Published online Oct 16, 2025. doi: 10.12998/wjcc.v13.i29.108411
Revised: May 23, 2025
Accepted: July 22, 2025
Published online: October 16, 2025
Processing time: 137 Days and 3.1 Hours
In this letter, we delve into the groundbreaking research by Lorente et al, which sheds light on the intricate relationship between low salivary uric acid levels and periodontitis. The study not only confirms previous observations of reduced salivary uric acid concentrations in periodontitis patients but also establishes, for the first time, an independent association between these two factors, even when controlling for traditional risk factors such as age, smoking status, and arterial hypertension. Moreover, the findings reveal a significant negative correlation between salivary uric acid levels and the severity of periodontitis, suggesting that this biomarker may serve as a valuable indicator of disease progression. These discoveries open new avenues for understanding the pathophysiology of periodontitis and pave the way for innovative diagnostic and therapeutic strategies. The potential clinical applications of salivary uric acid measurement, such as guiding personalized treatment plans and monitoring disease activity, warrant further exploration to enhance patient care and improve outcomes in this prevalent inflammatory condition.
Core Tip: This letter explores the relationship between low salivary uric acid levels and periodontitis, revealing a significant independent association and inverse correlation with disease severity. Salivary uric acid emerges as a potential biomarker for disease progression, offering new insights into the pathophysiology of periodontitis and paving the way for innovative diagnostic and therapeutic strategies in clinical practice.
- Citation: Hu ZQ, Wang Y. Low salivary uric acid levels and periodontitis: New insights and implications for clinical practice. World J Clin Cases 2025; 13(29): 108411
- URL: https://www.wjgnet.com/2307-8960/full/v13/i29/108411.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i29.108411
Periodontitis, a chronic inflammatory disease characterized by the destruction of the periodontal ligament, affects approximately 50% of the global population with mild forms and 7% with advanced stages. Despite significant ad
The development and progression of periodontitis are driven by the complex interplay between microbial infections and host inflammatory responses, with oxidative stress and tissue destruction at their core. Salivary uric acid, the most abundant antioxidant in saliva and accounting for approximately 70% of its total antioxidant capacity, has garnered attention. Previous studies and meta-analyses have indicated that patients with periodontitis exhibit significantly lower concentrations of salivary uric acid compared to healthy individuals. For instance, a systematic review by Uppin et al[2] noted that while serum uric acid levels are elevated in periodontitis patients, salivary uric acid levels are reduced, suggesting the potential of salivary uric acid (SUA) as a diagnostic and prognostic marker. Similarly, a meta-analysis by Ye et al[3] confirmed an association between periodontitis and elevated serum uric acid alongside reduced salivary uric acid. However, prior research has not thoroughly explored the independent association of SUA levels with periodontitis and its specific relationship with disease severity after adjusting for confounding factors.
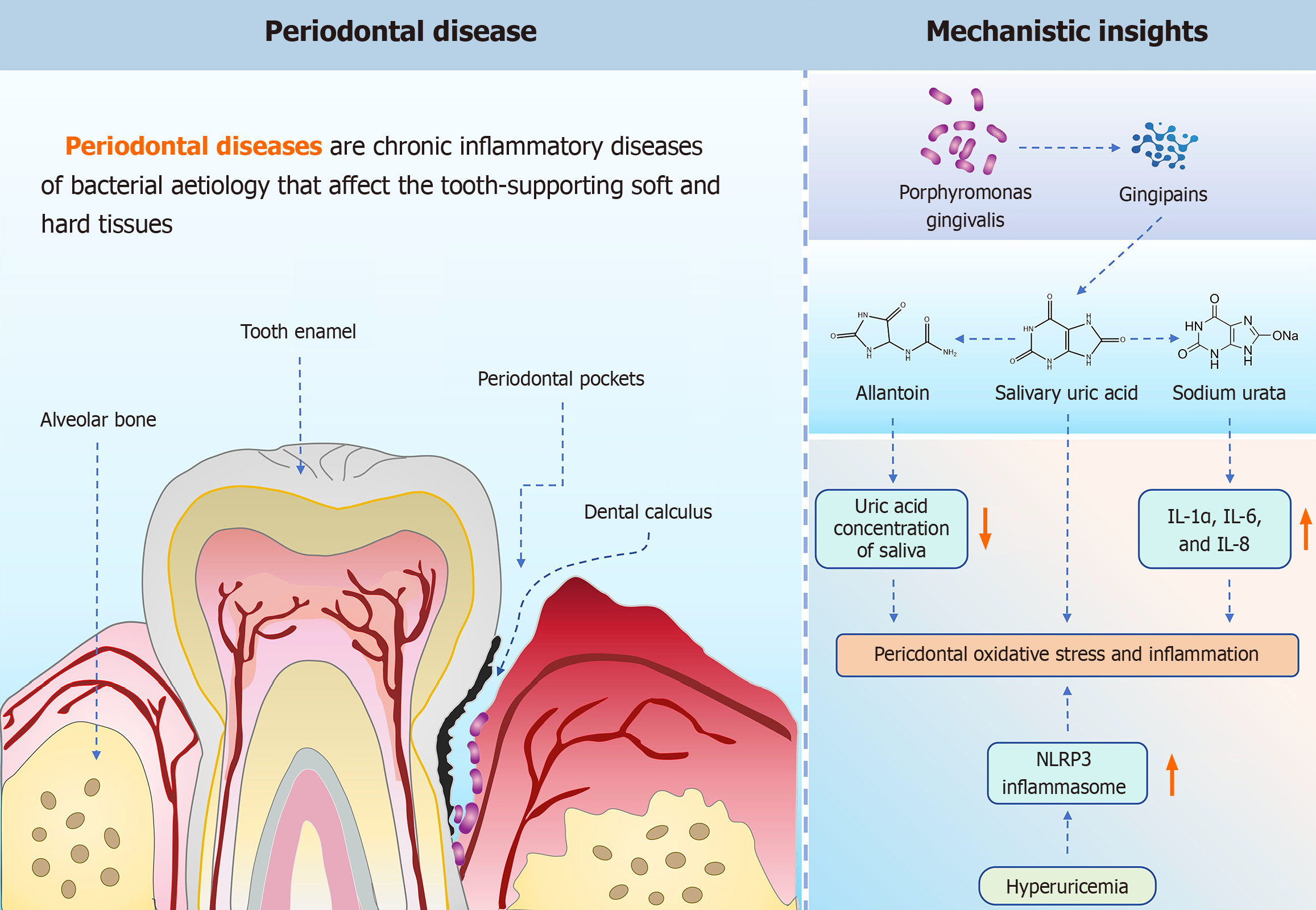
The inverse relationship between SUA levels and the severity of periodontitis raises important questions about underlying mechanisms. Figure 1 highlights the key role of uric acid in periodontal disease and illustrates some of the hot pathological mechanisms. One hypothesis posits that oxidative stress during periodontal infection may lead to the oxidation of uric acid to allantoin, thereby reducing its concentration in saliva. Another possibility is that uric acid serves as a substrate for microbial metabolism within dental plaque, further depleting its levels. Research by Jun et al[4] supports this perspective, indicating that Porphyromonas gingivalis, a key periodontal pathogen, induces uric acid generation through its gingipains, directly linking microbial activity to oxidative stress and inflammation. Furthermore, studies by Wu et al[5] demonstrated that hyperuricemia exacerbates experimental periodontitis by promoting NLRP3 inflammasome activation and increasing oxidative stress, suggesting a bidirectional relationship between uric acid metabolism and periodontal inflammation. Chen et al[6] found significantly higher levels of uric acid in the gingival tissues of periodontitis-induced mice compared to control groups, indicating a potential active role of uric acid in disease progression. These findings suggest that SUA levels may not only serve as biomarkers but also play an active role in disease progression.
Lorente et al[1] conducted a prospective observational study involving 121 participants, including 61 patients with periodontitis and 60 without (39 with periodontal health and 21 with localized gingivitis). Using a validated enzymatic method to measure SUA levels and employing multiple regression models to analyze their association with periodontitis, the study revealed two key findings that highlight the diagnostic potential of SUA levels. First, low SUA levels (< 111 nmol/mL) were significantly associated with periodontitis, with an odds ratio of 6.14 (95%CI: 2.015-18.721; P = 0.001), even after adjusting for age, smoking history, and arterial hypertension. Second, SUA levels exhibited a significant negative correlation with the severity of periodontitis (rho = -0.32; P < 0.001), suggesting that lower SUA levels may indicate more advanced disease stages. The area under the receiver operating characteristic curve was 66% (95%CI: 57%-75%; P < 0.001), indicating that while SUA levels cannot replace clinical and imaging assessments for diagnosis, they can serve as a complementary tool for evaluating antioxidant status and guiding personalized treatment strategies.
The findings of Lorente et al[1] hold significant clinical implications. First, SUA levels may help identify patients at higher risk of disease progression, particularly in cases where traditional risk factors are absent or inadequately controlled. Second, measuring SUA levels can guide adjunctive therapies, such as the use of antioxidant mouthwashes, to improve treatment outcomes in patients with low antioxidant capacity. Additionally, as a non-invasive biomarker, SUA levels can be used to monitor treatment responses and disease activity. For example, a study by Priya et al[7] indicated that salivary arginase levels serve as pro-inflammatory markers, while elevated salivary uric acid levels act as anti-inflammatory markers, highlighting their roles in assessing periodontal status and treatment efficacy. Furthermore, research by Toczewska et al[8] demonstrated that reduced glutathione levels in saliva can distinguish between different severities of periodontitis, underscoring the potential of salivary antioxidants as markers of disease progression.
Recent studies have further emphasized the role of salivary uric acid and oxidative stress in periodontitis. For instance, Vernerová et al[9] developed a high-performance liquid chromatography method combined with fluorescence and diode array detection, capable of quantifying inflammatory biomarkers, including uric acid, in saliva, proving its utility in monitoring early disease manifestations. Sredojevic et al[10] found significantly elevated levels of antioxidant enzymes in the saliva of patients with systemic sclerosis and periodontitis, indicating the significant role of oxidative stress in periodontal pathology. Chen et al[11], using surface-enhanced Raman spectroscopy to predict the prognosis of non-surgical periodontal therapy, identified uric acid as a potential biomarker. Collectively, these findings highlight the significant role of salivary uric acid in periodontal health and disease.
Although the study by Lorenteet al[1] provides valuable insights, several limitations should still be considered. The relatively small sample size and the exclusion of individuals under 18 years of age limit the generalizability of the findings. Future research should validate these results in larger, more diverse populations and further explore the mechanistic links between SUA and periodontal pathology. Additionally, investigating the potential of SUA as a therapeutic target, such as through the application of local antioxidants, may enhance its clinical utility.
The study by Lorente et al[1] makes a significant contribution to understanding the relationship between salivary uric acid and periodontitis. By revealing an independent association and inverse relationship between low SUA levels and disease severity, this study paves the way for innovative diagnostic and therapeutic approaches. As our understanding of the complex interplay between oxidative stress and periodontal health continues to evolve, salivary uric acid may emerge as a valuable tool for improving treatment outcomes in patients with this prevalent inflammatory condition.
We acknowledge Leonardo Lorente and colleagues for their pioneering research on the association between low salivary uric acid levels and periodontitis, which has drawn more attention to this emerging field.
| 1. | Lorente L, Hernández Marrero E, Abreu-gonzalez P, Lorente Martín AD, González-rivero AF, Marrero González MJ, Hernández Marrero C, Hernández Marrero O, Jiménez A, Hernández Padilla CM. Low salivary uric acid levels are independently associated with periodontitis. World J Clin Cases. 2025;13. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Uppin RB, Varghese SS. Estimation of Serum, Salivary, and Gingival Crevicular Uric Acid of Individuals With and Without Periodontal Disease: A Systematic Review and Meta-analysis. J Int Soc Prev Community Dent. 2022;12:393-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Ye LW, Zhao L, Mei ZS, Zhou YH, Yu T. Association between periodontitis and uric acid levels in blood and oral fluids: a systematic review and meta-analysis. BMC Oral Health. 2023;23:178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Jun HK, An SJ, Kim HY, Choi BK. Inflammatory response of uric acid produced by Porphyromonas gingivalis gingipains. Mol Oral Microbiol. 2020;35:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Wu Z, Zhao L, Guo Y, Lin C, Lu P, He Q, Zhou Y, Wang X, Yu T. Hyperuricemia Exacerbates Experimental Periodontitis via Uric Acid-Induced Periodontal Inflammation and Oxidative Stress. J Clin Periodontol. 2025;52:773-786. [PubMed] |
| 6. | Chen ZY, Ye LW, Zhao L, Liang ZJ, Yu T, Gao J. Hyperuricemia as a potential plausible risk factor for periodontitis. Med Hypotheses. 2020;137:109591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Priya KL, Mahendra J, Mahendra L, Kanakamedala A, Alsharif KF, Mugri MH, Varadarajan S, Alamoudi A, Hassan AAA, Alnfiai MM, Alzahrani KJ, Bahammam MA, Baeshen HA, Balaji TM, Bhandi S. Salivary Biomarkers in Periodontitis Post Scaling and Root Planing. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Toczewska J, Maciejczyk M, Zalewska A, Konopka T. Gingival fluid and saliva concentrations of selected non-enzymatic antioxidants in periodontitis. Dent Med Probl. 2022;59:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Vernerová A, Krčmová LK, Heneberk O, Radochová V, Strouhal O, Kašparovský A, Melichar B, Švec F. Chromatographic method for the determination of inflammatory biomarkers and uric acid in human saliva. Talanta. 2021;233:122598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Sredojevic SI, Dolijanovic SP, Dozic I, Pficer JK, Aleksic Z, Nikolic-Jakoba NS. Salivary Antioxidant Profile in Patients with Systemic Sclerosis and Periodontitis. Mediators Inflamm. 2023;2023:7886272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Chen S, Wu H, Chen C, Wang D, Yang Y, Zhou Z, Zhu R, He X, Pan Y, Li C. The prognostic prediction of periodontal non-surgery therapy in periodontitis patients based on surface-enhanced Raman measurements of pre-treatment saliva. Spectrochim Acta A Mol Biomol Spectrosc. 2023;288:122150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |