INTRODUCTION
Dystrophic cardiac calcification is often diffuse and occurs primarily in patients with chronic inflammatory diseases. Long term advancement of dystrophic calcification often lead to complicated valvular stenosis, cardiac arrhythmias, cardiac block and abnormal cardiac hemodynamic by effecting systolic and diastolic cardiac function, thus awareness, early detection and treatment of the underlying cause, and resulting complications is key to patient outcome.
CASE REPORT
A 55-year-old African-American woman with past medical history of hypertension, endstage renal failure on dialysis with failure of previous renal transplant presented for cardiac risk stratification prior to renal transplant surgery. Previous work up for renal transplant had been unremarkable except for elevated intact parathyroid hormone of 1346 pg/mL. Electrocardiogram showed normal sinus rhythm. By Teichholtz equation, transthoracic echocardiography showed a left ventricular ejection fraction of 75%. In the parasternal long axis view, the interventricular septal diameter was 1 cm, the left ventricular posterior wall diameter was 1.1 cm, the left ventricular end diastolic diameter was 4 cm, and the left ventricular mass index was greater than 86 g/m2. There was concentric remodeling by relative wall thickness calculation. Grade 2 diastolic dysfunction with pseudonormalization mitral inflow pattern was observed. The E:A was 1.4, lateral e’ of 8.68 cm/s, medial e’ of 6.14 cm/s, and a deceleration time of 200 ms. The left atrial size and volume was in the upper limits of normal. (Normal left atrial size by index is < 29 cm/m2)[1]. The left atrial wall, however, showed diffuse hyper-echogenicity not observed in the left ventricular walls. This hyper-echogenicity did not restrict the movement of the mitral valve leaflets (Figure 1). Tricuspid annular plane systolic excursion was 2.1 cm/s. A transesophageal echocardiogram was not performed in this case. However, in order to confirm our suspicion and diagnosis, the patient underwent a non-enhanced computed tomography (CT) of the chest which noted increased attenuation of the left atrial wall, consistent with calcification of majority of the left atrium and mild calcification of the ascending aorta (Figure 2). The CT unfortunately did not show the aorto-mitral continuity well. The Calcium-phosphorous product at time of evaluation was within normal limits, and patient had no history of tuberculosis or a positive Purified Protein Derivative.
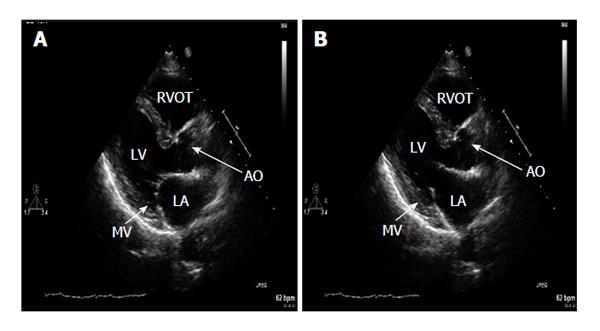
Figure 1 Transthoracic 2D parasternal long axis.
A: Systole shows diffuse echo dense signal significantly confined to the walls of the left atrium indicated by arrow. No mitral valve (MV) or mitral annulus involvement. Valve closes normally during systole; B: Diastole shows adequate MV opening. LA: Left atrium; AO: Aortic root.
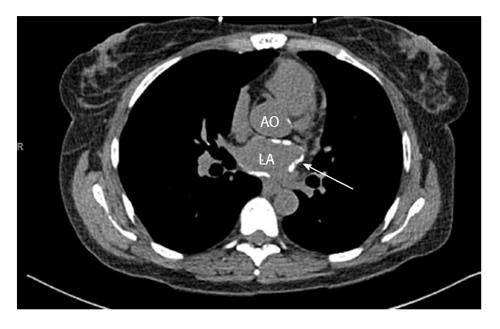
Figure 2 Cardiac computed tomography indicating calcium within the walls of the left atrium and mild calcification of the aortic root.
LA: Left atrium; AO: Aortic root.
DISCUSSION
Calcification seen in patients is typically either a product of metastatic calcification or dystrophic calcification. Metastatic calcification is typically seen in patients with a disturbance of Calcium and Phosphorus metabolism, often due to renal dysfunction. In our patient, although the intact parathyroid hormone level was elevated on previous workup for renal transplant, the pattern of calcification was that of a dense, finely speckled pattern. This indicates that the calcification in the left atrium is a product of dystrophic calcification and not that of metastatic calcification.
Dystrophic myocardial calcification which is increased intracellular calcium deposition driven by high serum calcium, is a well described process associated with conditions that cause systemic inflammation such as renal failure, rheumatic disease or cardiac surgery[2-5]. Calcium deposition often involves the left atrial appendage, free wall and septal wall, and mitral valve as well. In severe cases the entire atrium is involved with significant mitral valve stenos is or malcoaptation[6]. In cases where an entire atrium is involved, it is often referred to as a “coconut”[7] or “porcelain atrium”[8]. Although calcification of parts or the whole left atrium have been well documented, calcium deposition confined to the left atrial walls without valvular involvement is a unique variant.
There are several genes that have been linked to dystrophic cardiac calcification, including but not limited to the ATP-binding cassette transporter subtype 6 gene which was recently found to mediate myocardial necrosis and calcification[9-11], the Adiponectin gene which was linked to calcification of the aortic median[12,13], the alpha2-HS-glycoprotein/fetuin gene which has been linked to calcification of coronary artery plaques in patients with type II diabetes[14,15], the ectonucleotide pyrophosphatase/phosphodiesterase1 gene which has been linked to increased aortic arch calcifications in patients with type II diabetes and higher coronary calcification scores in patients with End Stage Renal Disease[16-20], and the Osteoprotegerin gene which has been linked to increased risk for coronary artery disease[21-25].
Women in their fifth and sixth decades are most often affected[6] and can be classified into one of three types which are associated with prior mitral valve dysfunction[26]. Type A is caused by mitral stenos is with calcification confined to the left atrial appendage causing an increased occurrence of thrombi within the appendage. Type B is the result of advanced mitral stenos is with calcification confined to the left atrial free wall and the mitral valve. Type C is the confined calcification of the posterior wall of the left atrium. This particular pattern of left atrial calcium deposition is known as MacCallum’s patch and is often the result of a regurgitant mitral jet[26]. The structure that is most often spared is the interatrial septum. When the septal wall is calcified as well, the term coconut atrium is used to describe the hyperdense white appearance of the complete left atrial calcification on imaging[6]. Multiple imaging techniques can be used to diagnose and assess the extent of calcification. On anterior-posterior chest radiograph mural calcification presents as a thin C shaped curvilinear density outlining the left atrium partially or completely with the opening of the C anterior to mitral annulus. Hyper-echogenicity within the walls of the left atrium, Restriction in atrial wall motion and mitral valve abnormalities can be well visualized with transthoracic and transesophageal echocardiography, however, CT is superior to all other imaging modalities for determining extent of calcification[20,27].
Dystrophic calcification of the left atrium can cause significant complications which can lead to hemodynamic compromise or even collapse. A common complication is the occurrence of mitral stenos is as the mitral leaflets become restricted due to calcification[20]. In cases of significant calcium deposition Arrhythmias can present especially if calcification occurs outside the left atrium involving the SA node, AV node or other points along the cardiac conduction system. Over time increased left atrial pressure can develop due to decreased compliance of the left atrial walls and can be transmitted through pulmonary veins resulting in derangements in right heart hemodynamics (Figure 3)[7,28].
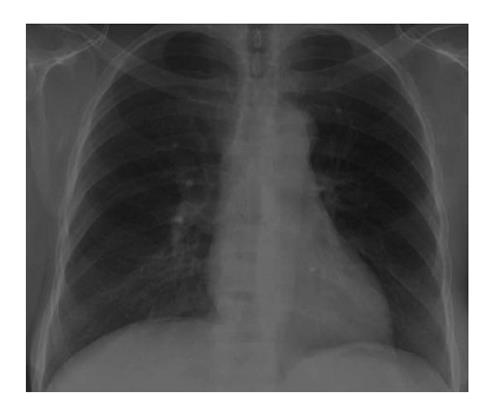
Figure 3 CXR shows a small amount of what appears to be pneumoperitoneum beneath the right hemidiaphragm that was present prior, otherwise, no change.
The accepted surgical treatment for left atrial calcification is endoatrioectomy with mitral valve replacement as the calcification usually does not extend beyond the endocardium[25]. Total endoatrioectomy of a calcified left atrium has been shown to be a technique with limited morbidity, however, not much follow up of atrial compliance has been done in patients who undergo this procedure in order to quantify improvement[29]. The two major contraindications to endoatrioectomy are calcification of the left atrial septal wall and mitral annulus. Septal calcification makes repair difficult and increases mortality due to the septum serving as a cleavage plane during surgery which helps prevent hemorrhage and embolization if thrombus is present. Caseous necrosis is known to cause mitral calcification, usually occurring along the posterior annulus[28,30].
Our case presentation is unique in that all of the walls of the left atrium are heavily calcified including the septal wall and left atrial appendage with minimal presence in the ascending aorta, however, there is no calcification of the mitral valve and no resultant mitral stenos is, mitral regurgitation, arrhythmias or hemodynamic compromise.
COMMENTS
Case characteristics
In a preoperative evaluation for renal transplant of an asymptomatic patient with a prior history of end stage renal disease (ESRD) and hypertension, there were no significant clinical or exam findings.
Clinical diagnosis
However, upon routine echocardiogram and chest X-ray, findings of dystrophic calcification was seen in the heart.
Differential diagnosis
Findings of dystrophic calcification lead to further concerns of tuberculosis, sarcoidosis, and other rare systemic inflammatory diseases.
Laboratory diagnosis
Calcium-phosphorous product at time of evaluation was within normal limits, and patient had no history of tuberculosis or a positive Purified Protein Derivative.
Imaging diagnosis
Further evaluation by computed tomography (CT) imaging confirmed a calcified left atrium and made sarcoidosis, and other more rare inflammatory disease less likely.
Pathological diagnosis
No further pathological studies were done, but the additional imaging studies confirmed dystrophic calcification in an individual with ESRD, a systemic inflammatory disease equivalent.
Treatment
The patient is at risk for complicated valvular stenosis, cardiac arrhythmias, cardiac block and abnormal cardiac hemodynamics due to this dystrophic calcification; thus close follow-up, and early treatment of these resulting complications are the key to the patient’s outcome.
Term explanation
An “atrium of stone”, thus refers to this dystrophic calcification almost isolated to the left atrium.
Experiences and lessons
ESRD patients often carry a higher mortality and morbidity than previous thought, which may not always be present on initial clinical and exam assessment.
Peer review
The authors present an unusual case of dystrophic left atrial calcification in the setting of end stage renal disease on hemodialysis diagnosed by echocardiography and CT. Calcium deposition is significantly confined within the walls of the left atrium with no involvement of the mitral valve or untoward effects on hemodynamics. This is an interesting case report for the clinical practice.