaya Z, Yaşar B, Özkara S, Kurdaş-Övünç AO. AST-platelet ratio index in the prediction of significant fibrosis and cirrhosis in patients with chronic hepatitis B. Turk J Gastroenterol. 2012;23:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Eminler AT, Ayyildiz T, Irak K, Kiyici M, Gurel S, Dolar E, Gulten M, Nak SG. AST/ALT ratio is not useful in predicting the degree of fibrosis in chronic viral hepatitis patients. Eur J Gastroenterol Hepatol. 2015;27:1361-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Liu J, Zhao J, Zhang Y, Ji Y, Lin S, Dun G, Guo S. Noninvasive Assessment of Liver Fibrosis Stage Using Ultrasound-Based Shear Wave Velocity Measurements and Serum Algorithms in Patients With Viral Hepatitis B: A Retrospective Cohort Study. J Ultrasound Med. 2017;36:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Yang L, Ding Y, Rao S, Chen C, Wu L, Sheng R, Fu C, Zeng M. Staging liver fibrosis in chronic hepatitis B with T1 relaxation time index on gadoxetic acid-enhanced MRI: Comparison with aspartate aminotransferase-to-platelet ratio index and FIB-4. J Magn Reson Imaging. 2017;45:1186-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Li Q, Li W, Huang Y, Chen L. The gamma-glutamyl transpeptidase-to-platelet ratio predicts liver fibrosis and cirrhosis in HBeAg-positive chronic HBV infection patients with high HBV DNA and normal or mildly elevated alanine transaminase levels in China. J Viral Hepat. 2016;23:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, Goldin R, Njai HF, Ndow G, Taal M, Cooke G, D'Alessandro U, Vray M, Mbaye PS, Njie R, Mallet V, Thursz M. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 39. | Zhou QQ, Hu YB, Zhou K, Zhang WW, Li MH, Dong P, Ding JG, Hong L, Du QW, Xie Y, Sun QF. Value of non-invasive models of liver fibrosis in judgment of treatment timing in chronic hepatitis b patients with ALT < 2x upperlimit ofnormal. Zhonghua Gan Zang Bing Za Zhi. 2016;24:665-670. |
| 40. | Li Q, Song J, Huang Y, Li X, Zhuo Q, Li W, Chen C, Lu C, Qi X, Chen L. The Gamma-Glutamyl-Transpeptidase to Platelet Ratio Does not Show Advantages than APRI and Fib-4 in Diagnosing Significant Fibrosis and Cirrhosis in Patients With Chronic Hepatitis B: A Retrospective Cohort Study in China. Medicine (Baltimore). 2016;95:e3372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, Kim DJ, Jun SY, Park CK. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008;40:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Liu Y, Dong CF, Yang G, Liu J, Yao S, Li HY, Yuan J, Li S, Le X, Lin Y, Zeng W, Lin H, Zhang X, Chen X. Optimal linear combination of ARFI, transient elastography and APRI for the assessment of fibrosis in chronic hepatitis B. Liver Int. 2015;35:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Salkic NN, Cickusic E, Jovanovic P, Denjagic MB, Iljazovic-Topcic S, Bevanda M, Ahmetagic S. Online combination algorithm for non-invasive assessment of chronic hepatitis B related liver fibrosis and cirrhosis in resource-limited settings. Eur J Intern Med. 2015;26:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Erdogan S, Dogan HO, Sezer S, Uysal S, Ozhamam E, Kayacetin S, Koca Y. The diagnostic value of non-invasive tests for the evaluation of liver fibrosis in chronic hepatitis B patients. Scand J Clin Lab Invest. 2013;73:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, Janssen HL, Lampertico P, Lau D, Bornstein JD, Schall RE, Dinh P, Yee LJ, Martins EB, Lim SG, Loomba R, Petersen J, Buti M, Marcellin P. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 46. | Başar O, Yimaz B, Ekiz F, Giniş Z, Altinbaş A, Aktaş B, Tuna Y, Çoban S, Delibaş N, Yüksel O. Non-invasive tests in prediction of liver fibrosis in chronic hepatitis B and comparison with post-antiviral treatment results. Clin Res Hepatol Gastroenterol. 2013;37:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Dong C, Feng LY, Li HY, Lu PX, Yao SM, Shan LB, Zhang XY, Chen X. Virtual touch tissue quantification and aspartate aminotransferase to platelet ratio for staging hepatic fibrosis in patients with chronic hepatitis B. Zhongguo Yixue Yingxiang Jishu. 2016;32:398-402. |
| 48. | Li Q, Lu C, Li W, Huang Y, Chen L. Impact of age on the diagnostic performances and cut-offs of APRI and FIB-4 for significant fibrosis and cirrhosis in chronic hepatitis B. Oncotarget. 2017;8:45768-45776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Wang J, Yan X, Yang Y, Chang H, Jia B, Zhao XA, Chen G, Xia J, Liu Y, Chen Y, Wang G, Wang L, Zhang Z, Ding W, Huang R, Wu C. A novel predictive model using routinely clinical parameters to predict liver fibrosis in patients with chronic hepatitis B. Oncotarget. 2017;8:59257-59267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Lin CL, Liu CH, Wang CC, Liang CC, Su TH, Liu CJ, Kao JH. Serum Biomarkers Predictive of Significant Fibrosis and Cirrhosis in Chronic Hepatitis B. J Clin Gastroenterol. 2015;49:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Ma XH, Zang X, You Y, Jiang LL, Zhao J, Ayiguli A, Nie ZG. Diagnostic value of apri combined with fib-4 for significant liver fibrosis in patients with chronic hepatitis b. Chin J Gastroenterol. 2017;22:544-547. [DOI] [Full Text] |
| 52. | Cao Z, Li Z, Wang H, Liu Y, Xu Y, Mo R, Ren P, Chen L, Lu J, Li H, Zhuang Y, Liu Y, Wang X, Zhao G, Tang W, Xiang X, Cai W, Liu L, Bao S, Xie Q. Algorithm of Golgi protein 73 and liver stiffness accurately diagnoses significant fibrosis in chronic HBV infection. Liver Int. 2017;37:1612-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Li Y, Cai Q, Zhang Y, Xie Q, Xu N, Jiang X, Li J, Li X, Zhang Z. Development of algorithms based on serum markers and transient elastography for detecting significant fibrosis and cirrhosis in chronic hepatitis B patients: Significant reduction in liver biopsy. Hepatol Res. 2016;46:1367-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Liu DP, Lu W, Zhang ZQ, Wang YB, Ding RR, Zhou XL, Huang D, Li XF. Comparative evaluation of GPR versus APRI and FIB-4 in predicting different levels of liver fibrosis of chronic hepatitis B. J Viral Hepat. 2018;25:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Noguchi R, Kaji K, Namisaki T, Moriya K, Kitade M, Takeda K, Kawaratani H, Okura Y, Aihara Y, Furukawa M, Mitoro A, Yoshiji H. Serum angiotensin-converting enzyme level for evaluating significant fibrosis in chronic hepatitis B. World J Gastroenterol. 2017;23:6705-6714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Ren T, Wang H, Wu R, Niu J. Gamma-Glutamyl Transpeptidase-to-Platelet Ratio Predicts Significant Liver Fibrosis of Chronic Hepatitis B Patients in China. Gastroenterol Res Pract. 2017;2017:7089702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Wu X, Cai B, Su Z, Li Y, Xu J, Deng R, Wang L. Aspartate transaminase to platelet ratio index and gamma-glutamyl transpeptidase-to-platelet ratio outweigh fibrosis index based on four factors and red cell distribution width-platelet ratio in diagnosing liver fibrosis and inflammation in chronic hepatitis B. J Clin Lab Anal. 2018;32:e22341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Yan LB, Zhang QB, Zhu X, He M, Tang H. Serum S100 calcium binding protein A4 improves the diagnostic accuracy of transient elastography for assessing liver fibrosis in hepatitis B. Clin Res Hepatol Gastroenterol. 2018;42:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Zhijian Y, Hui L, Weiming Y, Zhanzhou L, Zhong C, Jinxin Z, Hongyan W, Xiangbin D, Weizhi Y, Duoyun L, Xiaojun L, Qiwen D. Role of the Aspartate Transaminase and Platelet Ratio Index in Assessing Hepatic Fibrosis and Liver Inflammation in Adolescent Patients with HBeAg-Positive Chronic Hepatitis B. Gastroenterol Res Pract. 2015;2015:906026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Bonnard P, Sombié R, Lescure FX, Bougouma A, Guiard-Schmid JB, Poynard T, Calès P, Housset C, Callard P, Le Pendeven C, Drabo J, Carrat F, Pialoux G. Comparison of elastography, serum marker scores, and histology for the assessment of liver fibrosis in hepatitis B virus (HBV)-infected patients in Burkina Faso. Am J Trop Med Hyg. 2010;82:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Lesmana CR, Salim S, Hasan I, Sulaiman AS, Gani RA, Pakasi LS, Lesmana LA, Krisnuhoni E, Budihusodo U. Diagnostic accuracy of transient elastography (FibroScan) versus the aspartate transaminase to platelet ratio index in assessing liver fibrosis in chronic hepatitis B: the role in primary care setting. J Clin Pathol. 2011;64:916-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Liu HB, Zhou JP, Zhang Y, Lv XH, Wang W. Prediction on liver fibrosis using different APRI thresholds when patient age is a categorical marker in patients with chronic hepatitis B. Clin Chim Acta. 2011;412:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Liu XD, Wu JL, Liang J, Zhang T, Sheng QS. Globulin-platelet model predicts minimal fibrosis and cirrhosis in chronic hepatitis B virus infected patients. World J Gastroenterol. 2012;18:2784-2792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Sebastiani G, Castera L, Halfon P, Pol S, Mangia A, Di Marco V, Pirisi M, Voiculescu M, Bourliere M, Alberti A. The impact of liver disease aetiology and the stages of hepatic fibrosis on the performance of non-invasive fibrosis biomarkers: an international study of 2411 cases. Aliment Pharmacol Ther. 2011;34:1202-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 65. | Ucar F, Sezer S, Ginis Z, Ozturk G, Albayrak A, Basar O, Ekiz F, Coban S, Yuksel O, Armutcu F, Akbal E. APRI, the FIB-4 score, and Forn's index have noninvasive diagnostic value for liver fibrosis in patients with chronic hepatitis B. Eur J Gastroenterol Hepatol. 2013;25:1076-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 66. | Wang H, Xue L, Yan R, Zhou Y, Wang MS, Cheng MJ, Huang HJ. Comparison of FIB-4 and APRI in Chinese HBV-infected patients with persistently normal ALT and mildly elevated ALT. J Viral Hepat. 2013;20:e3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Wang Y, Xu MY, Zheng RD, Xian JC, Xu HT, Shi JP, Li SB, Qu Y, Dong YW, Lu LG. Prediction of significant fibrosis and cirrhosis in hepatitis B e-antigen negative patients with chronic hepatitis B using routine parameters. Hepatol Res. 2013;43:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Wu SD, Wang JY, Li L. Staging of liver fibrosis in chronic hepatitis B patients with a composite predictive model: a comparative study. World J Gastroenterol. 2010;16:501-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 69. | Li J, Yu J, Peng XY, Du TT, Wang JJ, Tong J, Lu GL, Wu XW. Acoustic Radiation Force Impulse (ARFI) Elastography and Serological Markers in Assessment of Liver Fibrosis and Free Portal Pressure in Patients with Hepatitis B. Med Sci Monit. 2017;23:3585-3592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Dong CF, Xiao J, Shan LB, Li HY, Xiong YJ, Yang GL, Liu J, Yao SM, Li SX, Le XH, Yuan J, Zhou BP, Tipoe GL, Liu YX. Combined acoustic radiation force impulse, aminotransferase to platelet ratio index and Forns index assessment for hepatic fibrosis grading in hepatitis B. World J Hepatol. 2016;8:616-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 71. | Dong DR, Hao MN, Li C, Peng Z, Liu X, Wang GP, Ma AL. Acoustic radiation force impulse elastography, FibroScan®, Forns' index and their combination in the assessment of liver fibrosis in patients with chronic hepatitis B, and the impact of inflammatory activity and steatosis on these diagnostic methods. Mol Med Rep. 2015;11:4174-4182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Friedrich-Rust M, Buggisch P, de Knegt RJ, Dries V, Shi Y, Matschenz K, Schneider MD, Herrmann E, Petersen J, Schulze F, Zeuzem S, Sarrazin C. Acoustic radiation force impulse imaging for non-invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2013;20:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Zhang D, Chen M, Wang R, Liu Y, Zhang D, Liu L, Zhou G. Comparison of acoustic radiation force impulse imaging and transient elastography for non-invasive assessment of liver fibrosis in patients with chronic hepatitis B. Ultrasound Med Biol. 2015;41:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Ozturker C, Karagoz E, Sivrioglu AK, Kara K. Clinical usefulness and performance of acoustic radiation force impulse in patients with chronic hepatitis B. Eur J Gastroenterol Hepatol. 2017;29:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Park MS, Kim SW, Yoon KT, Kim SU, Park SY, Tak WY, Kweon YO, Cho M, Kim BK, Park JY, Kim DY, Ahn SH, Han KH. Factors Influencing the Diagnostic Accuracy of Acoustic Radiation Force Impulse Elastography in Patients with Chronic Hepatitis B. Gut Liver. 2016;10:275-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Liu J, Ji Y, Ai H, Ning B, Zhao J, Zhang Y, Dun G. Liver Shear-Wave Velocity and Serum Fibrosis Markers to Diagnose Hepatic Fibrosis in Patients with Chronic Viral Hepatitis B. Korean J Radiol. 2016;17:396-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Liu F, Wei L, Wang S, Huang B. Comparison of FibroTouch and acoustic radiation force impulse in diagnosis of liver fibrosis in patients with chronic hepatitis B. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016;45:416-421. [PubMed] |
| 78. | Coskun BD, Altinkaya E, Sevinc E, Ozen M, Karaman H, Karaman A, Poyrazoglu O. The diagnostic value of a globulin/platelet model for evaluating liver fibrosis in chronic hepatitis B patients. Rev Esp Enferm Dig. 2015;107:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Zhang YF, Shi H, Chen LB, Xu QH. [Value of FIB-4 for the diagnosis of liver fibrosis in chronic hepatitis B]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2010;24:215-217. [PubMed] |
| 80. | Zhao J, Zhai F, Cheng J, He Q, Luo J, Yang X, Shao J, Xing H. Evaluating the Significance of Viscoelasticity in Diagnosing Early-Stage Liver Fibrosis with Transient Elastography. PLoS One. 2017;12:e0170073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Zeng X, Xu C, He D, Li M, Zhang H, Wu Q, Xiang D, Wang Y. Performance of several simple, noninvasive models for assessing significant liver fibrosis in patients with chronic hepatitis B. Croat Med J. 2015;56:272-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, Chu SH, Chan AW, Choi PC, Ahuja AT, Chan HL, Chu WC. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 83. | Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P; FIBROSTIC study group. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 84. | Cho HJ, Seo YS, Lee KG, Hyun JJ, An H, Keum B, Kim JH, Yim HJ, Jeen YT, Lee HS, Chun HJ, Um SH, Kim CD, Ryu HS. Serum aminotransferase levels instead of etiology affects the accuracy of transient elastography in chronic viral hepatitis patients. J Gastroenterol Hepatol. 2011;26:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Kim BK, Kim HS, Park JY, Kim DY, Ahn SH, Chon CY, Park YN, Han KH, Kim SU. Prospective validation of ELF test in comparison with Fibroscan and FibroTest to predict liver fibrosis in Asian subjects with chronic hepatitis B. PLoS One. 2012;7:e41964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Huang R, Jiang N, Yang R, Geng X, Lin J, Xu G, Liu D, Chen J, Zhou G, Wang S, Luo T, Wu J, Liu X, Xu K, Yang X. Fibroscan improves the diagnosis sensitivity of liver fibrosis in patients with chronic hepatitis B. Exp Ther Med. 2016;11:1673-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Kumar M, Rastogi A, Singh T, Bihari C, Gupta E, Sharma P, Garg H, Kumar R, Bhatia V, Tyagi P, Sarin SK. Analysis of discordance between transient elastography and liver biopsy for assessing liver fibrosis in chronic hepatitis B virus infection. Hepatol Int. 2013;7:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Kongtawelert P, Chanmee T, Pothaeharoen P, Wisedopa N, Kranokpiruk P, Poovorawan K, Poovorawan Y, Tangkijvanich P. Diagnostic accuracy of liver stiffness measurement and serum hyaluronic acid for detecting liver fibrosis in chronic hepatitis B with respect to alt levels. Asian Biomed. 2013;7:609-617. |
| 89. | Kim BK, Kim SU, Kim HS, Park JY, Ahn SH, Chon CY, Cho IR, Joh DH, Park YN, Han KH, Kim DY. Prospective validation of FibroTest in comparison with liver stiffness for predicting liver fibrosis in Asian subjects with chronic hepatitis B. PLoS One. 2012;7:e35825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Goyal R, Mallick SR, Mahanta M, Kedia S, Shalimar, Dhingra R, Sharma H, Das P, Datta Gupta S, Panda S, Acharya SK. Fibroscan can avoid liver biopsy in Indian patients with chronic hepatitis B. J Gastroenterol Hepatol. 2013;28:1738-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Cardoso AC, Carvalho-Filho RJ, Stern C, Dipumpo A, Giuily N, Ripault MP, Asselah T, Boyer N, Lada O, Castelnau C, Martinot-Peignoux M, Valla DC, Bedossa P, Marcellin P. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012;32:612-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 92. | Gaia S, Carenzi S, Barilli AL, Bugianesi E, Smedile A, Brunello F, Marzano A, Rizzetto M. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011;54:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 93. | Fung J, Lai CL, Chan SC, But D, Seto WK, Cheng C, Wong DK, Lo CM, Fan ST, Yuen MF. Correlation of liver stiffness and histological features in healthy persons and in patients with occult hepatitis B, chronic active hepatitis B, or hepatitis B cirrhosis. Am J Gastroenterol. 2010;105:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Lee HJ, Seo YS, Kim DJ, Kang HS, An H, Kim JH, Cheong JY, Yim HJ, Yeon JE, Lee HS, Byun KS, Cho SW, Kim DJ, Um SH, Kim CD, Ryu HS. Application of the HALF index obviates the need for liver biopsy in half of all patients with chronic hepatitis B. J Gastroenterol Hepatol. 2011;26:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Myers RP, Elkashab M, Ma M, Crotty P, Pomier-Layrargues G. Transient elastography for the noninvasive assessment of liver fibrosis: a multicentre Canadian study. Can J Gastroenterol. 2010;24:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Sporea I, Sirli R, Deleanu A, Tudora A, Popescu A, Curescu M, Bota S. Liver stiffness measurements in patients with HBV vs HCV chronic hepatitis: a comparative study. World J Gastroenterol. 2010;16:4832-4837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 98. | Dong H, Xu C, Zhou W, Liao Y, Cao J, Li Z, Hu B. The combination of 5 serum markers compared to FibroScan to predict significant liver fibrosis in patients with chronic hepatitis B virus. Clin Chim Acta. 2018;483:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 99. | Hennedige TP, Wang G, Leung FP, Alsaif HS, Teo LL, Lim SG, Wee A, Venkatesh SK. Magnetic Resonance Elastography and Diffusion Weighted Imaging in the Evaluation of Hepatic Fibrosis in Chronic Hepatitis B. Gut Liver. 2017;11:401-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Lee JE, Lee JM, Lee KB, Yoon JH, Shin CI, Han JK, Choi BI. Noninvasive assessment of hepatic fibrosis in patients with chronic hepatitis B viral infection using magnetic resonance elastography. Korean J Radiol. 2014;15:210-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Wu WP, Chou CT, Chen RC, Lee CW, Lee KW, Wu HK. Non-Invasive Evaluation of Hepatic Fibrosis: The Diagnostic Performance of Magnetic Resonance Elastography in Patients with Viral Hepatitis B or C. PLoS One. 2015;10:e0140068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 102. | Venkatesh SK, Wang G, Lim SG, Wee A. Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur Radiol. 2014;24:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 103. | Shi Y, Guo Q, Xia F, Dzyubak B, Glaser KJ, Li Q, Li J, Ehman RL. MR elastography for the assessment of hepatic fibrosis in patients with chronic hepatitis B infection: does histologic necroinflammation influence the measurement of hepatic stiffness? Radiology. 2014;273:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 104. | Chang W, Lee JM, Yoon JH, Han JK, Choi BI, Yoon JH, Lee KB, Lee KW, Yi NJ, Suh KS. Liver Fibrosis Staging with MR Elastography: Comparison of Diagnostic Performance between Patients with Chronic Hepatitis B and Those with Other Etiologic Causes. Radiology. 2016;280:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 105. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 106. | World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015; Available from: https://www.who.int/hiv/pub/hepatitis/hepatitisb-guidelines/en/. |
| 107. | Kim BK, Kim DY, Park JY, Ahn SH, Chon CY, Kim JK, Paik YH, Lee KS, Park YN, Han KH. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 108. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S, Herrmann E. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 109. | Sporea I, Sirli R, Popescu A, Danilă M. Acoustic Radiation Force Impulse (ARFI)--a new modality for the evaluation of liver fibrosis. Med Ultrason. 2010;12:26-31. [PubMed] |
| 110. | Lockhart M, Moore JW. Classical differential and operant conditioning in rabbits (Oryctolagus cuniculus) with septal lesions. J Comp Physiol Psychol. 1975;88:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 896] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 111. | Xu X, Su Y, Song R, Sheng Y, Ai W, Wu X, Liu H. Performance of transient elastography assessing fibrosis of single hepatitis B virus infection: a systematic review and meta-analysis of a diagnostic test. Hepatol Int. 2015;9:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 112. | Xu XY, Kong H, Song RX, Zhai YH, Wu XF, Ai WS, Liu HB. The effectiveness of noninvasive biomarkers to predict hepatitis B-related significant fibrosis and cirrhosis: a systematic review and meta-analysis of diagnostic test accuracy. PLoS One. 2014;9:e100182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |