Published online Jun 16, 2017. doi: 10.12998/wjcc.v5.i6.238
Peer-review started: January 16, 2017
First decision: March 28, 2017
Revised: March 30, 2017
Accepted: April 18, 2017
Article in press: April 19, 2017
Published online: June 16, 2017
Processing time: 150 Days and 4.7 Hours
Hepatocellular carcinoma (HCC) is one of the most common cancers and the third highest cause of cancer-associated mortality worldwide. The treatment of HCC is complicated by its variable biological behavior and the frequent coexistence of chronic liver disease, particularly cirrhosis. To date, multiple treatment modalities have been developed according to the stage of the tumor and the hepatic functional reserve, including transarterial treatments such as transarterial chemoembolization, transarterial oily chemoembolization (TOCE), and hepatic arterial infusion chemotherapy (HAIC). We conducted a phase I and II study of the combination therapy with double platinum agents, miriplatin and cisplatin, and confirmed its safety and efficacy. Here, we describe two cases of unresectable HCC who were successfully treated by miriplatin-TOCE/cisplatin-HAIC combination therapy, resulting in complete responses with no significant adverse events. This report will provide that the combination therapy can be the therapeutic option for HCC patients in the advanced stage.
Core tip: To date, multiple treatment modalities of hepatocellular carcinoma (HCC) exist; however, only liver transplantation or surgical resection is curative. Hepatic resection remains the most frequently performed treatment modality, but curative resection cases are limited. Therefore, palliative treatment options are necessary, including transarterial chemoembolization, transarterial oily chemoembolization (TOCE), and hepatic arterial infusion chemotherapy (HAIC). We conducted a clinical trial of combination therapy with miriplatin-TOCE and cisplatin-HAIC and demonstrated its safety and efficacy for patients with unresectable HCC. Here, we presented two cases who showed good response after the several sessions of the combination therapy.
- Citation: Ogawa K, Kamimura K, Watanabe Y, Motai Y, Kumaki D, Seki R, Sakamaki A, Abe S, Kawai H, Suda T, Yamagiwa S, Terai S. Effect of double platinum agents, combination of miriplatin-transarterial oily chemoembolization and cisplatin-hepatic arterial infusion chemotherapy, in patients with hepatocellular carcinoma: Report of two cases. World J Clin Cases 2017; 5(6): 238-246
- URL: https://www.wjgnet.com/2307-8960/full/v5/i6/238.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i6.238
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third highest cause of cancer-associated mortality worldwide[1,2]. The treatment of HCC is complicated by its variable biological behavior and the frequent coexistence of chronic liver disease, particularly cirrhosis. To date, multiple treatment modalities exist; however, only liver transplantation or surgical resection is curative. Hepatic resection remains the most frequently performed treatment modality, but curative resection cases are limited[3,4]. Another curative treatment is ablation therapy, including radiofrequency ablation (RFA). These treatments have been adopted for patients who are in Child-Pugh A or B cirrhosis states and have fewer than three tumors, all of which are less than 3 cm in diameter[5]. For many patients whose diagnosis does not fit these criteria, palliative treatment options are necessary, including transarterial treatments such as transarterial chemoembolization (TACE), transarterial oily chemoembolization (TOCE), and hepatic arterial infusion chemotherapy (HAIC).
Among these therapeutic options, TOCE showed equivalent therapeutic effect to TACE for many HCC patients with low hepatic reserve in a randomized phase III trial using zinostatin stimalamer dissolved in lipiodol[6]. Additionally, miriplatin, a third-generation platinum agent that forms a stable suspension with lipiodol[7], was developed for TOCE in HCC and approved for clinical use in Japan as a novel chemotherapeutic agent[8-14]. On the other hand, HAIC can affect larger areas of the liver without reducing hepatic arterial flow[15]. As the chemotherapeutic agent used in HAIC, a multicenter phase II study in patients with unresectable HCC using cisplatin (CDDP), a first-generation platinum agent, revealed that the response rate with CDDP powder (DDP-H, IA-call®; Nippon Kayaku, Tokyo, Japan) was better than that with other chemotherapeutic agents, such as epirubicin[16] and mitomycin C[17]. Based on these results, we decided to combine TOCE using miriplatin with HAIC using CDDP to improve the efficacy and decrease the risk of hepatic failure. We conducted a phase I study of the combination therapy and determined that both miriplatin and DDP-H can be administered up to the maximum tolerated dose of each drug without additional adverse effects[18]. Along with these results, we conducted a phase II study and confirmed the efficacy and safety of the combination therapy in cases with multiple HCC[19]. Here, we report two cases of patients with unresectable HCC who were successfully treated by miriplatin-TOCE/DDP-H-HAIC combination therapy, resulting in complete responses with no significant adverse events.
Case 1: A 37-year-old Japanese woman diagnosed with multiple liver tumors was referred to our hospital. She had been a heavy drinker for 20 years. Her physical examination revealed no remarkable changes, and laboratory analysis provided the following results (Table 1): All viral markers, including hepatitis B virus (HBV) and hepatitis C virus (HCV), were negative (HBsAg 0.32 mIU/mL, anti-HBs 0.01 IU/mL, anti-HBc 0.04 S/CO, anti-HCV 0.03 S/CO). Platelets (7.4 × 104/μL) showed a mild decrease, but serum aspartate aminotransferase (28 IU/L), alanine aminotransferase (20 IU/L), total bilirubin (0.8 mg/dL), albumin (4.5 g/dL), and prothrombin time (PT) (85%) were within normal ranges. Her Child-Pugh grade was A with a score of 5 points. There was no specific familial history of diseases.
| Hematology | Biochemistry | ||||
| WBC | 5100/μL | TP | 7.3 g/dL | Na | 142 mEq/L |
| RBC | 399 × 104/μL | Alb | 4.5 g/dL | K | 2.9 mEq/L |
| Hb | 14.1 g/dL | BUN | 7 mg/dL | Cl | 101 mEq/L |
| Ht | 41.80% | Cre | 0.44 mg/dL | CRP | 0.50 mg/dL |
| PLT | 7.4 × 104/μL | T-Bil | 0.8 mg/dL | AFP | 7 ng/mL |
| Coagulation | D-Bil | 0.1 mg/dL | AFP-L3 | < 0.5% | |
| PT | 85% | AST | 28 IU/L | DCP | 24 mAU/mL |
| ALT | 20 IU/L | CEA | 5.8 ng/mL | ||
| ChE | 211 IU/L | CA 19-9 | 68 U/mL | ||
| LDH | 181 IU/L | ||||
| γ-GTP | 256 IU/L |
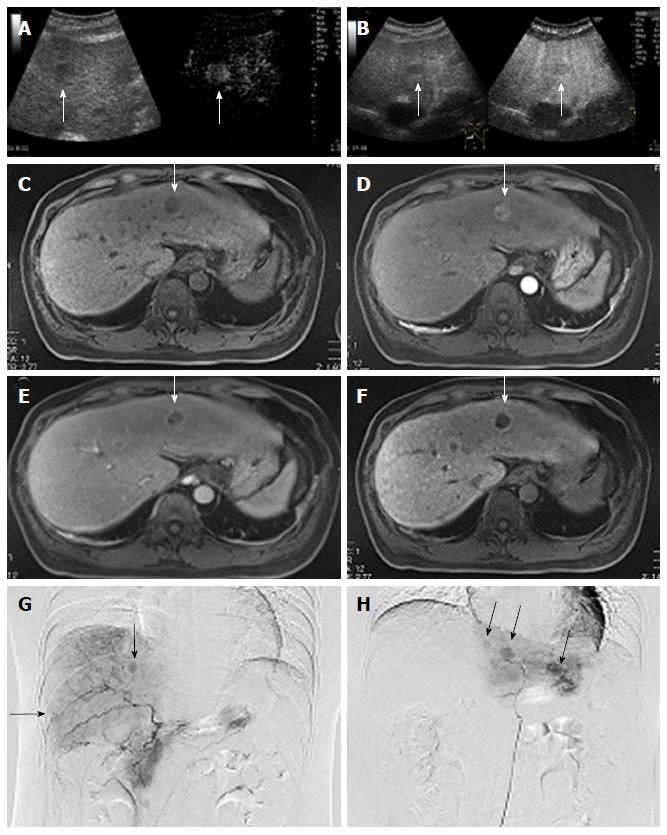
Contrast-enhanced ultrasonography (CEUS) using a perflubutane microbubble agent (Sonazoid, Daiichi Sankyo, Tokyo, Japan), angiography, and Gd-EOB-DTPA-enhanced magnetic resonance imaging (EOB-MRI) were performed and showed multiple tumors in the both lobes of the liver (Figure 1). Ultrasonography showed multiple low echoic masses of various sizes, with the maximum diameter of 2.1 cm in the left lobe. CEUS performed for the real-time observation of tumors and hepatic blood flow showed blood flow in the vascular arterial phase in the tumorous lesions with low echoic areas in the Kupffer phase (Figure 1A and B). MRI showed T1 hypointense tumors in both lobes of the liver (Figure 1C). EOB-MRI showed hyperintense tumors with hepatic blood perfusion in the arterial phase (Figure 1D). These tumors were clearly shown to have a drainage of blood perfusion in the delayed phase (Figure 1E), and marked as typical hypointensity tumors in the hepatobiliary phase (Figure 1F). Angiography showed multiple hypervascular tumor staining by the selective hepatic angiogram through the feeding arteries (Figure 1G and H). Although the imaging studies showed typical images for HCC, to confirm the histological diagnosis, we performed a liver biopsy of the tumorous area in the left lobe and confirmed it to be HCC.
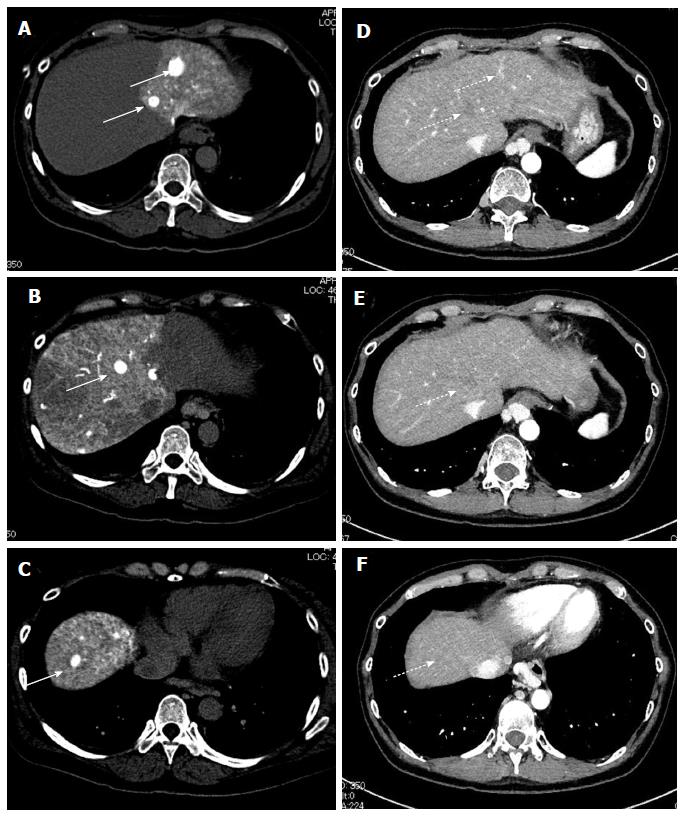
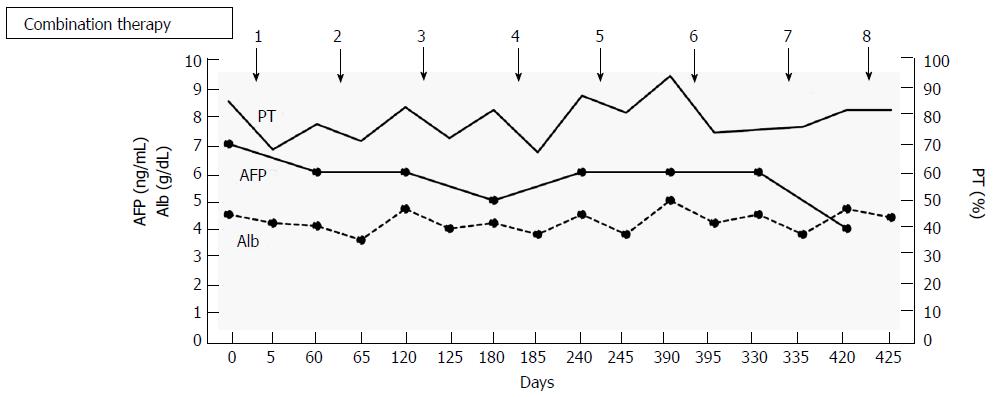
Based on these results, the patient was diagnosed with multiple HCCs with liver cirrhosis due to alcohol abuse. Because of the bilaterality and multiplicity of the tumors, we performed transhepatic arterial chemotherapy using a combination therapy. DDP-H was administered as HAIC through the hepatic arteries covering the lobes of the liver with tumors at a concentration of 1.43 mg/mL in saline at a rate of 3 mg/min. Miriplatin was administered through the tumor feeders at a concentration of 20 mg/mL in lipiodol up to 120 mg/body. No other medication was adopted during the procedure. To date, we have performed this treatment in eight sessions without severe adverse events in any session. Dynamic contrast-enhanced CT demonstrated significant improvements in the HCCs after eight sessions of treatment (Figure 2). Based on the REIST classification, her clinical course was classified as a complete response (CR), and no recurrences were seen 2 years after the last therapy, the period of follow-up. The clinical course and time-dependent changes of serum biochemical analyses are shown in Figure 3. AFP showed decreasing whereas albumin, and PT did not change significantly during the eight sessions of combination therapy. This result suggests that this combination therapy can be performed safely and repeatedly without severe adverse events.
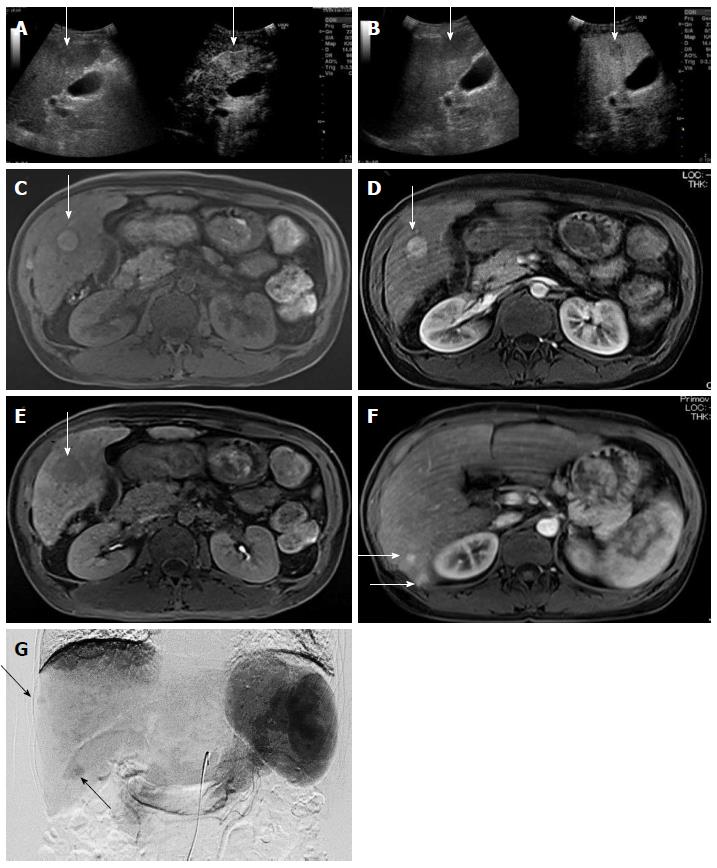
Case 2: A 43-year-old Japanese woman was referred to our hospital for rupture of esophageal varices. We performed endoscopic variceal ligation for the varices. CT revealed multiple tumors in bilateral lobes of the liver. CEUS and EOB-MRI showed clear enhancement in the arterial phase with detection as low echoic areas in Kupffer phase (Figure 4A and B). T1-weighted MRI revealed a hyperintense tumor with film structure (Figure 4C). EOB-MRI showed a hypointensity tumor in the hepatobiliary phase in the S5 lesion with hepatic blood perfusion in the arterial phase (Figure 4D and E). Several tumorous lesions in the liver besides the S5 lesion were detected as enhanced tumors in the arterial phase of the EOB-MRI study (Figure 4F). Because tumor markers were normal, including carcinoembryonic antigen, carbohydrate antigen 19-9, alpha-fetoprotein (AFP), and des-γ-carboxy prothrombin, angiography was performed for the evaluation of these tumorous lesions which showed multiple hypervascular areas of tumor staining by the selective hepatic angiograms (Figure 4G) followed by the histological diagnosis of HCC by tumor biopsy.
Based on these findings, we diagnosed the patient with multiple HCCs. Laboratory results before the treatment of these tumors (Table 2) showed reduced levels of hemoglobin (10.2 g/dL), platelets (8.6 × 104/μL), cholinesterase (124 IU/L), prothrombin (52%), and albumin (3.1 g/dL) and increased aspartate aminotransferase (63 IU/L), alanine aminotransferase (49 IU/L), γ-glutamyl transpeptidase (83 IU/L), and alkaline phosphatase (471 IU/L). The physical examination showed no remarkable findings and no signs of hepatic encephalopathy or flapping tremor. Based on these results, her Child-Pugh grade was determined to be B with a score of 7 points. All viral markers, including HBV and HCV, were negative (HBs Ag 0.02 mIU/mL, anti-HBs 0.01 IU/mL, anti-HBc 0.02 S/CO, and anti-HCV 0.11 S/CO).
| Hematology | Biochemistry | ||||
| WBC | 3980 /μL | TP | 7.0 g/dL | Na | 138 mEq/L |
| RBC | 344 × 104/μL | Alb | 3.1 g/dL | K | 3.5 mEq/L |
| Hb | 10.2 g/dL | BUN | 11 mg/dL | Cl | 106 mEq/L |
| Ht | 30.00% | Cre | 0.45 mg/dL | CRP | 0.16 mg/dL |
| PLT | 8.6 × 104/μL | T-Bil | 0.7 mg/dL | AFP | 4 ng/mL |
| Coagulation | D-Bil | 0.1 mg/dL | AFP-L3 | < 0.5% | |
| PT | 52% | AST | 63 IU/L | DCP | < 15 mAU/mL |
| ALT | 49 IU/L | CEA | 2.2 ng/mL | ||
| ChE | 124 IU/L | CA 19-9 | 40 U/mL | ||
| LDH | 143 IU/L | ||||
| γ-GTP | 83 IU/L |
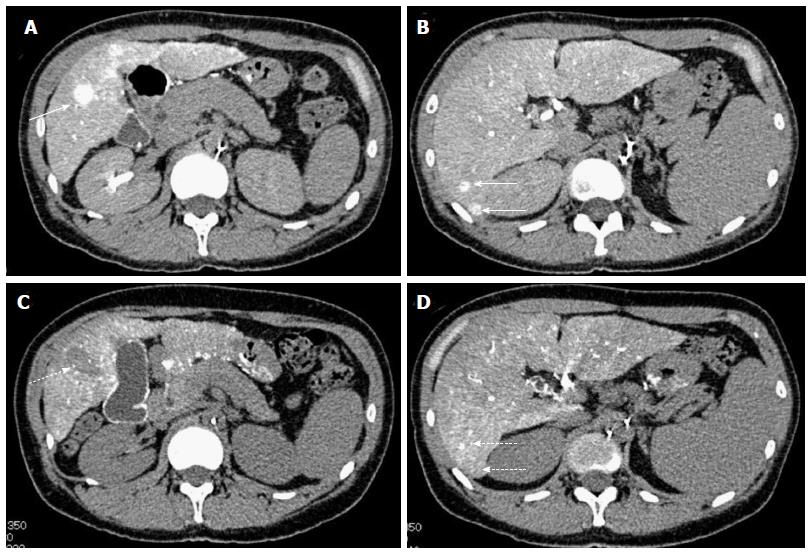
Based on the multiplicity of tumors and the poor hepatic reserve function, we decided to perform combination therapy with the same protocol as described for case 1. Dynamic contrast-enhanced CT demonstrated significant improvement after three sessions of treatment and because this combination therapy was successful for all other tumors, we performed an additional RFA for the tumor in S5 lesion, suspected of retaining a small part of the tumor (Figure 5). To date, the RECIST evaluation achieved CR, no recurrences were seen for 1 year, the period of follow-up, and no adverse events have been seen.
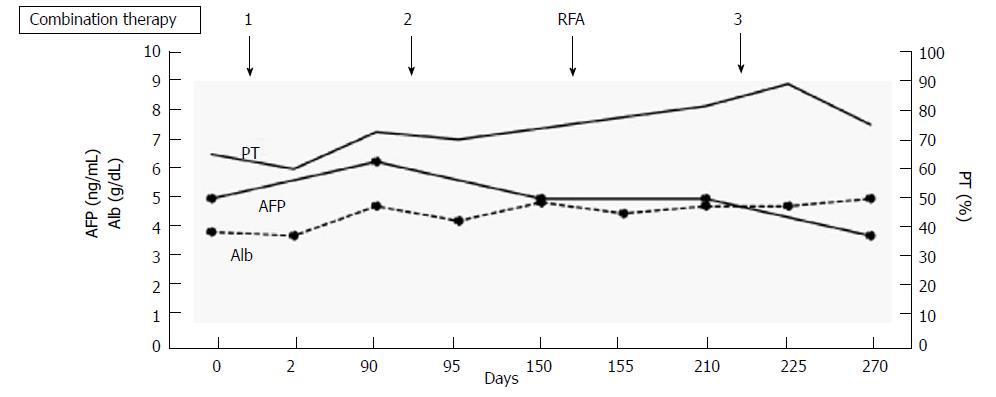
The clinical course and time-dependent changes of serum biochemical analyses are shown in Figure 6. There were no significant changes in AFP, albumin, or PT during the three sessions of combination therapy or during RFA using Cool-tip® (Medtronic, MN, United States). This result suggests that we can perform this combination therapy safely and repeatedly without adverse events, as with case 1.
In this report, we have shown two representative cases with unresectable HCC that were successfully treated by miriplatin-TOCE/DDP-H-HAIC combination therapy, resulting a CR. Because the therapeutic strategy is determined according to the stage of the tumor and the hepatic functional reserve, several therapeutic options, including surgical resection, RFA, and heavy particle radiotherapy, can be adopted as loco-regional control for cases of limited HCC[20,21]. The use of TACE is strictly limited because it requires a higher hepatic functional reserve[22-26]. However, in a randomized phase III trial of zinostatin stimalamer dissolved in lipiodol, TOCE demonstrated a therapeutic effect, with no difference in the anti-tumor effect in comparison with TACE, for HCC patients with low hepatic reserve[6] TOCE has served as a therapeutic option for patients in advanced stages of HCC with poor hepatic reserve. Miriplatin was developed for TOCE in HCC because it gradually releases active derivatives in situ, enabling the continuous release of platinum in the tumor while avoiding systemic release and toxicity[7]. It has been used since 2010. To date, there have been some reports of the safety and efficacy of miriplatin-TOCE in patients with HCC[27]. Otsuji recently reported that the efficacy and safety of miriplatin for HCC were on the same level as the efficacy and safety of DDP-H in a randomized controlled trial[28]. Compared with TACE and TOCE, HAIC can affect larger areas of the liver without reducing hepatic arterial flow because no embolization was combined. Although various chemotherapeutic agents have been used for treating HCC[16,17], platinum agents achieved a better response rate in a multicenter phase II study enrolling patients with unresectable HCC[29]. Based on these results and the fact that miriplatin showed no cross-resistance with different generations of platinum agents[30], we conducted a phase I study[18] and a phase II study[19] of combined therapy with miriplatin-TOCE and DDP-H-HAIC and demonstrated its safety and efficacy for patients with unresectable HCC. In fact, we could repeat the miriplatin-TOCE/DDP-H-HAIC combination therapy for the two cases in the present study without severe adverse effects. The combination therapy may have some adverse events for specific cases with poor hepatic functional reserve. A further study should be conducted to confirm the efficacy and safety of miriplatin-TOCE/DDP-H-HAIC combination therapy for a larger number of patients with poor hepatic functional reserve.
In conclusion, we report two representative cases of patients with unresectable HCC that was successfully controlled by miriplatin-TOCE/DDP-H-HAIC combination therapy. This report suggests that the combination therapy can be performed safely and repeatedly for patients with advanced HCC.
Two cases with unresectable hepatocellular carcinoma (HCC).
HCC diagnosed with multiple imaging modalities.
The imaging modalities clearly demonstrated the typical patterns of HCC.
Mild liver injury and increase of tumor markers including alpha-fetoprotein and des-γ-carboxy prothrombin.
Contrast-enhanced ultrasonography showed blood flow in the vascular arterial phase in the tumorous lesions with low echoic areas in the Kupffer phase. Magnetic resonance imaging showed T1 hypointense and hyperintense with hepatic blood perfusion in the arterial phase. Hypointense in the delayed phase and marked as typical hypointensity tumors in the hepatobiliary phase.
HCC.
Combination therapy of miriplatin-transarterial oily chemoembolization and cisplatin-hepatic arterial infusion chemotherapy.
This report suggests that the combination therapy can be performed safely and repeatedly for patients with advanced HCC.
These cases appear to have responded which may be an important finding.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Cerwenka HR, Fiorentini G, Smith RC S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: From diagnosis to treatment. Surg Oncol. 2016;25:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 333] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 3. | Balogh J, Victor D, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 807] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 4. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 5. | European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 6. | Okusaka T, Kasugai H, Shioyama Y, Tanaka K, Kudo M, Saisho H, Osaki Y, Sata M, Fujiyama S, Kumada T. Transarterial chemotherapy alone versus transarterial chemoembolization for hepatocellular carcinoma: a randomized phase III trial. J Hepatol. 2009;51:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Kishimoto S, Ohtani A, Fukuda H, Fukushima S, Takeuchi Y. Relation between intracellular accumulation and cytotoxic activity of cis-[((1R, 2R)-1, 2-cyclohexanediamine-N, N’)bis(myristato)]platinum(II) suspended in Lipiodol. Biol Pharm Bull. 2003;26:683-686. [PubMed] |
| 8. | Shimakura J, Fujimoto K, Komuro S, Nakano M, Kanamaru H. Long-term disposition of a novel lipophilic platinum complex SM-11355 in dog after intrahepatic arterial administration: highly sensitive detection of platinum and radioactivity. Xenobiotica. 2002;32:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Hanada M, Baba A, Tsutsumishita Y, Noguchi T, Yamaoka T, Chiba N, Nishikaku F. Intra-hepatic arterial administration with miriplatin suspended in an oily lymphographic agent inhibits the growth of tumors implanted in rat livers by inducing platinum-DNA adducts to form and massive apoptosis. Cancer Chemother Pharmacol. 2009;64:473-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Hanada M, Baba A, Tsutsumishita Y, Noguchi T, Yamaoka T. Intra-hepatic arterial administration with miriplatin suspended in an oily lymphographic agent inhibits the growth of human hepatoma cells orthotopically implanted in nude rats. Cancer Sci. 2009;100:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Hanada M, Takasu H, Kitaura M. Acquired resistance to miriplatin in rat hepatoma AH109A/MP10 is associated with increased Bcl-2 expression, leading to defects in inducing apoptosis. Oncol Rep. 2010;24:1011-1018. [PubMed] |
| 12. | Fujiyama S, Shibata J, Maeda S, Tanaka M, Noumaru S, Sato K, Tomita K. Phase I clinical study of a novel lipophilic platinum complex (SM-11355) in patients with hepatocellular carcinoma refractory to cisplatin/lipiodol. Br J Cancer. 2003;89:1614-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Okusaka T, Okada S, Nakanishi T, Fujiyama S, Kubo Y. Phase II trial of intra-arterial chemotherapy using a novel lipophilic platinum derivative (SM-11355) in patients with hepatocellular carcinoma. Invest New Drugs. 2004;22:169-176. [PubMed] |
| 14. | Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Sakamoto A, Henmi S, Ishikawa T, Saito S, Kita R, Kimura T. A case of advanced hepatocellular carcinoma with portal vein tumor thrombus refractory to epirubicin that showed marked decrease in tumor markers after transcatheter arterial infusion with miriplatin. Case Rep Oncol. 2011;4:327-335. [PubMed] |
| 15. | Niguma T, Mimura T, Tutui N. Adjuvant arterial infusion chemotherapy after resection of hepatocellular carcinoma with portal thrombosis: a pilot study. J Hepatobiliary Pancreat Surg. 2005;12:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Nagasue N, Yukaya H, Okamura J, Kuroda C, Kubo Y, Hirai K, Tanikawa K, Okita K, Ando K, Tamura K. Intra-arterial administration of epirubicin in the treatment of non-resectable hepatocellular carcinoma. Epirubicin Study Group for Hepatocellular Carcinoma. Gan To Kagaku Ryoho. 1986;13:2786-2792. [PubMed] |
| 17. | Ishikawa T. Future perspectives on the treatment of hepatocellular carcinoma with cisplatin. World J Hepatol. 2009;1:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Kamimura K, Suda T, Tamura Y, Takamura M, Yokoo T, Igarashi M, Kawai H, Yamagiwa S, Nomoto M, Aoyagi Y. Phase I study of miriplatin combined with transarterial chemotherapy using CDDP powder in patients with hepatocellular carcinoma. BMC Gastroenterol. 2012;12:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Kamimura K, Suda T, Yokoo T, Kamimura H, Kanefuji T, Tsuchiya A, Takamura M, Kawai H, Waguri N, Yamagiwa S. Transhepatic arterial infusion chemotherapy using a combination of miriplatin and CDDP powder versus miriplatin alone in the treatment of hepatocellular carcinoma: a randomized controlled trial. BMC Cancer. 2017;17:322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 21. | Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 22. | Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 618] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 23. | Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 24. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2271] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 25. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 26. | Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;CD004787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Yukisawa S, Ishii H, Kasuga A, Matsuyama M, Kuraoka K, Takano K, Ozaka M. A transcatheter arterial chemotherapy using a novel lipophilic platinum derivative (miriplatin) for patients with small and multiple hepatocellular carcinomas. Eur J Gastroenterol Hepatol. 2012;24:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Otsuji K, Takai K, Nishigaki Y, Shimizu S, Hayashi H, Imai K, Suzuki Y, Hanai T, Ideta T, Miyazaki T. Efficacy and safety of cisplatin versus miriplatin in transcatheter arterial chemoembolization and transarterial infusion chemotherapy for hepatocellular carcinoma: A randomized controlled trial. Hepatol Res. 2015;45:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Yoshikawa M, Ono N, Yodono H, Ichida T, Nakamura H. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol Res. 2008;38:474-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Watanabe S, Nitta N, Ohta S, Sonoda A, Otani H, Tomozawa Y, Nitta-Seko A, Tsuchiya K, Tanka T, Takahashi M. Comparison of the anti-tumor effects of two platinum agents (miriplatin and fine-powder cisplatin). Cardiovasc Intervent Radiol. 2012;35:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |