Published online Jun 16, 2015. doi: 10.12998/wjcc.v3.i6.533
Peer-review started: December 18, 2014
First decision: January 22, 2015
Revised: February 14, 2015
Accepted: April 10, 2015
Article in press: April 14, 2015
Published online: June 16, 2015
Processing time: 184 Days and 6.3 Hours
A 75-year-old male presented with difficult defecation and increasing urinary frequency over a few months. He had a significant history of previous partial gastrectomy for gastric carcinoma 20 years prior. Computed tomography of the abdomen and pelvis showed extensive lymphadenopathy, a gastric mass and rectal as well as bladder wall thickening with bilateral ureterohydronephrosis. Normal looking serosal surfaces of the bladder and bowel were seen on laparoscopy and a defunctioning ileostomy was created. Gastroscopy revealed a malignant mass while cystoscopy and sigmoidscopy found extensive tumour growth lining the mucosal surfaces. Biopsies from all sites were compatible with intestinal type adenocarcinoma of gastric origin with few signet ring cells. Metabolic response to palliative chemotherapy was good and the patient’s symptoms have improved on follow-up four months post ileostomy. We discuss the immunohistochemical profile of the tumour and review the literature.
Core tip: Although exceedingly rare, metastases to the colorectum and bladder can occur with primary gastric adenocarcinoma. Unusual sites of spread are more often associated with diffuse type or signet ring cell gastric carcinoma but can occur with intestinal type as well. Site specific symptoms should alert the clinician to the possible locations of spread so as to allow prompt diagnosis. CK7 and CK20 profiles may help to establish the origin of the metastatic tumour if in doubt.
- Citation: Seow-En I, Seow-Choen F. Intestinal type gastric adenocarcinoma with unusual synchronous metastases to the colorectum and bladder. World J Clin Cases 2015; 3(6): 533-537
- URL: https://www.wjgnet.com/2307-8960/full/v3/i6/533.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i6.533
Gastric adenocarcinoma commonly spreads to the peritoneum, liver, lungs and intra-abdominal lymph nodes. Multiple as well as unusual sites of metastases have been described more often with diffuse type or signet ring cell gastric cancer. We describe the first case of intestinal type gastric adenocarcinoma with few signet ring cells presenting with synchronous colorectal and vesical metastases. Laparoscopic findings were also unusual as the serosal surfaces looked grossly normal but extensive intraluminal masses were found on endoscopy. Metabolic response to palliative chemotherapy was good and the patient’s symptoms have improved on follow-up four months post defunctioning ileostomy.
A 75-year-old Indonesian male presented to our clinic with the complaints of difficult defaecation and increasing frequency of micturition for a few months’ duration. He had a history of previous partial gastrectomy for gastric cancer in Indonesia more than 20 years prior. Full histology from the initial surgery was unavailable. Abdominal examination was unremarkable apart from mild abdominal distension. On rectal examination, extra-anal nodular masses were felt causing tight stricturing of the anal canal extending proximally. The anal mucosa felt intact.
Serum CEA and CA 19-9 were markedly elevated at 31.6 μg/L (normal 0-5 μg/L) and 30523 U/mL (normal 0-37 U/mL) respectively. AFP level was normal. Contrast enhanced computed tomography of the abdomen and pelvis revealed extensive retroperitoneal lymphadenopathy including para-aortic and aortocaval nodes as well as bilateral iliac lymphadenopathy. In addition a gastric mass was suspected. Rectal wall thickening causing luminal constriction and bladder wall thickening with bilateral ureterohydronephrosis were noted. The liver appeared normal.
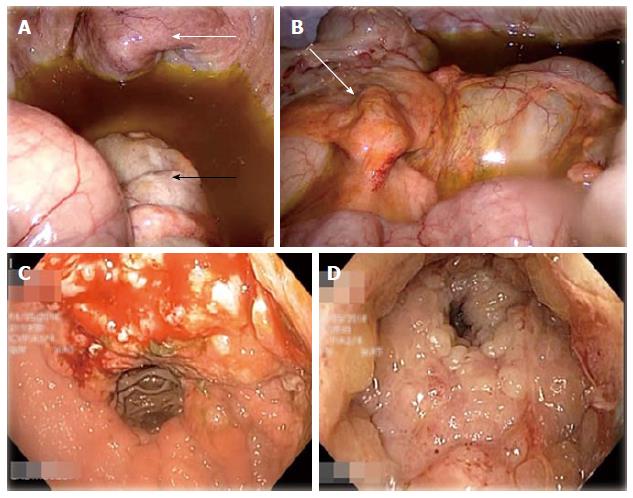
During diagnostic laparoscopy, the transverse colon down to the visualised rectum was found to be thickened and rigid. The bladder appeared normal externally (Figure 1) but was thickened with intravesical masses palpable via laparoscopic manipulation. The appendices epiploicae of the transverse colon and the median umbilical ligament also appeared thickened (Figure 1) and biopsies were taken from both sites. Moderate greenish ascites was noted. No peritoneal or serosal nodules were seen and the small intestine and stomach were grossly unremarkable on laparoscopy. The previous gastrojejunostomy anastamosis site looked normal. A defunctioning ileostomy was created to alleviate intestinal obstruction.
As no definite intra-abdominal masses were found on laparoscopy, oesophago-gastroduodenoscopy (OGD) and sigmoidoscopy were performed at the same sitting. OGD showed a mass at the lesser curve of the stomach and sigmoidoscopy revealed anal stricturing and extensive tumour lining the rectal mucosa (Figure 1). The scope was unable to pass beyond 8 cm from the anal verge due to the tight intraluminal growths. A cystoscopy was done which showed the entire bladder mucosa to be covered by mucosal tumour growth.
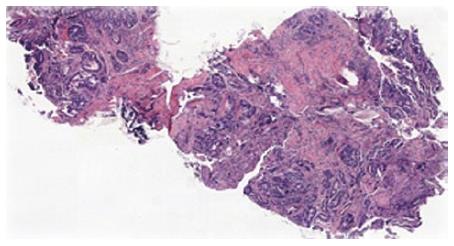
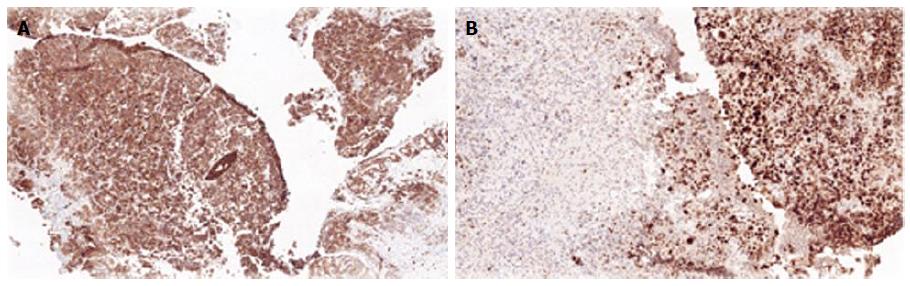
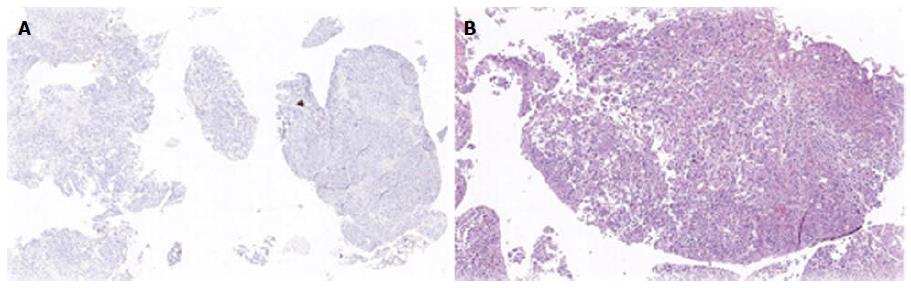
Biopsies from all sites including the gastric, rectal and bladder tumours as well as the appendices epiploicae and median umbilical ligament were compatible with adenocarcinoma of gastric origin (Figure 2). This was of intestinal type based on both the Lauren as well as world health organization (WHO) classification. In view of the length of time between the patients’s initial gastric tumour and the current primary lesion occurring in the remnant stomach it was not felt to be a recurrence. Immunohistochemical studies revealed the tumour cells to be positive for CK7, CK19 and CDX2 while negative for CK20 and CK5 (Figure 3). Mucicarmine staining showed intracytoplasmic mucin positivity in only few tumour cells (Figure 4). Uroplakin and HER2/neu were negative. KRAS and BRAF gene mutations were not detected. No features of a second primary tumour were found.
F-18 fluorodeoxyglucose positron emission tomography coupled with a multi-slice low-dose computed tomography scan (FDG PET/CT) was performed with FDG uptake at the site of gastric tumour and retroperitoneal lymph nodes. Additionally, hypermetabolic para-oesophageal, anterior mediastinal, para-tracheal and left supraclavicular lymph nodes were seen. There were also foci of increased FDG uptake in the T2 and T3 vertebrae consistent with bone metastasis. There was no significant FDG uptake seen in the liver.
The patient underwent initial chemotherapy with 5-FU and bevacizumab. Three further cycles of chemotherapy was given using 5-FU, bevacizumab and oxaliplatin with paclitaxel added to the second cycle.
FDG PET/CT prior to the third cycle showed good metabolic response with interval decreased FDG uptake in the stomach. Previously noted FDG avid nodes in the neck, thorax, abdomen and pelvis all showed interval resolution, metabolic resolution or decreased uptake. Interval resolution was also observed in T2 and T3 vertebrae. Serum CA 19-9 also came down to 3900 U/mL.
The patient showed clinical improvement with resolution of urinary symptoms and decrease in abdominal distension with good ileostomy output 4 mo from surgery at the time of writing. He has been on regular follow-up and is planned for further chemotherapy.
Gastric adenocarcinoma is the fourth most common cancer worldwide, accounting for 8% of the 12.7 million new cases of cancer diagnosed in 2008[1]. More than 50% of these patients present with unresectable locally advanced or metastatic cancer[2], and out of those resected with curative intent, 40%-65% of cancers will recur[3]. Seventy-nine percent of recurrences occur within the first 2 years of resection, 94% within 4 years and rarely after[4].
Patterns of gastric metastasis have been well studied. The most common sites of metastasis include the liver due to portal drainage, the lungs due to lymphatic drainage, the peritoneum, and the intra-abdominal lymph nodes. Reports of spread to colonic or bladder mucosa have been rare in literature. 39 cases of gastric linitis plastica with metastases to the colon were described from 1936 to 1989; the bladder was involved in three cases[5]. To our knowledge, only four cases of gastric cancer with colorectal metastases[6-9] and five cases with vesical metastases[10-13] were described in the English language literature in the past 10 years. No other reports of concomitant colorectal and vesical metastases were found.
Six out of these nine recent cases of gastric adenocarcinoma with colorectal or vesical metastases were poorly differentiated diffuse type based on the Lauren classification with predominant signet ring cells. Two of the remaining cases were well differentiated intestinal type[6,10], while the last was an undifferentiated type with signet ring and intestinal differentiation[8]. Diffuse type adenocarcinoma have been shown to have an increased propensity for disseminated spread to multiple organs, in particular to the peritoneum, while intestinal type carcinoma more frequently spreads to the liver. Frequency of abdominal lymph node involvement was similar for both types[14]. In a 2001 radiological study of 23 patients with intestinal metastases from gastric adenocarcinoma, 70% were linitis plastica and 74% were poorly differentiated or signet ring cell type[15]. It is clear that an association exists between linitis plastica/diffuse type or signet ring type histology and unusual sites of metastases.
This report is the first described with synchronous colorectal and vesical metastases from intestinal type gastric adenocarcinoma. Few signet ring cells were present in the histological specimens and less than the required 50% to be labelled as signet ring carcinoma as per the WHO classification. This case was also unusual as the rectal and bladder metastases were only obvious intraluminally and initially suggestive of de novo carcinoma. While the serosal biopsies were positive for cancer, no obvious masses were visible on the serosal aspect during laparoscopy.
Immunohistochemical analysis can be useful in establishing location of the primary tumour if in doubt. In this case the CK7+/CK20- profile of the tumour was indicative of a primary gastric carcinoma as opposed to a colorectal origin, with more than 90% of rectal primaries expressing CK20[16]. CDX2 is a sensitive and specific marker for adenocarcinoma of the gastrointestinal tract, and is associated with both gastric intestinal metaplasia and intestinal type gastric carcinoma. CDX2 positive gastric adenocarcinoma have been demonstrated to have better outcomes and prognosis as compared to CDX2 negative tumours[17]. A CK7+/CK20-/CDX2+ profile was compatible with adenocarcinoma of pancreaticobiliary origin as well; this was ruled out by PET/CT.
In conclusion, albeit very rare, colorectal and vesical metastases can occur from a primary gastric adenocarcinoma. Although previously more significantly associated with linitis plastica, diffuse type or signet ring cell carcinoma, multiple sites of metastases to unusual locations can also occur with intestinal differentiation. Further studies can be done to shed light on the mechanism of such spread and may likely be derived only from analyses of isolated case reports. Site specific symptoms should alert the clinician to the possible locations of spread so as to allow prompt diagnosis and appropriate treatment.
A 75-year-old male presented with difficult defecation and increasing urinary frequency over a few months.
Rectal examination revealed nodular masses causing tight stricturing of the anal canal while endoscopy showed a gastric mass and intravesical tumour growth.
Metastatic gastric adenocarcinoma vs de novo rectal or bladder carcinoma.
Serum CEA and CA 19-9 were markedly elevated at 31.6 μg/L (normal 0-5 μg/L) and 30523 U/mL (normal 0-37 U/mL) respectively.
Computed tomography showed a gastric mass, rectal and bladder thickening as well as extensive lymphadenopathy.
Histological analyses of all biopsy specimens were compatible with metastatic gastric adenocarcinoma of intestinal type differentiation based on Lauren classification.
A defunctioning ileostomy was performed to relieve impending obstruction and the patient underwent palliative chemotherapy with good response.
Incidences in literature of colorectal or bladder metastases from a primary gastric adenocarcinoma are rare. Most reported cases are associated with diffuse type adenocarcinoma or linitis plastica rather than intestinal type.
Cytokeratins are intermediate filament proteins found in normal epithelium, which have preserved expression in neoplastic cells. This allows the detection of specific cytokeratins to be useful tools in determining the origin of metastatic carcinoma.
Multiple sites of metastases to unusual locations can occur with intestinal type gastric adenocarcinoma and site specific symptoms should alert the clinician to the possible locations of spread.
It is a very unsual case of gastric Ca. with long time distal relapse in colon, bladder and huge abdominal lymph nodes. Specially remarkable is that primary tumor ocurred 20 year ago.
P- Reviewer: Fernandez-Pello S, Kai K S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
| 1. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013; Available from: http://globocan.iarc.fr. |
| 2. | Thompson GB, van Heerden JA, Sarr MG. Adenocarcinoma of the stomach: are we making progress? Lancet. 1993;342:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2435] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 4. | D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 487] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 5. | Katon RM, Brendler SJ, Ireland K. Gastric linitis plastica with metastases to the colon: a mimic of Crohn’s disease. J Clin Gastroenterol. 1989;11:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Tural D, Selçukbiricik F, Erçalışkan A, Inanç B, Günver F, Büyükünal E. Metachronous rectum metastases from gastric adenocarcinoma: a case report. Case Rep Med. 2012;2012:726841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Lim SW, Huh JW, Kim YJ, Kim HR. Laparoscopic low anterior resection for hematogenous rectal metastasis from gastric adenocarcinoma: a case report. World J Surg Oncol. 2011;9:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Pace U, Contino G, Chiappa A, Bertani E, Bianchi PP, Fazio N, Renne G, Di Meglio G, Andreoni B. Metachronous Colon Metastases from Gastric Adenocarcinoma: A Case Report. Case Rep Oncol. 2009;2:92-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Lee HC, Yang MT, Lin KY, Tu HY, Zhang TA, Chen PH. Metastases from gastric carcinoma to colon in the form of multiple flat elevated lesions: a case report. Kaohsiung J Med Sci. 2004;20:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | András C, Tóth L, Pósán J, Csiki E, Tanyi M, Csiki Z, Garami Z, Enyedi A, Flaskó T, Horváth Z. Occurrence of bladder metastasis 10 years after surgical removal of a primary gastric cancer: a case report and review of the literature. J Med Case Rep. 2013;7:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Sharma PK, Vijay MK, Das RK, Chatterjee U. Secondary signet-ring cell adenocarcinoma of urinary bladder from a gastric primary. Urol Ann. 2011;3:97-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Neves TR, Covita A, Soares M, Monteiro P, Nogueira R, Lima MS, Monteiro H. Bladder metastasis of gastric adenocarcinoma. Report of 2 cases and bibliographic review. Arch Esp Urol. 2011;64:544-550. [PubMed] |
| 13. | Antunes AA, Siqueira TM, Falcão E. Vesical metastasis of gastric adenocarcinoma. Int Braz J Urol. 2004;30:403-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Duarte I, Llanos O. Patterns of metastases in intestinal and diffuse types of carcinoma of the stomach. Hum Pathol. 1981;12:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Jang HJ, Lim HK, Kim HS, Cho EY, Lee SJ, Kim KA, Choi D. Intestinal metastases from gastric adenocarcinoma: helical CT findings. J Comput Assist Tomogr. 2001;25:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Lee MJ, Lee HS, Kim WH, Choi Y, Yang M. Expression of mucins and cytokeratins in primary carcinomas of the digestive system. Mod Pathol. 2003;16:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Saad RS, Ghorab Z, Khalifa MA, Xu M. CDX2 as a marker for intestinal differentiation: Its utility and limitations. World J Gastrointest Surg. 2011;3:159-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |