Published online Jul 26, 2019. doi: 10.12998/wjcc.v7.i14.1775
Peer-review started: March 20, 2019
First decision: May 7, 2019
Revised: June 17, 2019
Accepted: July 2, 2019
Article in press: July 3, 2019
Published online: July 26, 2019
Processing time: 128 Days and 14.1 Hours
Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) is a useful procedure that enables reliable pathological diagnoses of pancreatobiliary diseases, subepithelial lesions, and swollen lymph nodes. In recent years, a pathological diagnosis based on EUS-FNA has made it possible to provide accurate treatment methods not only in these fields, but also in respiratory organs and otorhinolaryngology. This review discusses the latest topics pertaining to EUS-FNA as well as procedural tips.
Core tip: In the era of cyto-pathological diagnosis of various malignant diseases, endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) represents the most promising procedure for diagnosing various malignant diseases. However, to date, no reports have compared the utility, faults, and techniques of this procedure. In this review we highlight the recent topics and technical tips of EUS-FNA in the diagnostic process of various diseases, especially those which require tissue-based diagnosis to determine treatment.
- Citation: Matsumoto K, Takeda Y, Onoyama T, Kawata S, Kurumi H, Koda H, Yamashita T, Isomoto H. Endoscopic ultrasound-guided fine-needle aspiration biopsy - Recent topics and technical tips. World J Clin Cases 2019; 7(14): 1775-1783
- URL: https://www.wjgnet.com/2307-8960/full/v7/i14/1775.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i14.1775
In patients with difficult to reach lesions, where no histo-cytological tissue is obtainable, diagnosis has conventionally been determined using imaging techniques. Endoscopic ultrasonography (EUS) is a widely accepted modality for detecting pancreatobiliary diseases and, for visualizing lesions more precisely than other imaging modalities.
EUS has two different shaped scopes, radial and longitudinal. The radial EUS has a viewing angle of 360 degrees, so the positional relationship with surrounding organs can be easily understood. On the other hand, the longitudinal EUS has the advantage that the relationship between the lesion and the blood vessel can be easily grasped since the blood vessel is easily matched with the axis of the scope and endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) can be carried out. Following a basic investigation by Harada et al[1] in 1991 using dogs, EUS-FNA was first clinically applied to subepithelial lesions (SEL) of the stomach[2], followed by use in cases with pancreatic cancer, resulting in qualified pathological diagnoses. With its usefulness confirmed, EUS-FNA is currently used worldwide before determining a treatment strategy for various diseases[3].
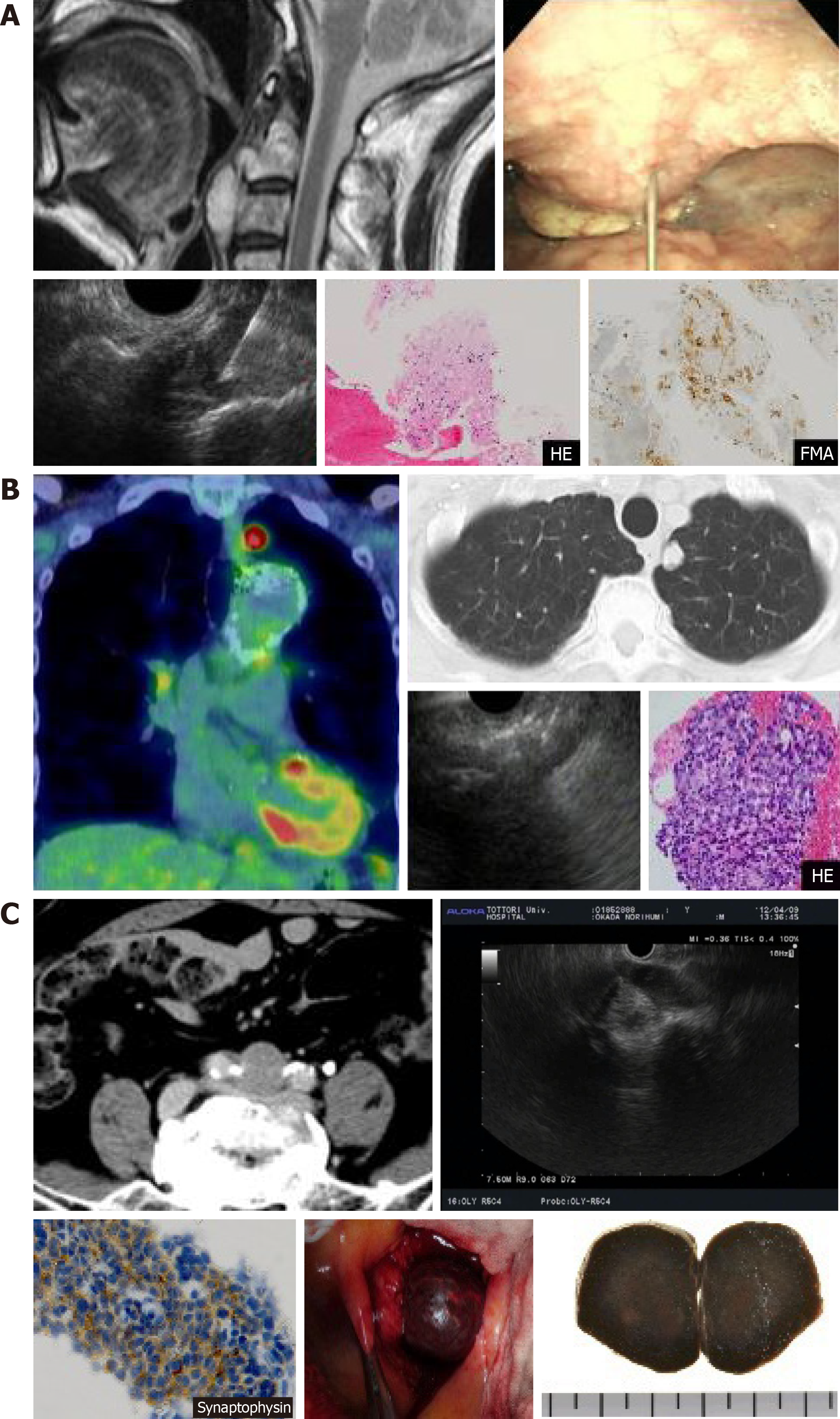
We have also selected treatments based on a pathological diagnosis using EUS-FNA for diseases in the gastrointestinal area, mainly pancreatobiliary disease, but also cervical spine chordoma, adenocarcinoma of the lung, and metastasis of liver neuroendocrine tumors to lymph nodes of the bifurcation of the common iliac artery (Figure 1 A-C). Currently, there are many different puncture needles available on the market that improve lesion accessibility and puncture performance, and devices have been developed that even beginners can use (Table 1).
| Release year1 | Endoscopic ultrasound-guided fine-needle aspiration biopsy needles |
| 2000 | Echo Tip Ultra (Cook) |
| 2001 | NA-11J-KB (OLYMPUS) |
| 2003 | EZ Shot (OLYMPUS) |
| 2004 | Quick-Core (Cook) |
| 2011 | Expect (Boston) |
| 2011 | EZ Shot (OLYMPUS) |
| 2012 | Echo Tip Procore (Cook) |
| 2012 | SONO tip Pro Control (Medi-Globe) |
| 2012 | Expect 19 G Flex Needle (Boston) |
| 2013 | EUS Sonopsy CY (HAKKO) |
| 2016 | EZ Shot 3 Plus (OLYMPUS) |
| 2016 | Echo Tip Procore 20 G (Cook) |
| 2016 | Acquire (Boston) |
However, according to the (first and second) “Survey on actual condition of pancreatic tumor diagnosis in Tottori Prefecture”[4], the proportion of cases that have been diagnosed with unresectable progressive pancreatic cancer and were undergoing chemotherapy where pathological evidence was acquired by EUS-FNA was 65% in the first survey (2009-2011, n = 272), but failed to improve in the second survey at 59% (2012-2014, n = 339). The number of facilities in Japan where EUS-FNA is performed is increasing, but remains at only about 1/6 when compared to facilities performing endoscopic retrograde cholangiopancreatography (ERCP). Facilities should be more proactive and include EUS-FNA to ensure treatments are more suitable for diseases.
Many prospective studies and meta-analyses that have evaluated the selection of a procedure or device for EUS-FNA have assessed factors that affect diagnostic power (Table 2). More specifically, it has been reported that regarding scopes, a cap-attached forward-viewing echoendoscope is useful for EUS-FNA of small SEL[5]. Regarding needle diameter and the stylet, the presence or absence of a stylet has no impact on the diagnostic power of EUS-FNA[6]; where no stylet is present, a 22-G needle and 25-G needle have equivalent diagnostic power[7]. A meta-analysis showed that 25-G needles have significantly better sensitivity for pancreatic tumors than 22-G needles[8] and 19-G needles have a significantly better correct diagnostic rate for pancreatic tumors than 22-G needles[9]. In terms of the shape of the needle tip, one meta-analysis has reportedly shown that the number of punctures is reduced using puncture needles with a side hole[10] and that EUS-guided through-the-needle forceps biopsy is useful[11]. With regard to the method of aspiration, there are some scattered reports on pancreatic tumors, where wet suction[12] and a high negative pressure provided improved cellularity compared to the typical methods[13]. It has also been reported that there is no difference between the stylet slow-pull and standard suction in diagnostic power[14]. Reports on puncture methods have stated that fanning lowers the number of punctures[15], and that the door-knock technique yields improved cellularity in transgastric punctures when compared to the typical methods[16]. Regarding post-puncture treatment, it has also been reported in a meta-analysis that rapid on-site evaluation (ROSE) was useful[17]. In addition, EUS-FNA combined with ROSE and fine-needle biopsy have equivalent diagnostic power[18], in which macroscopic on-site quality evaluation (MOSE) is useful[19]. Cellvizio[20] and TSCI[21] are also reportedly useful devices to assist with post-puncture treatment.
| Factors influencing diagnostic ability | Result |
| Scope | A forward-viewing echoendoscope is useful[5] |
| Stylet | The presence or absence of a stylet has no impact on the diagnostic power of EUS-FNA[6] |
| Needle diameter | A 22-G needle and 25-G needle have equivalent diagnostic power[7]. A 25-G needle has significantly better sensitivity for pancreatic tumors than a 22-G needle[8] A 19-G needle has a significantly better correct diagnostic rate for pancreatic tumors than a 22-G needle[9] |
| The shape of the needle tip | The number of punctures is reduced using puncture needles with a side hole[10] EUS-guided through-the-needle forceps biopsy is useful[11] |
| Suction | Wet suction[12] and a high negative pressure provided improved cellularity compared to the typical methods[13]. There is no difference between the stylet slow-pull and standard suction in diagnostic power[14] |
| Puncture | Fanning lowers the number of punctures[15] The door-knock technique improved cellularity in transgastric punctures when compared to the typical methods[16] |
| Post puncture-treatment | FNA combined with ROSE and fine-needle biopsy have equivalent diagnostic power[17] Macroscopic on-site quality evaluation is useful[18] The ROSE was useful[19] Cellvizio[20] and TSCI[21] are useful devices to assist with post-puncture treatment |
More widespread use of EUS-FNA in pathological diagnoses will require establishing simpler techniques that are easy, even for doctors with little experience with such cases, based on the factors that affect the diagnostic power of EUS-FNA.
EUS-FNA is fundamentally indicated for all diseases where collecting cells from the lesion makes it possible to determine a treatment strategy. Specific examples include histological evidence of cancer when chemotherapy or chemoradiotherapy is being selected, differential diagnosis of benignancy/malignancy (selecting surgery/non-surgery, selecting a surgical procedure, determining whether or not follow-up observation is possible for disease where differentiating between benignancy/ malignancy is challenging), and accurate diagnosis of the degree of progression of malignant tumors (lymph node metastasis, low volume of ascites). Initially, pancreatic lesions, lymph nodes, and SEL were considered to be covered, but recently biliary tract disease, lung tumors, head and neck tumors, and gastrointestinal lesions where biopsy using a conventional endoscope does not yield a diagnosis have also been included. Lesions 10 mm in size or less have previously been regarded as posing a challenge for sample collection, but improvements in puncture needle visibility, puncture performance, and sample collection ability have recently brought about diagnostic power with a sensitivity of 89.3% and a correct diagnosis rate of 91.7% for pancreatic tumors, where the lesion is smaller than 10 mm[22].
Procedural adverse events with EUS-FNA include abdominal pain, bleeding, dissemination, pancreatitis, and infectious disease[23]. Piriform sinus injuries caused by scope insertion in the pre-stage of EUS-FNA occur at a frequency of 0.06% (4/4894)[24], as does digestive tract perforation, at 0.02% (2/10941)[25,26]. Preventing piriform sinus injury requires careful, gentle insertion with observation inside the mouth. Gastrointestinal tract perforation is often found to occur mainly during insertion into the descending part of the duodenum, and insertion should be performed while the gastrointestinal lumen is being checked to prevent complications.
EUS-FNA is contraindicated if bleeding diathesis is observed, EUS fails to clearly render the lesion, or there is a strong risk of EUS-FNA causing a procedural accident. With regard to dissemination due to EUS-FNA, there is believed to be a risk of dissemination even with solid tumors, such as invasive ductal carcinoma of the pancreas; this, though possible, does not affect the survival rate[27]. With solid pancreatic tumors, invasive ductal carcinoma of the pancreas has a frequency of less than 80%[23], and thus the benefit of acquiring pathological evidence before deciding on a treatment strategy outweighs the risk of dissemination. Countries across the world vary significantly regarding whether EUS-FNA is indicated for pancreatic cystic lesions such as intraductal papillary mucinous neoplasms (IPMN) or mucinous cystic neoplasms[28]. One report on the usefulness of EUS-FNA for pancreatic cyst lesions included an analysis of cells obtained in a facility with ample experience with EUS-FNA, and diagnosis by cytology yielded diagnostic value for cases with relatively small BD-IPMN where there were no “worrisome features”. Another study where diagnosis of high-grade epithelial atypia or high-grade dysplasia of cells in the mucinous cystic fluid had a sensitivity of 72% and a positive predictive value of 80%, 30% more cancers were detected in small branch duct-IPMN cases than “worrisome features”[28]. Reported complications for pancreatic cysts include intracystic hemorrhage[29] and dissemination. With regard to dissemination in particular, one report[30] recommends not performing EUS-FNA on cysts because of a “high-risk stigmata” or “worrisome features”, for fear that the puncture could cause cystic fluid to leak out, resulting in peritoneal dissemination, or could allow cancer to invade the gastric wall at the puncture route. However, another report[31] has indicated that preoperative EUS-FNA performed on patients with IPMN was not linked to any increase in dissemination. Thus, cytological analysis by EUS-FNA of pancreatic cyst lesions should only be performed at facilities with plenty of experience, and increasing the use of this method will require accumulating data pertaining to diagnostic power and safety.
EUS-FNA is absolutely contraindicated if there is a high risk of a procedural accident. Specifically, in the presence of significant respiratory fluctuations, where the puncture needle could cause organ damage, or blood vessels clearly present on the puncture line[32]. Here, we describe cases of difficult EUS-FNA that we have experienced, where an innovative technique enabled us to ensure the puncture route and perform EUS-FNA to reach a pathological diagnosis.
The “abdominal compression” method, where respiratory fluctuations are limited by manual compression of the abdomen.
Light manual compression of the upper abdomen in cases with significant respiratory fluctuations restricts the breadth of the respiratory fluctuations, which makes puncturing easier. However, excessive compression of the abdomen creates an oppressive suffocating sensation that temporarily elevates the patient’s breathing, which may cause the position of the needle to fluctuate, requiring careful attention.
The “pull-out” method to prevent punctures in the main pancreatic duct.
It is difficult to ensure the normal transgastric puncture route in cases where invasive ductal carcinoma of the pancreas produces expansion/meandering of the main pancreatic duct (Figure 2 A). However, scanning after the scope has been pulled out from the duodenum makes it possible to ensure a safe puncture route to the lesion while still avoiding the main pancreatic duct (Figure 2 B). Scope manipulation to ensure the puncture route may be useful in some cases, but even minute fluctuations in the position of the scope may create an offset in ultrasound images, and thus, a scope operation requires meticulous attention.
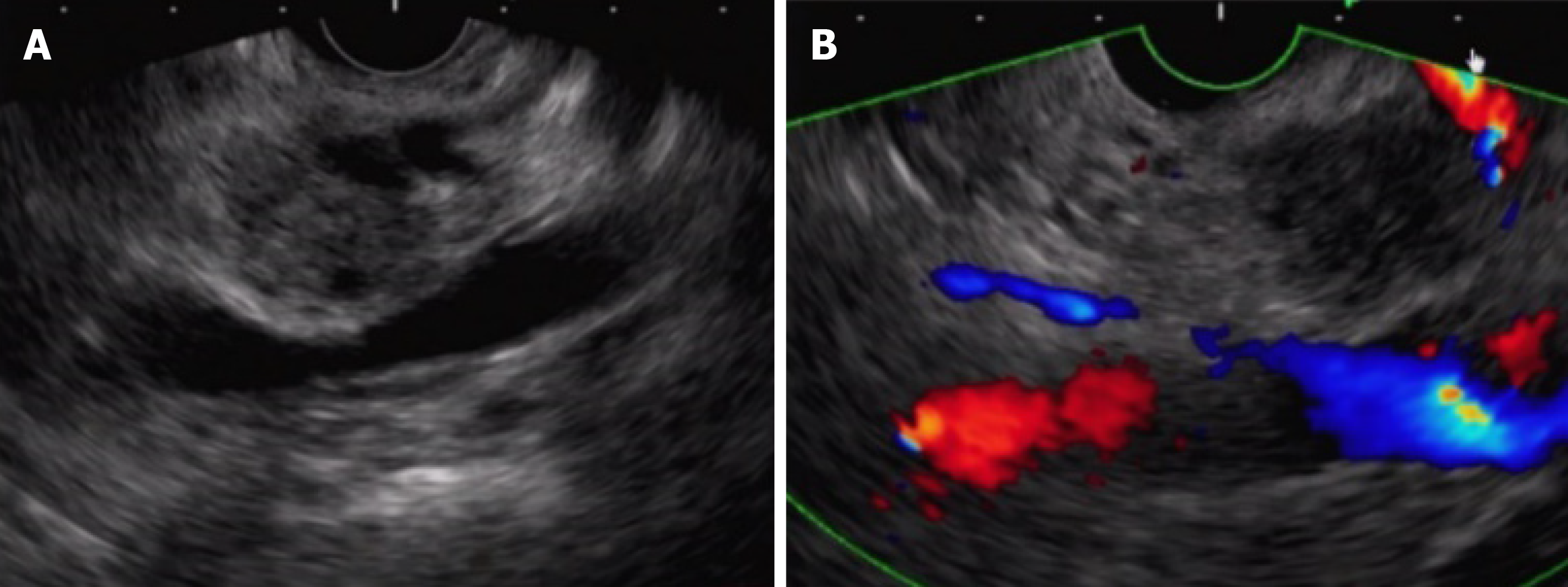
The “blood vessel push-aside” method to ensure the puncture route while also displacing blood vessels around the lesion.
In a case with distal cholangiocarcinoma, a puncture by EUS-FNA appeared to be difficult due to the presence of several blood vessels around the lesion (Figure 3 A). Lifting the raising base and applying an up-angle while also pushing the puncture needle up against the far side of the blood vessels (left side as seen in the EUS image), made it possible to push the blood vessels aside to follow the puncture route (Figure 3 B-D). Tissue could be collected by the door-knocking method, with attention being paid to the portal vein located deep in the lesion.
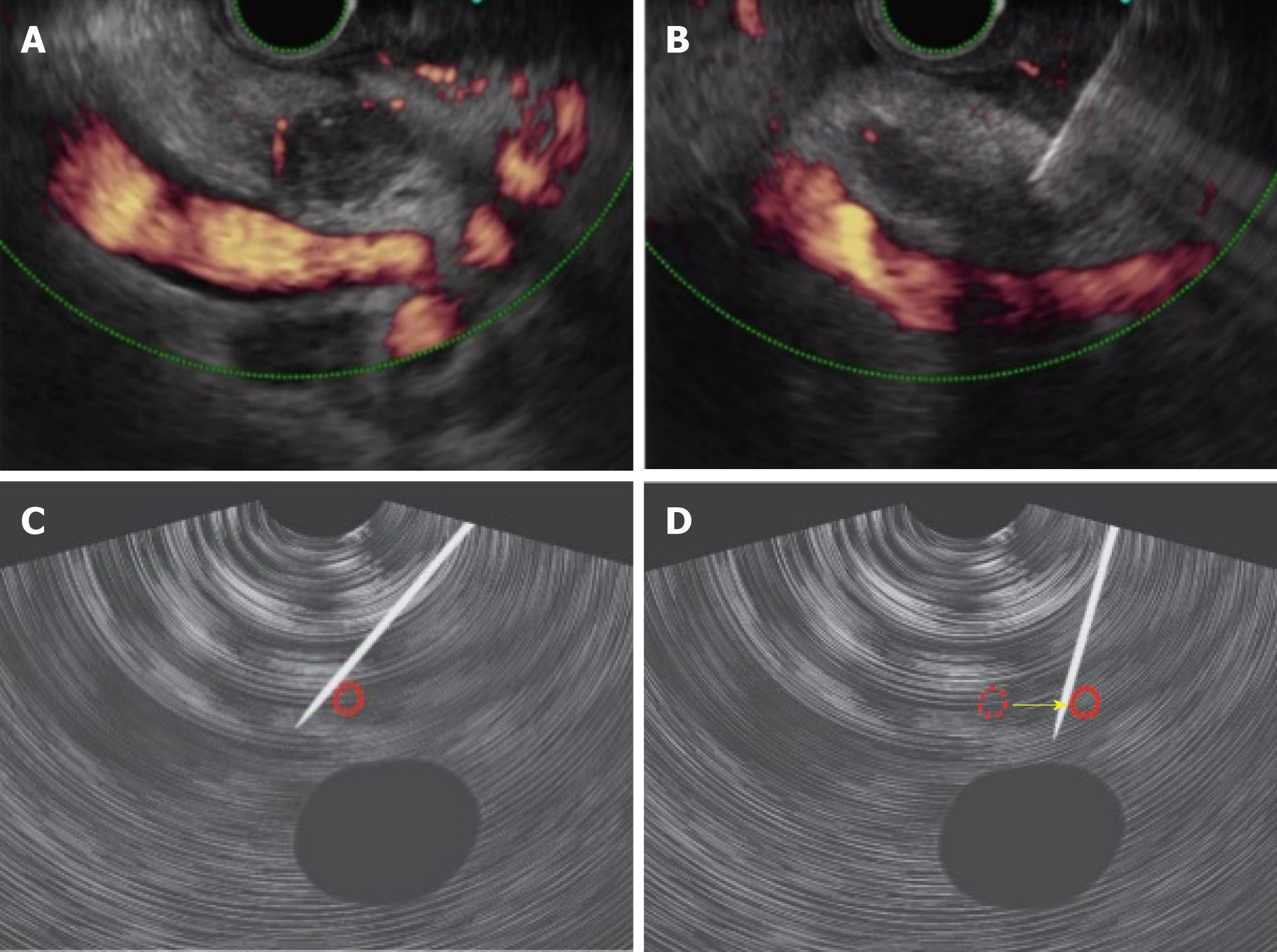
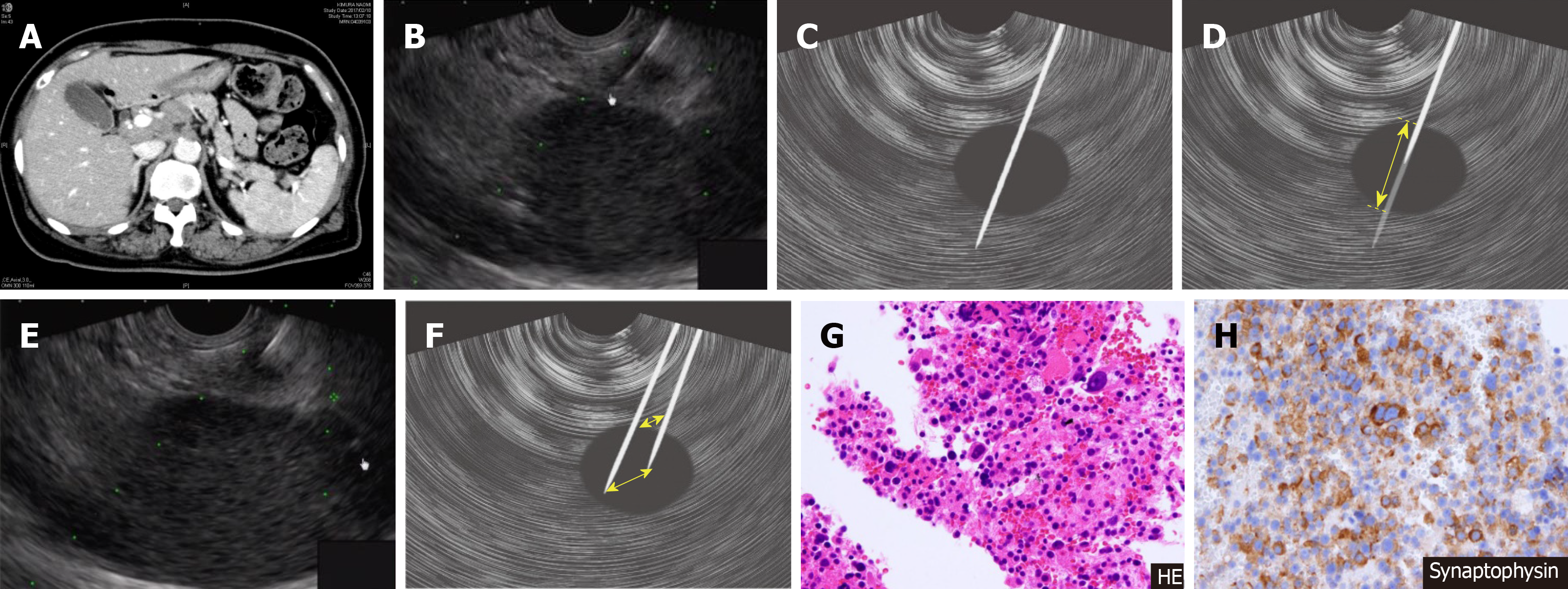
“Skewering + respiratory fluctuations” making the puncture with a puncture needle and respiratory fluctuations.
In this case with a mass measuring 7 mm in the tail of the pancreas (Figure 4 A and B), a skewering method (Figure 4 C and D) was applied, but it was difficult to ensure the stroke width because of the adjacent location of the kidneys. There were also significant respiratory fluctuations. With the needle tip retained at the same position after puncture of the mass, respiratory fluctuations were prevented via fanning, enabling extensive tissue collection (Figure 4 E and F). In this case, sample tissue was also collected for immunohistological staining even though the needle was not stroked, yielding a diagnosis of neuroendocrine tumor of the pancreas (Figure 4 G and H).
Previous reports state that EUS-FNA has a sensitivity of approximately 85%-89% for pancreatic disease[23], 25%-100% for biliary duct disease[33-36], and a diagnostic power of 85.7% to 86.0% for SEL[37,38]. Techniques that are reportedly useful for supplementing this diagnostic power include pancreatic juice cytology for pancreatic disease[39-41], transpapillary bile duct biopsy, and bile cytology for biliary tract disease[42-44], and EUS-FNA with a forward-viewing linear echoendoscope for SEL[45], as well as endoscopic submucosal dissection and endoscopic snare resection[46-49].
Although the number of facilities practicing EUS-FNA has been on the rise in recent years, some facilities may still perceive hurdles in implementing EUS-FNA, perhaps due to the impression that the procedure is difficult. EUS-FNA provides treatment choices based on pathological diagnosis not only in the gastrointestinal area but also in many more areas. This is a technique where diagnostic power improves by simple solutions for the puncture method or the specimen treatment method after puncturing, and a greater number of facilities should be more proactive in performing EUS-FNA in the future.
Manuscript source: Invited Manuscript
Manuscript source: Invited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: de Moura DTH, Grillo F, Massironi S S-Editor: Cui LJ L-Editor: Webster JR E-Editor: Wu YXJ
| 1. | Harada N, Kouzu T, Ohshima I, Ichinose M, Arima M, Hishikawa E, Ishijima H, Sakuma Y, Tanaka H, Muraoka M, Miyazaki S, Ninomiya E, Isono K. A trial of endoscopic ultrasound-guided puncture technique. Gastroenterol Endosc. 1991;33:1657-1663. |
| 2. | Caletti GC, Brocchi E, Ferrari A, Bonora G, Santini D, Mazzoleni G, Barbara L. Guillotine needle biopsy as a supplement to endosonography in the diagnosis of gastric submucosal tumors. Endoscopy. 1991;23:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 410] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 4. | Matsumoto K, Harada K, Onoyama T, Kawata S, Murawaki Y, Tanaka H, Tanaka K, Miura M, Kanbe T, Takeda Y. Survey of pancreatic tumor treatment in San-in resion. J. Tottori med. Ass. 2015;43:90-93. |
| 5. | Kida M, Kawaguchi Y, Miyata E, Hasegawa R, Kaneko T, Yamauchi H, Koizumi S, Okuwaki K, Miyazawa S, Iwai T, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Endoscopic ultrasonography diagnosis of subepithelial lesions. Dig Endosc. 2017;29:431-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Abe Y, Kawakami H, Oba K, Hayashi T, Yasuda I, Mukai T, Isayama H, Ishiwatari H, Doi S, Nakashima M, Yamamoto N, Kuwatani M, Mitsuhashi T, Hasegawa T, Hirose Y, Yamada T, Tanaka M, Sakamoto N; Japan EUS-FNA Stylet Study Group. Effect of a stylet on a histological specimen in EUS-guided fine-needle tissue acquisition by using 22-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2015;82:837-844.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Gimeno-García AZ, Elwassief A, Paquin SC, Gariépy G, Sahai AV. Randomized controlled trial comparing stylet-free endoscopic ultrasound-guided fine-needle aspiration with 22-G and 25-G needles. Dig Endosc. 2014;26:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Madhoun MF, Wani SB, Rastogi A, Early D, Gaddam S, Tierney WM, Maple JT. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy. 2013;45:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Song TJ, Kim JH, Lee SS, Eum JB, Moon SH, Park DY, Seo DW, Lee SK, Jang SJ, Yun SC, Kim MH. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Nakai Y, Isayama H, Chang KJ, Yamamoto N, Mizuno S, Mohri D, Kogure H, Matsubara S, Tada M, Koike K. A pilot study of EUS-guided through-the-needle forceps biopsy (with video). Gastrointest Endosc. 2016;84:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Villa NA, Berzosa M, Wallace MB, Raijman I. Endoscopic ultrasound-guided fine needle aspiration: The wet suction technique. Endosc Ultrasound. 2016;5:17-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Kudo T, Kawakami H, Hayashi T, Yasuda I, Mukai T, Inoue H, Katanuma A, Kawakubo K, Ishiwatari H, Doi S, Yamada R, Maguchi H, Isayama H, Mitsuhashi T, Sakamoto N; Japan EUS-FNA Negative Pressure Suction Study Group. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030-1037.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Saxena P, El Zein M, Stevens T, Abdelgelil A, Besharati S, Messallam A, Kumbhari V, Azola A, Brainard J, Shin EJ, Lennon AM, Canto MI, Singh VK, Khashab MA. Stylet slow-pull versus standard suction for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic lesions: a multicenter randomized trial. Endoscopy. 2018;50:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Mukai S, Itoi T, Ashida R, Tsuchiya T, Ikeuchi N, Kamada K, Tanaka R, Umeda J, Tonozuka R, Fukutake N, Hoshi K, Moriyasu F, Gotoda T, Irisawa A. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for EUS-FNA of solid pancreatic masses (with videos). Gastrointest Endosc. 2016;83:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, Eltoum IA. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 18. | Khan MA, Grimm IS, Ali B, Nollan R, Tombazzi C, Ismail MK, Baron TH. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363-E375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 19. | Iwashita T, Yasuda I, Mukai T, Doi S, Nakashima M, Uemura S, Mabuchi M, Shimizu M, Hatano Y, Hara A, Moriwaki H. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: a single-center prospective pilot study (MOSE study). Gastrointest Endosc. 2015;81:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 20. | Kadayifci A, Atar M, Basar O, Forcione DG, Brugge WR. Needle-Based Confocal Laser Endomicroscopy for Evaluation of Cystic Neoplasms of the Pancreas. Dig Dis Sci. 2017;62:1346-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Matsumoto K, Ueki M, Takeda Y, Harada K, Onoyama T, Kawata S, Ikebuchi Y, Imamoto R, Horie Y, Murawaki Y. Development of a device for detecting target specimens from EUS-guided FNA samples. Endosc Int Open. 2015;3:E662-E664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Sugiura R, Kuwatani M, Hirata K, Sano I, Kato S, Kawakubo K, Sakamoto N. Effect of Pancreatic Mass Size on Clinical Outcomes of Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Dig Dis Sci. 2019;64:2006-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Matsumoto K, Takeda Y, Onoyama T, Kawata S, Kurumi H, Ueki M, Miura N, Isomoto H. Role of the preoperative usefulness of the pathological diagnosis of pancreatic diseases. World J Gastrointest Oncol. 2016;8:656-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Eloubeidi MA, Tamhane A, Lopes TL, Morgan DE, Cerfolio RJ. Cervical esophageal perforations at the time of endoscopic ultrasound: a prospective evaluation of frequency, outcomes, and patient management. Am J Gastroenterol. 2009;104:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 26. | ASGE Standards of Practice Committee; Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf RN, Shergill AK, Cash BD. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 27. | Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1150] [Article Influence: 143.8] [Reference Citation Analysis (1)] |
| 29. | Bang JY, Varadarajulu S. Management of acute hemorrhagic pancreatitis after EUS-guided FNA of a pancreatic cyst. Gastrointest Endosc. 2016;83:1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, Okada N, Itoh T, Kawashima H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Yoon WJ, Daglilar ES, Fernández-del Castillo C, Mino-Kenudson M, Pitman MB, Brugge WR. Peritoneal seeding in intraductal papillary mucinous neoplasm of the pancreas patients who underwent endoscopic ultrasound-guided fine-needle aspiration: the PIPE Study. Endoscopy. 2014;46:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Yamao K, Sawaki A, Mizuno N, Takahashi K, Nakamura T, Tajika M, Kawai H, Isaka T, Imaoka M, Okamoto Y, Inoue H, Aoki M. Can we change the choice of treatment for gastrointestinal diseases by EUS-FNAB? Endoscopia Digestiva. 2004;16:1242-1246. |
| 33. | Weilert F, Bhat YM, Binmoeller KF, Kane S, Jaffee IM, Shaw RE, Cameron R, Hashimoto Y, Shah JN. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | Ohshima Y, Yasuda I, Kawakami H, Kuwatani M, Mukai T, Iwashita T, Doi S, Nakashima M, Hirose Y, Asaka M, Moriwaki H. EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J Gastroenterol. 2011;46:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Rösch T, Hofrichter K, Frimberger E, Meining A, Born P, Weigert N, Allescher HD, Classen M, Barbur M, Schenck U, Werner M. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Onda S, Ogura T, Kurisu Y, Masuda D, Sano T, Takagi W, Fukunishi S, Higuchi K. EUS-guided FNA for biliary disease as first-line modality to obtain histological evidence. Therap Adv Gastroenterol. 2016;9:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Lee JH, Cho CJ, Park YS. EUS-guided 22-guagefine needle biopsy for the diagnosis of gastric subepithelial tumors larger than 2 cm. Scand J Gastroenterol. 2016;51:485–92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Lee M, Min BH, Lee H. Feasibility and diagnostic yield of endoscopic ultrasonography-guided fine needle biopsy with a new core biopsy needle device in patients with gastric subepithelial tumors. Medicine. 2015;94:1–7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Matsumoto K, Takeda Y, Harada K, Horie Y, Yashima K, Murawaki Y. Effect of pancreatic juice cytology and/or endoscopic ultrasound-guided fine-needle aspiration biopsy for pancreatic tumor. J Gastroenterol Hepatol. 2014;29:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Matsumoto K, Takeda Y, Harada K, Onoyama T, Kawata S, Horie Y, Sakamoto T, Ueki M, Miura N, Murawaki Y. Clinical Impact of the KL-6 Concentration of Pancreatic Juice for Diagnosing Pancreatic Masses. Biomed Res Int. 2015;2015:528304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Takeda Y, Matsumoto K, Kurumi H, Koda H, Yamashita T, Onoyama T, Kawata S, Horie Y, Isomoto H. Efficacy and safety of pancreatic juice cytology by using synthetic secretin in the diagnosis of pancreatic ductal adenocarcinoma. Dig Endosc. 2018;30:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res. 2013;184:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Navaneenthan U, Njei B, Lourdusamy V, Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo J, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 44. | Onoyama T, Matsumoto K, Koda H, Yamashita T, Kurumi H, Kawata S, Takeda Y, Harada K, Yashima K, Isomoto H. Diagnostic usefulness of KL-6 concentration of bile in biliary tract cancer. Mol Clin Oncol. 2018;8:567–570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Larghi A, Fuccio L, Chiarello G, Attili F, Vanella G, Paliani GB, Napoleone M, Rindi G, Larocca LM, Costamagna G, Ricci R. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy. 2014;46:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | de la Serna-Higuera C, Pérez-Miranda M, Díez-Redondo P, Gil-Simón P, Herranz T, Pérez-Martín E, Ochoa C, Caro-Patón A. EUS-guided single-incision needle-knife biopsy: description and results of a new method for tissue sampling of subepithelial GI tumors (with video). Gastrointest Endosc. 2011;74:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Mimura T, Kuramoto S, Hashimoto M, Yamasaki K, Kobayashi K, Kobayashi M, Oohara T. Unroofing for lymphangioma of the large intestine: a new approach to endoscopic treatment. Gastrointest Endosc. 1997;46:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Lee CK, Chung IK, Lee SH, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Ihara E, Matsuzaka H, Honda K, Hata Y, Sumida Y, Akiho H, Misawa T, Toyoshima S, Chijiiwa Y, Nakamura K, Takayanagi R. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc. 2013;5:191-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |