Published online Mar 16, 2014. doi: 10.12998/wjcc.v2.i3.67
Revised: December 27, 2013
Accepted: February 18, 2014
Published online: March 16, 2014
Processing time: 153 Days and 16.1 Hours
Hutchinson-Gilford progeria syndrome (HGPS) is a rare dysmorphic syndrome characterized by several features of premature aging with clinical involvement of the skin, bones, and cardiovascular system. HGPS has an estimated incidence of one in four million to one in eight million births. The main clinical features of HGPS include short stature, craniofacial dimorphism, alopecia, bone fragility, and cardiovascular disorders. The most frequent cause of death is myocardial infarction at a mean age of 13 years old. Dental manifestations include delayed development and eruption of teeth, discoloration, crowding and rotation of teeth, and displaced teeth. Cone beam computed tomography images revealed the absence of the sphenoid, frontal, and maxillary sinus, flattening of the condyles and glenoid fossa, and bilateral hypoplasia of the mandibular condyles. The disease is caused by mutations in lamin A/C (LMNA). Here, we present a case report of an 11-year-old boy with classical features of HGPS, which was caused by a de novo germ-line mutation (C1824T, G608G) in exon 11 of the LMNA gene. Some uncommon HGPS-associated features in our patient, such as alterations in the facial sinuses and hypoplasia of the condyles, contributed to the expansion of the phenotypic spectrum of this syndrome from a dentomaxillofacial perspective.
Core tip: Hutchinson-Gilford progeria syndrome (HGPS) is a rare genetic syndrome characterized by the accelerated appearance of aging in children. We report a case of an 11-year-old boy with HGPS with uncommon HGPS-associated dentomaxillofacial features. Alterations in the facial sinuses and hypoplasia of the condyles were recognized in our patient, expanding the phenotypic spectrum of this syndrome.
- Citation: Alves DB, Silva JM, Menezes TO, Cavaleiro RS, Tuji FM, Lopes MA, Zaia AA, Coletta RD. Clinical and radiographic features of Hutchinson-Gilford progeria syndrome: A case report. World J Clin Cases 2014; 2(3): 67-71
- URL: https://www.wjgnet.com/2307-8960/full/v2/i3/67.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i3.67
Hutchinson-Gilford progeria syndrome (HGPS; OMIN #176670) is an uncommon genetic disorder characterized by accelerated aging with clinical involvement of the skin, bones, and cardiovascular system[1,2]. The prevalence of HGPS is one in four million to one in eight million live births; males are more frequently affected than females, and the intellect of the affected children is unimpaired[3]. Clinically, individuals with HGPS demonstrate short stature, prominent eyes, micrognathia, craniofacial disproportion, loss of subcutaneous fat, alopecia, beaked nose, coxa valga, pathologic bone fractures, radiolucent terminal phalanges, hearing loss, photophobia, hypertension, hyperlipidemia, atherosclerosis, and cardiovascular disorders. The most frequent cause of death is myocardial infarction at a mean age of 13 years old[4-11]. Oral alterations include high rates of tooth decay, crowding, delayed tooth development and eruption, tooth discoloration, hypodontia, maxillary and mandibular hypoplasia, and small mouth opening[5-7,12,13]. Recognition of dentomaxillofacial features of HGPS may allow oral health problems to be readily identified and aid in implementation of preventative treatment plans to improve quality of life[14,15]. Although HGPS demonstrates both autosomal dominant and autosomal recessive modes of inheritance, most cases are due to sporadic mutations[16]. Mutations in the lamin A/C (LMNA) gene are responsible for HGPS[17-19].
Here we report a case of HGPS in an 11-year-old boy with an uncommon phenotype and a de novo heterozygous silent mutation at amino acid 608 (G608G) of the LMNA gene.
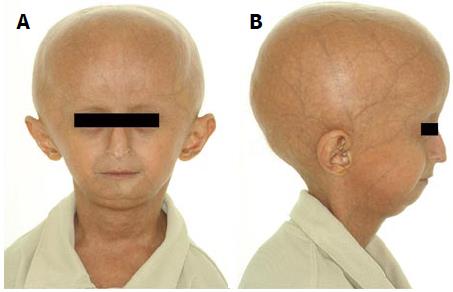
An 11-year-old boy with a clinical diagnosis of HGPS was referred to the Clinical Department, School of Dentistry, Federal University of Pará, Brazil for oral health care. He was suffering from angina, peptic ulcer disease, and limited joint mobility. His current medications were pravastatin (5 mg/d) to prevent cardiovascular disease and ranitidine (150 mg/d) for the treatment of the peptic ulcer. The patient had normal neurodevelopment and showed the classical clinical features of HGPS, including short stature, low weight/height ratio, thin and inelastic skin, eyes slightly open when sleeping, photophobia, osteoporosis in the femur region, generalized alopecia, prominent scalp veins, small face with a beaked nose, and high-pitched voice (Figure 1). The patient had no apparent hearing loss. Echocardiogram and electrocardiogram results, blood pressure, pulse, and oxygen saturation were within normal limits. A hand-wrist radiograph showed radiolucencies of the terminal phalanges and the skeletal maturity of a 14-year-old boy with a chronological age of 11 years and 8 mo.
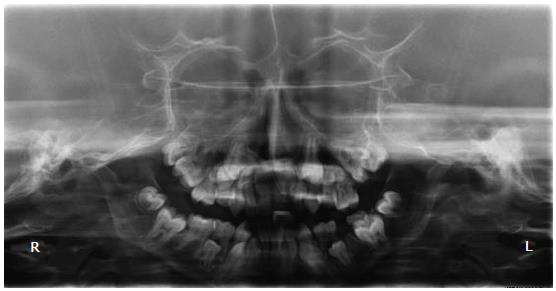
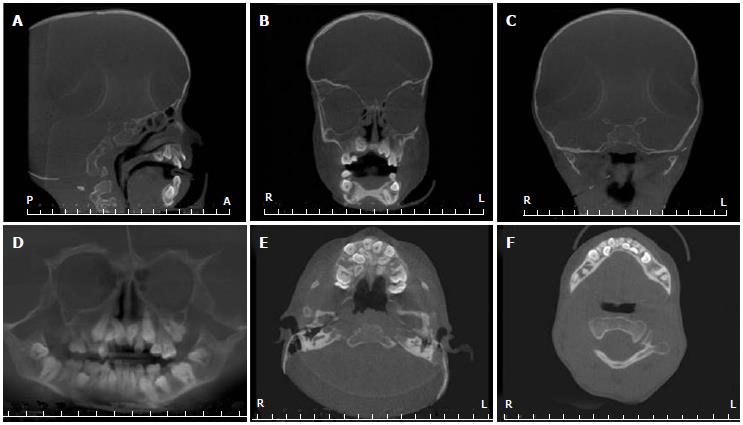
His height and weight were 1.1 m and 17.4 kg, respectively, which is well below the 3rd percentile for his age and only 4.4 kg greater than expected for a normal 4.5-year-old boy. Oral examination revealed micrognathia, class II malocclusion, and chronic trimus. Erupted teeth were of normal size, shape and color, but the permanent incisors were lingually erupted. The patient had gingivitis and low salivary flow, but had no dental caries and brushed his teeth while supervised by the mother. An orthopantomographic radiograph showed reduced dimensions of both arches with consequent lack of space for the correct positioning of the permanent teeth, mandible with a steep mandibular angle, eruption of the permanent teeth, and congenitally missing left upper second premolar and both lower second premolars (Figure 2). To better visualize the craniofacial features, cone beam computed tomography (CBCT) was performed. CBCT images revealed the absence of the sphenoid, frontal, and maxillary sinuses (Figure 3A and B), flattening of the condyles and glenoid fossa, and bilateral hypoplasia of the mandibular condyles (Figure 3C). Panoramic and axial images confirmed the dental alterations (Figure 3D-F).
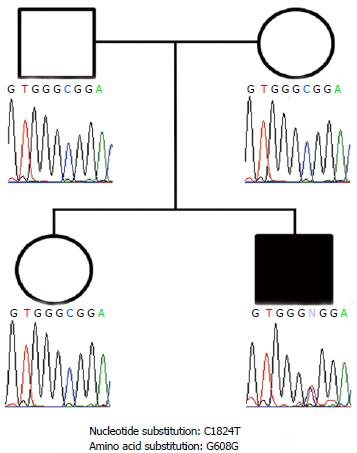
To confirm the clinical diagnosis of HGPS, DNA sequence analysis was performed. The parents gave informed consent before the genetic study began. Mutation analysis of the LMNA gene with genomic DNA extracted from oral mucosa cells was performed according to a published protocol[20]. The patient demonstrated a heterozygous C-to-T transition at nucleotide 1824 in exon 11 of LMNA, which created a silent point mutation at codon 608 (GGC>GGT, G608G) (Figure 4). A similar mutation was not observed in the patient’s parents or sister.
This case highlights some common and uncommon dentomaxillofacial features associated with HGPS. Tooth size was essentially normal but the eruption sequence was complicated by both incomplete mandibular and maxillary growth and micrognathia, which contributed to dental impactions. Despite radiographic evidence of normal root development, tooth eruption appeared to be delayed by three years. In addition, the permanent incisors had erupted lingually, and two premolars were absent (hypodontia). Delayed eruption, malocclusion associated with lower anterior dentition crowding, and hypodontia are consistent findings in patients with HGPS, as well as enamel hypoplasia and discoloration[15,20-22]. The permanent teeth in the current case were macroscopically normal in shape and color. Patients who do not present alterations in the joints of the hands can carry out oral hygiene perfectly, but adult supervision is required along with the use of a toothbrush with a small head due to the small oral cavity and limited mouth opening. Interestingly, CBCT images revealed the absence of the sphenoid, frontal and maxillary sinuses, shallow glenoid fossae, and bilateral hypoplasia of the mandibular condyles and articular eminences. After evaluating radiographs of 21 children aged newborn to 14.6 years old, Gordon et al[14] concluded that articulation deformities are not a common feature of HGPS. Chen et al[11] reported a similar case to ours and highlighted that craniofacial anomalies of HGPS contribute to increased number of caries, severe malocclusion, and problems with swallowing, feeding, and speech. However, Ullrich et al[23] evaluated 25 patients with HGPS and identified short mandibular rami in combination with flattened mandibular condyles, shallow glenoid fossae, and hypoplastic or absent articular eminences. The significance of the sinus alterations was unclear; however, patient- and parent-related chronic trismus can occur after a long period of regular dental treatment. Thus, HGPS patients should be evaluated for temporomandibular joint (TMJ) disorders, and dentists need to be aware of the possible TMJ complications after a long period of regular dental treatment.
Despite the reported clinical characteristics, HGPS may be confused with other syndromes that include some features of premature aging, including neonatal progeroid syndrome (Weidemann-Rautenstrauch syndrome), acrogeria, Cockayne syndrome, Hallermann-Streif syndrome, gerodermia osteodysplastica, Berardinelli-Seip congenital lipodystrophy (congenital generalized lipodystrophy), Petty-Laxova-Weidemann progeroid syndrome, Ehlers-Danlos syndrome, progeroid form, and Werner syndrome[10,21]. Since an overlap in the clinical features of the patients affected by progeroid syndromes is common, the diagnosis of HGPS is based on the recognition of common clinical features and the detection of mutations in the LMNA gene and eventually in the ZMPSTE2 gene, a metallopeptidase involved in processing of lamin A. LMNA mutations are present in more than 95% of cases, and genetic testing should start with analysis of the p.G608G mutation at exon 11, in which 62% of the defects reside[12]. DNA sequencing from the patient reported here revealed the p.G608G silent mutation. Although this mutation does not change the encoded amino acid, it results in the activation of a cryptic splice site and causes a truncated lamin A protein (50 amino acids shorter than normal), which is essential for the conversion of normal lamin A from prelamin A[18]. Since HGPS patients develop severe atherosclerosis and death usually occurs as a result of the complications of cardiac or cerebrovascular diseases during adolescence, early diagnosis of HGPS is important. To promote survival of HGPS patients, annual analysis of the vascular status is recommended using baseline electrocardiogram, echocardiogram, and carotid duplex scans to evaluate stenosis and intimal thickness. Additional tests include a skeletal X-ray to evaluate common associated features (e.g., acroosteolysis, clavicular resorption, and coxa valga), dual-energy X-ray absorptiometry to assess bone mineral density, standard goniometry to assess global joint mobility, and nutritional assessment to optimize caloric intake[24].
In summary, we report one patient affected by HGPS who demonstrated unusual features, including the absence of the sphenoid, frontal and maxillary sinuses and bilateral hypoplasia of the mandibular condyles. Proper characterization of the clinical features and genetic defects is of utmost importance for correct diagnosis and timely clinical management. Furthermore, early intervention by a multidisciplinary team can increase the quality of life and survival of HGPS patients.
An 11-year-old boy with a diagnosis of Hutchinson-Gilford progeria syndrome (HGPS) presented with a need for oral health care.
The patient exhibited classical clinical features of HGPS, including short stature, low weight/height ratio, thin and inelastic skin, eyes slightly open when sleeping, photophobia, generalized alopecia, prominent scalp veins, small face with a beaked nose, and high-pitched voice.
Differential diagnosis included neonatal progeroid syndrome (Weidemann-Rautenstrauch syndrome), acrogeria, Cockayne syndrome, Hallermann-Streif syndrome, gerodermia osteodysplastica, Petty-Laxova-Weidemann progeroid syndrome, and Werner syndrome.
Cone beam computed tomography (CBCT) images revealed absence of the sphenoid, frontal and maxillary sinuses, flattening of the condyles and glenoid fossa, and bilateral hypoplasia of the mandibular condyles.
DNA sequence analysis and mutation analysis of the lamin A/C (LMNA) gene was performed with genomic DNA extracted from oral mucosa cells. The patient demonstrated a heterozygous C-to-T transition at nucleotide 1824 in exon 11 of LMNA, which created a silent point mutation at codon 608 (GGC>GGT, G608G).
The patient received medical and dental treatment to improve his quality of life.
Recognition of dentomaxillofacial features of HGPS may allow for early identification of oral health problems and for the development of preventive treatment plans to improve quality of life.
Hutchinson-Gilford progeria syndrome is an uncommon genetic disorder characterized by accelerated aging with clinical involvement of the skin, bones, and cardiovascular system.
Proper characterization of the clinical features and genetic defects of HGPS is of utmost importance for correct diagnosis and initiation of timely clinical management; early intervention by a multidisciplinary team can increase the quality of life and survival of these patients.
This article reports a case of Hutchinson-Gilford progeria syndrome in an 11-year-old boy with an uncommon phenotype and a de novo heterozygous silent mutation at amino acid 608 (G608G) in the LMNA gene.
P- Reviewers: Franco AL, Rattan V, Worman HJ S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
| 1. | Badame AJ. Progeria. Arch Dermatol. 1989;125:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Brown WT, Zebrower M, Kieras FJ. Progeria, a model disease for the study of accelerated aging. Basic Life Sci. 1985;35:375-396. [PubMed] |
| 3. | Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet. 2006;7:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 4. | Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006;140:2603-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 380] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 5. | Russo-Menna I, Arancibias C. The Hutchinson-Gilford Progeria Syndrome: a case report. Minerva Anestesiol. 2010;76:151-154. [PubMed] |
| 6. | The Progeria Handbook: A Guide for Families and Health Care Providers of Children with Progeria. Accessed January 25, 2011. Available from: http://www.progeriaresearch.org/patient_care.html. |
| 7. | Cleveland RH, Gordon LB, Kleinman ME, Miller DT, Gordon CM, Snyder BD, Nazarian A, Giobbie-Hurder A, Neuberg D, Kieran MW. A prospective study of radiographic manifestations in Hutchinson-Gilford progeria syndrome. Pediatr Radiol. 2012;42:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Gordon CM, Gordon LB, Snyder BD, Nazarian A, Quinn N, Huh S, Giobbie-Hurder A, Neuberg D, Cleveland R, Kleinman M. Hutchinson-Gilford progeria is a skeletal dysplasia. J Bone Miner Res. 2011;26:1670-1679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Zhang H, Chen X, Guo Y, Liang J, Tang L, Yu H, Yao Z. Hutchinson-Gilford progeria syndrome: report of 2 cases and a novel LMNA mutation of HGPS in China. J Am Acad Dermatol. 2013;69:e175-e176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Pereira S, Bourgeois P, Navarro C, Esteves-Vieira V, Cau P, De Sandre-Giovannoli A, Lévy N. HGPS and related premature aging disorders: from genomic identification to the first therapeutic approaches. Mech Ageing Dev. 2008;129:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Chen CP, Lin SP, Lin DS, Liu YP, Hsu LJ, Wang W. Clinical imaging findings in a girl with Hutchinson-Gilford progeria syndrome. Genet Couns. 2012;23:1-7. [PubMed] |
| 12. | Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358:592-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 510] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 13. | Yu QX, Zeng LH. Progeria: report of a case and review of the literature. J Oral Pathol Med. 1991;20:86-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Gordon LB, McCarten KM, Giobbie-Hurder A, Machan JT, Campbell SE, Berns SD, Kieran MW. Disease progression in Hutchinson-Gilford progeria syndrome: impact on growth and development. Pediatrics. 2007;120:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Domingo DL, Trujillo MI, Council SE, Merideth MA, Gordon LB, Wu T, Introne WJ, Gahl WA, Hart TC. Hutchinson-Gilford progeria syndrome: oral and craniofacial phenotypes. Oral Dis. 2009;15:187-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Mazereeuw-Hautier J, Wilson LC, Mohammed S, Smallwood D, Shackleton S, Atherton DJ, Harper JI. Hutchinson-Gilford progeria syndrome: clinical findings in three patients carrying the G608G mutation in LMNA and review of the literature. Br J Dermatol. 2007;156:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Coutinho HD, Falcão-Silva VS, Gonçalves GF, da Nóbrega RB. Molecular ageing in progeroid syndromes: Hutchinson-Gilford progeria syndrome as a model. Immun Ageing. 2009;6:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1602] [Cited by in RCA: 1575] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 19. | De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1052] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 20. | Moulson CL, Fong LG, Gardner JM, Farber EA, Go G, Passariello A, Grange DK, Young SG, Miner JH. Increased progerin expression associated with unusual LMNA mutations causes severe progeroid syndromes. Hum Mutat. 2007;28:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Liessmann CD. Anaesthesia in a child with Hutchinson-Gildford progeria. Paediatr Anaesth. 2001;11:611-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Wesley RK, Delaney JR, Litt R. Progeria: clinical considerations of an isolated case. ASDC J Dent Child. 1979;46:487-492. [PubMed] |
| 23. | Ullrich NJ, Silvera VM, Campbell SE, Gordon LB. Craniofacial abnormalities in Hutchinson-Gilford progeria syndrome. AJNR Am J Neuroradiol. 2012;33:1512-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Gordon LB, Brown WT, Collins FS. Hutchinson-Gilford progeria syndrome. GeneReviews™ [Internet]. Seattle (WA): University of Washington, Seattle, 1993- 2014; . |