Published online Aug 16, 2025. doi: 10.12998/wjcc.v13.i23.107096
Revised: April 5, 2025
Accepted: May 8, 2025
Published online: August 16, 2025
Processing time: 81 Days and 4.6 Hours
Acinar cystic transformation (ACT) of the pancreas is a rare non-neoplastic transformation of the pancreas. Adult women are the majority of patients with ACT, but few cases have been reported in pediatric patients. Given that there are currently no guidelines for the treatment of ACT, current treatment is based primarily on expert opinions and clinical experiences. Here, we report the case of the youngest child with ACT to date. Additionally, a literature review on pediatric ACT cases was performed to summarize previous clinical experience and treatment methods.
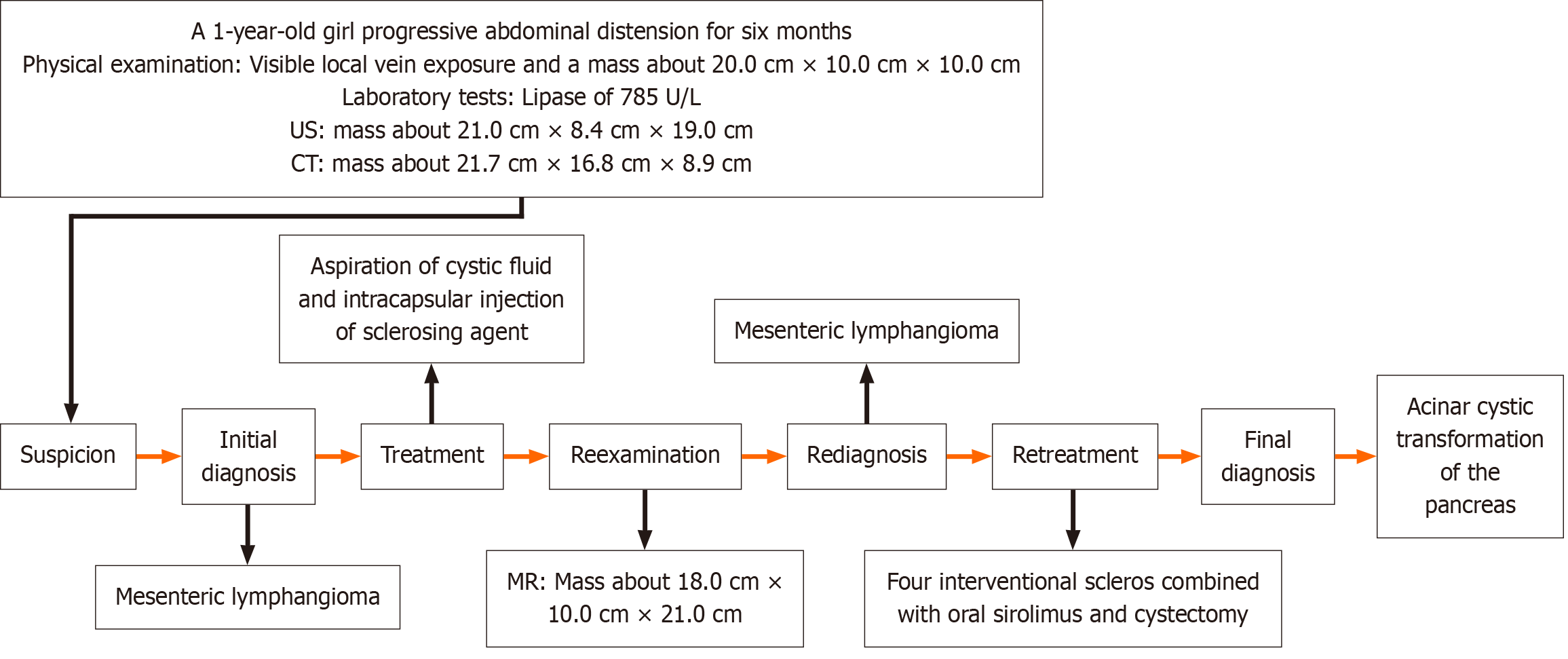
A 1-year-old Chinese girl presented with progressive abdominal distension for 6 months. A detailed consultation revealed an uneventful history. The patient showed no signs of fever or abdominal pain and had a good appetite and normal feces. A mass of about 20 cm × 10 cm × 10 cm in size was detected in the abdomen. Both abdominal ultrasound and computed tomography examination revealed a multilocular cystic mass about 21.7 cm × 16.8 cm × 8.9 cm in size. At first, due to the large size and the possible retroperitoneal origin of the cyst, a total resection of the lesion was not possible. A single-port laparoscopic lymphangioma puncture and Pingyangmycin injection were performed in March 2023. One month after surgery, the abdominal cyst rapidly enlarged to its pre-operative size. After consulting with the experts in the angiology department and interventional department, sclerotherapy combined with oral sirolimus was performed in May 2023. After confirming that the tumor was not sensitive to sclerotherapy combined with oral sirolimus, our surgical team performed tumor reduction in August 2023. This surgery confirmed that the polycystic mass originated from the head of the pancreas, and pathological and immunohistochemical findings diagnosed pancreatic ACT. The patient showed no signs of cyst lesions after 6 months of follow-up and remains in good health up to the time of this report.
ACT is a rare non-neoplastic transformation of the pancreas, more rarely seen in children. Manifestation and examinations show no specificity for diagnosis, and final diagnosis is mainly based on histological findings. To reach a specific diagnosis and rule out malignancy is a priority in clinical practice, and repeated biopsy or radical surgery should be considered before malignancy is ruled out. However, once a diagnosis of ACT is made, a conservative treatment with consecutive follow-up is recommended until symptoms present or obvious enlargement occurs because ACT is considered a slow-growing and benign tumor.
Core Tip: Acinar cystic transformation (ACT) of the pancreas is a rare non-neoplastic transformation of the pancreas, more rarely seen in children. Diagnosis is based on histological findings and therapy should be individualized. We report a rare case of a female child undergoing tumor reduction surgery. ACT is a heterogeneous entity, including a wide spectrum of lesions with different potential etiopathogenetic aspects. Because the clinical course is almost invariably benign, upfront surgical resection is not supported for asymptomatic lesions. We conclude that, because ACT is a slow-growing benign tumor, the principle of treatment is to follow up the child closely rather than perform overly aggressive surgery when imaging and cytological results are mutually supportive.
- Citation: Zhong XY, Liang ZJ, Lan ML, Xu XG, Yuan L, Zeng JX. Acinar cystic transformation of the pancreas: A rare case report. World J Clin Cases 2025; 13(23): 107096
- URL: https://www.wjgnet.com/2307-8960/full/v13/i23/107096.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i23.107096
Acinar cystic transformation (ACT) of the pancreas, also known as acinar cell cystadenoma, is a rare non-neoplastic transformation of the pancreas. Among the many different types of pancreatic cystic diseases, ACT is the most controversial and difficult to diagnose before surgery[1,2]. In April 2002, Albores Saaveda accidentally discovered a 9 cm multilocular cystic lesion in the body and tail of the pancreas during an autopsy and first described it as an acinar cystadenoma[3]. This cystic lesion is microscopically filled with eosinophils and lamellar tubercules (called lactosomes), lined with clearly differentiated acinar epithelium, and lacks nuclear atypia or mitosis.
The average age at diagnosis is 43.5 years, with the oldest being 82 years[4-8]. Half of ACT patients come from North America, followed by Europe and Asia[1]. ACT patients are predominantly female (65.3%), with a male-to-female ratio of about 2: 1. Although ACT is generally thought to affect adult women, there are still a small number of published case reports of ACT in children[9,10]. In view of the lack of current treatment guidelines for ACT, the diagnosis and treatment of children with ACT are still based on clinical experience described in the literature. Herein, we report a case of ACT that is the youngest child case worldwide to date. Additionally, a literature review was conducted on ACT in children to summarize clinical experiences and available treatments.
A 1-year-old Chinese girl was admitted to Guangzhou Women and Children Medical Center affiliated with Guangzhou Medical University in March 2023 with progressive abdominal distension for 6 months. Symptoms began 6 months before presentation with progressive abdominal distension.
The patient showed no signs of fever or abdominal pain, and had a good appetite and normal feces.
The patient showed no signs of fever or abdominal pain, and had a good appetite and normal feces. A detailed consultation revealed an uneventful history.
The patient’s guardian denied any family history of malignant tumors.
Physical examination revealed abdominal distention with visible local vein exposure and a soft, well-defined, and mobile mass about 20.0 cm × 10.0 cm × 10.0 cm in size was found in the abdomen.
Laboratory tests showed that routine blood values, hepatic and renal function, and blood gas analysis were normal, but an extremely high lipase value of 785 U/L was detected.
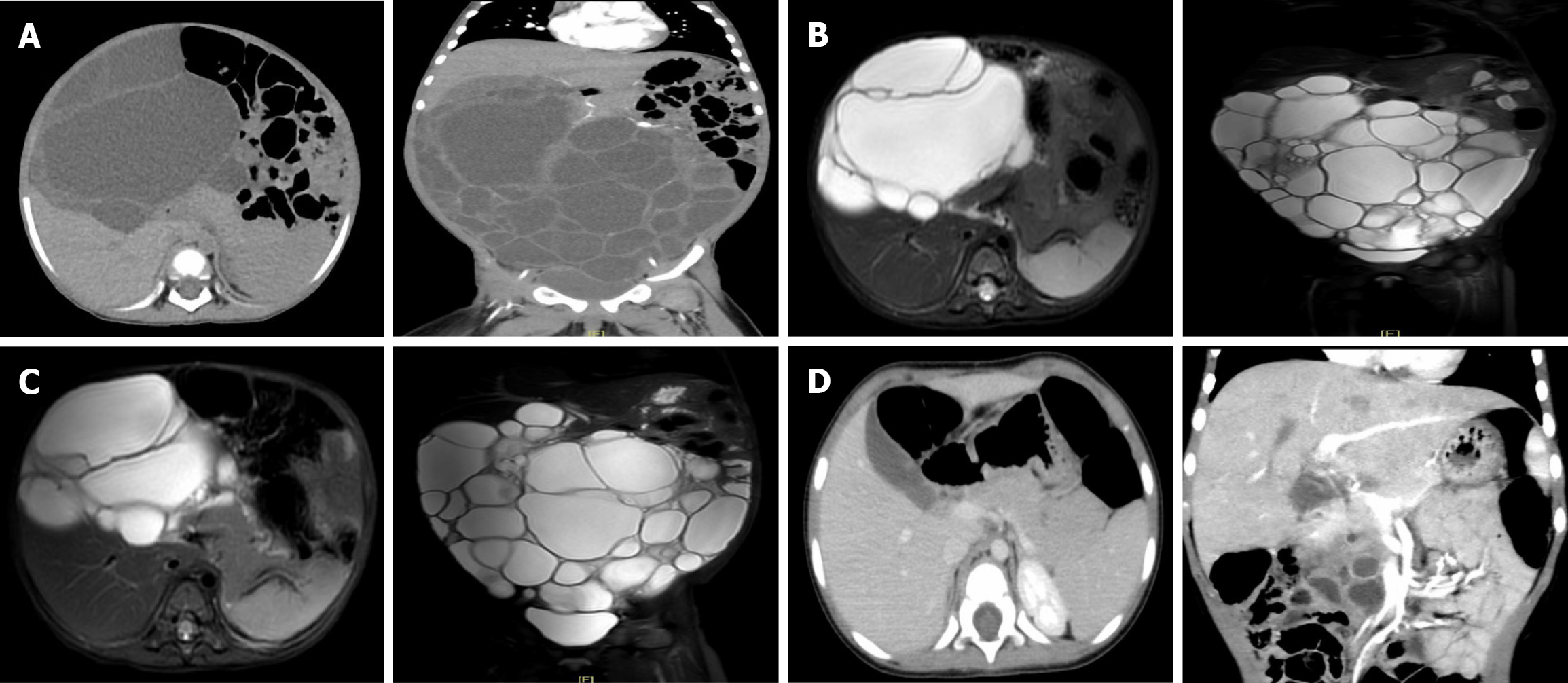
Ultrasound examination revealed a large cystic mass about 21.0 cm × 8.4 cm × 19.0 cm in size in the abdominal and pelvic cavity. Subsequently, an abdominal computed tomography (CT) scan revealed a massive multilocular cystic mass about 21.7 cm × 16.8 cm × 8.9 cm in size in the abdomen and pelvis, with uneven internal partition thickness and varying density (Figure 1A).
ACT of the pancreas.
Diagnostic criteria: (1) Macroscopic Findings: A polycystic mass protruded from the head of the pancreas; (2) Microscopic Findings: The lesion was lined by a single layer of cuboidal to columnar epithelium with focal acini; and (3) Immunohistochemical analysis: Trypsin(+), CK19(+).
Based on the clinical manifestation, our diagnosis was more inclined to mesenteric lymphangioma originating from the retroperitoneal cavity. The differential diagnoses included chronic pancreatitis and a pancreatic cystic disease. In March 2023, a single-port laparoscopic exploration was performed in the pediatric surgery department of our hospital. During the operation, a diffuse retroperitoneal cystic lesion was found in the abdominal cavity. Only about 800 mL of yellow-tinted non-viscous fluid were aspirated from polycystic mass. Cyst fluid analysis revealed that lymphocytes accounted for 9% and neutrophils accounted for 91%. Based on the above results, the diagnosis of mesenteric lymphangioma was made. As the size of the cyst was large and originated from the retroperitoneal cavity, the total resection of the lesion was not possible. Therefore, a resection was not performed and 6 mL of Pingyangmycin mixture was injected into the sac after the completion of sac fluid suction.
However, the symptoms of bloating remained unrelieved. Abdominal distension worsened with an obviously swollen abdomen and visible veins on the abdominal wall within 1 month. A more comprehensive evaluation of the cystic mass using magnetic resonance imaging (MR) was recommended. One month after the first surgery, MR showed a large irregular polycystic mass about 18.0 cm × 10.0 cm × 21.0 cm in size in the abdomen and pelvis. This large mass produced a hypointense signal on the T1-weighted imaging and a hyperintense signal on the T2-weighted imaging (Figure 1B). The abdominal cyst rapidly enlarged to the size it had been before the first operation. The serum lipase was tested again, and it was found that the lipase level was lower than before at 116 U/L. Less pancreatic inflammation was present. Because the cystic mass in the abdominal cavity of the child was too large, after consultation with the experts in the angiology department and interventional department, our team considered sclerotherapy combined with oral sirolimus to reduce the volume of the cystic mass by extracting the cyst fluid and killing the endothelial cells with secretory function on the cyst wall. In May 2023, an intraperitoneal lymphatic injection was performed in the interventional department of our hospital. During the operation, the tumor was found to be cystic and solid. About 750 mL of light yellow clear cyst fluid were extracted through puncture, and the patient then received four applications of sclerosis treatment with 4 mg Pingyangmycin hydrochloride (Harbin Leibotong Pharmaceutical Co., Ltd.) each time.
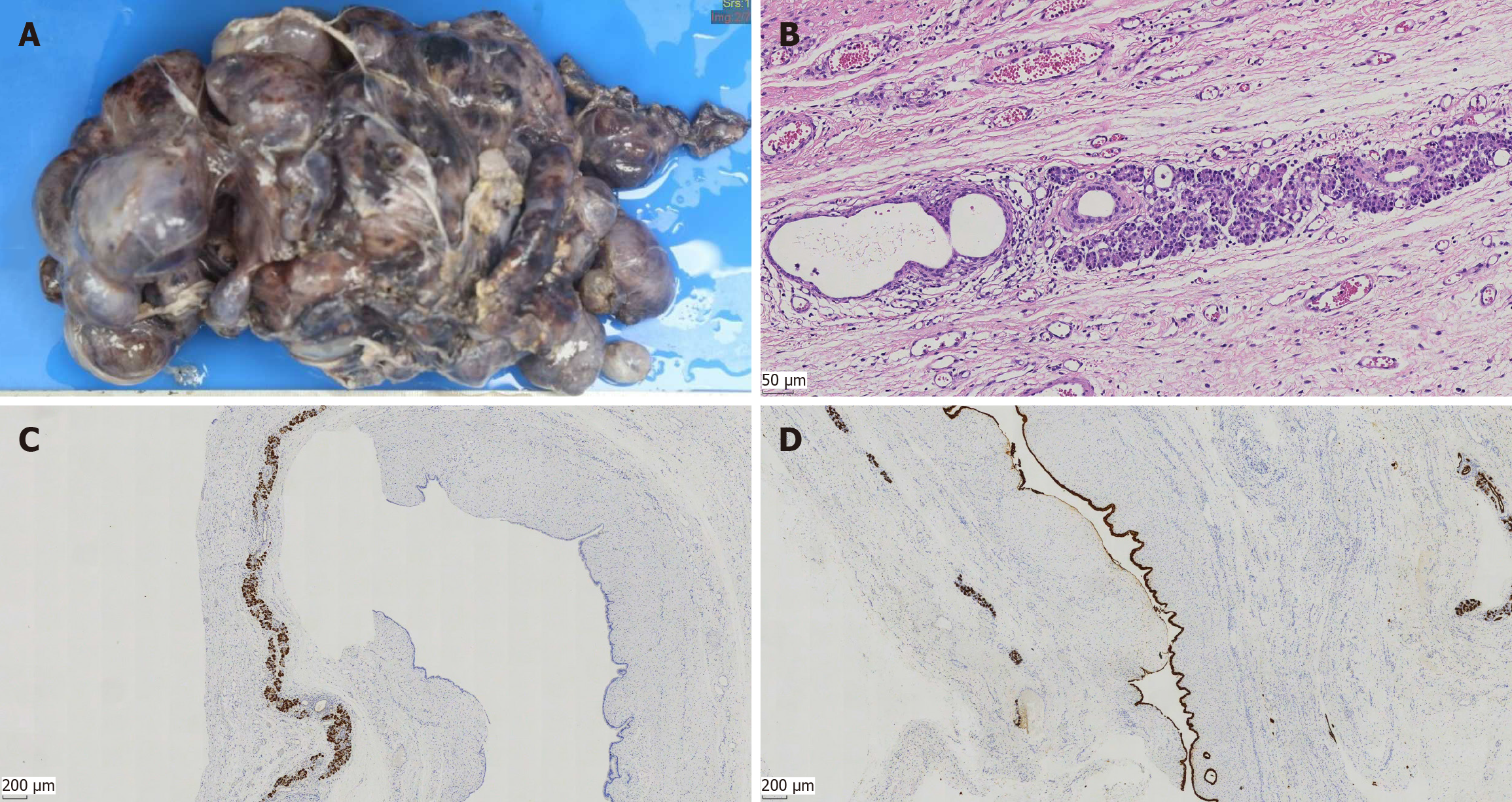
After the second surgery, 1 mL of oral sirolimus was administered twice daily at a steady serum concentration of 5–15 ng/mL for 3 months. The serum lipase was tested again and was found to be normal at 52.5 U/L. MR in June 2023 still showed an irregular polycystic mass in the abdomen and pelvic cavity measuring 17.9 cm × 11.0 cm × 21.0 cm, similar in size to the previous mass (Figure 1C). The serum lipase returned to normal levels. As the cystic mass was not sensitive to sclerotherapy combined with oral sirolimus, our surgical team performed a third surgery in August 2023. The surgery aimed to verify the accuracy of the diagnosis of mesenteric lymphangioma, reduce the burden of the tumor, and relieve the compression symptoms of the child as much as possible. The third operation showed that the tumor consisted of a polycystic mass, about 30.0 cm × 20.0 cm × 10.0 cm in size, emanating from the head of the pancreas, which was closely attached to the posterior peritoneum and mesentery. Macroscopically, the multiple cystic components consisted of a multilocular cystic lesion with cysts ranging in size from 2.0 cm × 1.5 cm × 1.0–3.5 cm × 3.0 cm × 2.5 cm (Figure 2A). Each cyst contained a smooth white wall filled with clear yellow fluid. Microscopically, the lesion consisted of multiple variable-sized cysts separated by fibrous stroma and residual islands of pancreatic tissue the cysts, which were lined by a single layer of cuboidal to columnar epithelium with focal acini (Figure 2B). Immunohistochemical analysis showed that the cysts endothelial cells were positive for trypsin (Figure 2C) and CK19 (Figure 2D). Postoperatively, the patient’s abdominal discomfort was resolved and the patient was discharged on postoperative day 17. The diagnosis and treatment flow chart is shown in Figure 3.
The patient's symptoms resolved after the surgery, and she was placed under close ultrasound and CT surveillance. One month after the operation, ultrasound revealed a multilocular cystic mass (6.6 cm × 5.0 cm × 3.3 cm) around the head of the pancreas with a larger cystic cavity of about 3.2 cm × 2.3 cm × 2.7 cm. Three months after the operation, ultrasound revealed that the cystic mass in the head of pancreas measured 7.3 cm × 5.4 cm × 2.6 cm. No obvious enlargement of cystic mass was observed. Six months after surgery, CT reexamination revealed multiple cystic lesions in the head of the pancreas (4.6 cm × 4.3 cm × 2.0 cm) (Figure 1D). The cyst was smaller than before, and no symptoms of the lesion were documented.
ACT is an extremely rare benign pancreatic cystic neoplasm, which is more common in adult women between 20 and 50 years of age and rarely diagnosed in children[11,12]. Our study reported the youngest patient with ACT in the literature. We also collected adult ACT cases published in the past 5 years and seven published pediatric cases of ACT in seven articles, and the clinical manifestations and imaging examinations of the two cases were summarized and compared to explore clinical treatment strategies.
We reviewed 11 adult cases and eight pediatric cases in the literature, with detailed information presented in Table 1 and Table 2, respectively. More than half of patients were female (male to female ratio: 0.71:1), with only five male patients. The mean age at diagnosis was 46 years, ranging from 25 to 69 years[2,4-5,7,12-18]. The most frequent symptom was abdominal pain (50%)[2,5,13-14,16], while almost half of patients (41.7%) did not report significant symptoms[4,7,12,15,17]. ACTs did not originate from a specific location within the pancreas; five cases were located in the tail of the pancreas[4,7,13-14,16], with 25% of cases arising in the head region[15,17], while 8.3% of the cases showed a diffuse involvement of the pancreatic gland[5]. Most cases presented as multiple cystic lesions (41.7%)[2,4-5,7,14,17], while solitary cysts were found in 16.7% of cases[12,16]. In children, ACT was predominantly female (male to female ratio: 0.75:1) and ranged in age from 9–18 years[5,11,19-22]. Abdominal pain was the most common symptom in all patients. ACT can occur in all parts of the pancreas, with about two-thirds of cases located in the head of the pancreas[5,9,11,20]. Only one case of diffuse enlargement of the pancreas has been reported[22]. In general, 85% of cases are multilocular[5,9,11,19] and only 25% are single-locular[20]. The size ranges from 3.5 to 11.7 cm[5,9,11,19-22].
| Ref. | Age (year) | Sex | Symptoms | Mass size (cm) | Cyst | Location | Preoperative diagnosis | Diagnosis | Treatment | Follow-up (M) |
| Rift et al[13], 2020 | 57 | F | Abdominal pain | 1.2 | - | Tail | IPMN | Histopathology | DP | NEOD (4) |
| Lee et al[4], 2021 | 51 | F | No | 8.0 | Multilocular | Tail | SC | Histopathology | DP | NEOD |
| Büyük et al[5], 2022 | 37 | M | Abdominal pain | 5.0 × 4.0 × 3.0 | Multilocular | Uncinate | IPMN | Histopathology | PD | NEOD (6) |
| Büyük et al[5], 2022 | 43 | F | Abdominal pain | <1.0 | - | Diffuse | Diffuse small cysts | Histopathology | TP | NEOD (86) |
| Ghio et al[7], 2022 | 30 | F | No | 8.0 | Multilocular | Body and tail | SC | Histopathology | DP+ splenectomy | NEOD (12) |
| Steinkraus et al[2], 2023 | 37 | M | Abdominal pain | - | Multilocular | - | IPMN | Histopathology | PD | NE |
| Narwal et al[14], 2023 | 69 | M | Abdominal pain | 14.5 × 9.0 × 4.0 | Multilocular | Tail | MCN | Histopathology | DP | NEOD (1.5) |
| Sergi et al[15], 2023 | 57 | F | No | 3.0 | - | Head | IPMN | Histopathology | PD | NE |
| Tatsumi et al[16], 2023 | 25 | M | Abdominal pain | 0.5 | Unilocular | Tail | IPMN | Histopathology | DP+ splenectomy | NEOD (12) |
| Sebastian et al[17], 2023 | 51 | F | No | 6.9 | Multilocular | Head | SC | Histopathology | PD | NE |
| Pritchard et al[18], 2024 | 50s | M | Iliac fossa pain | 2.8 × 3.3 × 2.6 | - | Head | IPMN | Histopathology | PD | NEOD (6) |
| Ahmed et al[12], 2024 | 45 | F | No | 6.3 × 6.9 | Unilocular | Body | MCN | Histopathology | DP | NE |
| Ref. | Age (year) | Sex | Symptoms | Mass size (cm) | Cyst | Location | Preoperative diagnosis | Diagnosis | Treatment | Follow-up (M) |
| Zamboni et al[9], 2002 | 16 | F | Abdominal pain | 7.5 | Multilocular | Head | - | Histopathology | PD | NEOD (30) |
| McEvoy et al[19], 2010 | 9 | M | Acute abdominal pain | 11.7 × 7.2 × 4.4 | Multilocular | - | Appendicitis | Histopathology | Cystectomy | NEOD (7) |
| Singhi et al[20], 2013 | 18 | F | Abdominal pain | 10 | Unilocular | Head | - | Histopathology | Cystectomy | NEOD (94) |
| Cosgrove et al[21], 2016 | 16 | M | Abdominal pain | 3.5 × 4.0 × 5.0 | - | Tail | Cystic pancreatic mass | Histopathology | Cystectomy | NE |
| Wen et al[11], 2020 | 10 | F | Intermittent abdominal pain | 4.0 × 3.0 | Multilocular | Head | Retroperitoneal lymphangioma | Histopathology | Cystectomy | EOD (180) |
| Büyük et al[5], 2022 | 15 | F | Intermittent abdominal pain | 4.0 × 3.0 | Multilocular | Head | Serous cystadenoma/IPMN | Histopathology | PD | NEOD (20) |
| Guo et al[22], 2023 | 17 | M | Intermittent abdominal pain | - | - | Diffuse involvement | - | Histopathology | Cystectomy | NE |
Due to the lack of specific clinical manifestations and imaging features of ACT, preoperative diagnosis is extremely challenging for clinicians. Most ACT patients are misdiagnosed as cystic tumor cases based on preoperative imaging in both adult and pediatric patients[4-5,11,16,18,23]. Based on the reported child cases, 87.5% of the children received inaccurate preoperative diagnosis, including appendicitis[19], cystic pancreatic mass[21], retroperitoneal lymphangioma[11], serous cystadenoma, intraductal papillary mucinous neoplasia (IPMN)[5], and mesenteric lymphangioma in our case. Among the adult cases collected, malignant diagnosis was more likely due to age and imaging findings. Of these, 50% of patients were diagnosed with intraductal papillary mucinous neoplasia[2,5,13,15-16,18], three with serous cystadenoma[4,7,17], two with mucinous cystic neoplasm[12,14], and one with no definite diagnosis[5].
Delavaud et al[24] found some imaging features on CT or MR that may be able to distinguish ACT from intraductal papillary mucinous neoplasia (IPMNs): (1) Five or more cysts; (2) Clustered peripheral small cysts; (3) Presence of cyst calcifications; and (4) Absence of communication with the main pancreatic duct. We also retrospectively re-evaluated the imaging data of our case and found similar features: (1) A multilocular cystic mass; (2) The density of each chamber is different and calcification can be seen; and (3) Lack of connection with the main pancreatic duct. These imaging features of ACT may serve as a key to presurgical diagnosis and should receive more attention.
The diagnosis of ACT is based on histological results because the clinical symptoms and examinations cannot exclude a malignant lesion. In clinical practice, a clear diagnosis and the exclusion of pancreatic malignancy are the top priority. Endoscopic ultrasound (EUS) with fine-needle aspiration or needle-based confocal endomicroscopy with biopsy can be initially performed[12]. If malignant lesions cannot be completely excluded, surgery should be actively performed. Aggressive radical surgery is more common in adult cases because pancreatic cancers are fatal and progressive and delayed treatment would have irremediable results[4,7]. In children cystectomy was preferred[11,19,21] to minimize surgical trauma and protect pancreatic function, as children are still in the growth and development stage.
After the diagnosis of ACT is made, management with a conservative preference and close follow-up should be considered for both adults and children until symptoms develop or the cyst rapidly expands. This recommendation is mainly based on the consensus that ACT has an almost invariably benign clinical course with few cases of cyst enlargement or cancer[25,26]. Our literature review showed similar results in clinical cases. Among the seven children mentioned in the article with a mean follow-up of 5.5 years (7 months to 15 years), only one patient showed progression. Wen et al[11] reported a patient with ACT who was followed for 15 years. At the age of 10 years, the child developed abdominal pain and CT examination indicated a 4 cm × 3 cm cystic lesion on the head of the pancreas. Laparotomy and cyst drainage were then performed. The symptoms were relieved after surgery and the patient was closely followed up. Within 15 years, the patient had no symptoms or complications, and the cyst diameter increased by only 2 cm. Fifteen years and 5 months later, the patient was unable to tolerate repeated pain and finally underwent a pyloric-sparing pancreaticoduodenectomy. The patient we reported was also placed under close ultrasound monitoring after surgery, and no significant trend of enlargement of the cystic mass in the head of the pancreas was observed during a two-year follow-up. The surveillance interval period was 1 month for the first follow-up, 3 months for the second follow-up, 6 months for the third follow-up, and the extended to a year[5].
In the course of the literature review, we found one neonatal ACT case of a 3-day-old boy with subglottic stenosis, complete jejunal atresia, and hepatic iron-overload[27]. We suspect that the child had a congenital genetic defect or a currently unreported syndrome involving ACT and the case was therefore not included in the literature review. As the neonatal patient soon died after birth and did not undergo ACT treatment, this limitation in our study may not lead to severe bias.
ACT is a rare non-neoplastic transformation of the pancreas, more rarely seen in children. The clinical symptoms and examinations are not specific and diagnosis is based on histological findings. For clinicians, a clear diagnosis and the exclusion of pancreatic malignancy are of top priority. Repeated EUS with fine-needle aspiration or needle-based confocal endomicroscopy with biopsy or radical surgery should be considered before the exclusion of malignant tumors. Once diagnosed with ACT, conservative treatment and continuous follow-up are recommended until symptoms appear or the size of the tumor significantly expands, as ACT is considered a slow-growing benign tumor.
| 1. | Mattiolo P, Wang H, Basturk O, Brosens LAA, Hong SM, Adsay V, Scarpa A, Luchini C. Comprehensive characterisation of acinar cystic transformation of the pancreas: a systematic review. J Clin Pathol. 2023;76:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Steinkraus KC, Mühlberger M, Schmidt SA, Kornmann M. Acinar cystic transformation of the pancreas-a rare case in a young patient. J Surg Case Rep. 2023;2023:rjad077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Albores-Saavedra J. Acinar cystadenoma of the pancreas: a previously undescribed tumor. Ann Diagn Pathol. 2002;6:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Lee JH, Jung SJ, Park YH, Park SJ, Choi JS. Pancreatic Acinar Cell Cystadenoma Mimicking Pancreatic Serous Cystadenoma. Korean J Gastroenterol. 2021;78:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Büyük M, Gündüz M, Berker N, Serin K, Çetin S, Özdemir H, Acunaş B, Güllüoğlu M. Acinar Cystic Transformation of the Pancreas: Report of Three Cases. Int J Surg Pathol. 2022;30:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Yang HJ, Sun K, Teng XD. [Acinar cystic transformation of the pancreas: report of two cases]. Zhonghua Bing Li Xue Za Zhi. 2022;51:548-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Ghio M, Vijay A. Asymptomatic Pancreatic Acinar Cystic Transformation With Intraepithelial Neoplasia in a Patient Presenting for Donor Nephrectomy. Pancreas. 2022;51:e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Luchini C, Mattiolo P, Basturk O, Mafficini A, Ozcan K, Lawlor RT, Hong SM, Brosens LA, Marchegiani G, Pea A, Manfrin E, Sciacca G, Zampieri F, Polati R, De Robertis R, Milella M, D'Onofrio M, Malleo G, Salvia R, Adsay V, Scarpa A. Acinar Cystic Transformation of the Pancreas: Histomorphology and Molecular Analysis to Unravel its Heterogeneous Nature. Am J Surg Pathol. 2023;47:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Zamboni G, Terris B, Scarpa A, Kosmahl M, Capelli P, Klimstra DS, Lam PW, Klöppel G. Acinar cell cystadenoma of the pancreas: a new entity? Am J Surg Pathol. 2002;26:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Fahlbusch T, Tannapfel A, Uhl W, Braumann C. Acinar Cell Cystadenoma - a Rarity in Advanced von Hippel-Lindau Disease: A Case Report. Visc Med. 2018;34:73-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Wen X, Bandovic J. Fifteen-Year Follow-Up of a Patient with Acinar Cystic Transformation of the Pancreas and Literature Review. Case Rep Pathol. 2020;2020:8847550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Ahmed N, Cao T, Chen W, Krishna SG. Acinar Cystic Transformation of the Pancreas With Main Pancreatic Duct Dilation and Distal Pancreatic Atrophy. ACG Case Rep J. 2024;11:e01286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Rift CV, Hasselby JP, Hansen CP, Federspiel B. Acinar cystic transformation of the pancreas: Report of a case and a review of the literature. Pathol Res Pract. 2020;216:152928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Narwal A, Panwar R, Madhusudhan KS, Pal S, Das P. Acinar cystic transformation of the pancreatic body and tail in an elderly male patient: A case report. Indian J Pathol Microbiol. 2024;67:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Sergi W, D'Ugo S, Libia A, Depalma N, Marchese T, Garritano S, Vadrucci S, Stasi E, Botrugno I, Manoochehri F, Spampinato M, Sergi W. Symptomatic acinar cell cystadenoma of the pancreas. J Surg Case Rep. 2023;2023:rjad360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Tatsumi M, Anazawa T, Masano Y, Yoh T, Nishino H, Yamane K, Nagai K, Uchida Y, Yoshizawa A, Hatano E. Acinar cystic transformation in the pancreatic tail. Clin J Gastroenterol. 2023;16:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Sebastian E, Longano A, Wei MYK, Goonawardena J, Bull N, Hassen S. Acinar cystic transformation of the pancreas causing progressive main pancreatic duct dilation: a diagnostic dilemma. J Surg Case Rep. 2023;2023:rjad154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Pritchard M, Boue AA, Samra J, Chou A, Mittal A. Acinar cystic transformation of the pancreas. BMJ Case Rep. 2024;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | McEvoy MP, Rich B, Klimstra D, Vakiani E, La Quaglia MP. Acinar cell cystadenoma of the pancreas in a 9-year-old boy. J Pediatr Surg. 2010;45:e7-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Singhi AD, Norwood S, Liu TC, Sharma R, Wolfgang CL, Schulick RD, Zeh HJ, Hruban RH. Acinar cell cystadenoma of the pancreas: a benign neoplasm or non-neoplastic ballooning of acinar and ductal epithelium? Am J Surg Pathol. 2013;37:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Cosgrove N, DiPalma J, Katz D, Kowalski T. A Rare Case of Acinar Cell Cystadenoma in a 14-Year-Old Adolescent: A Case Report. Case Rep Pancreat Cancer. 2016;2:3-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Guo L, Qian Y. Diffuse acinar cystic transformation of the pancreas: a rare case in a young male patient. Rev Esp Enferm Dig. 2024;116:290-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Gumus M, Ugras S, Algin O, Gundogdu H. Acinar cell cystadenoma (acinar cystic transformation) of the pancreas: the radiologic-pathologic features. Korean J Radiol. 2011;12:129-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Delavaud C, d'Assignies G, Cros J, Ruszniewski P, Hammel P, Levy P, Couvelard A, Sauvanet A, Dokmak S, Vilgrain V, Vullierme MP. CT and MR imaging of multilocular acinar cell cystadenoma: comparison with branch duct intraductal papillary mucinous neoplasia (IPMNs). Eur Radiol. 2014;24:2128-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 893] [Article Influence: 127.6] [Reference Citation Analysis (1)] |
| 26. | Wang G, Ji L, Qu FZ, Li L, Cao CL, Li ZB, Zhu H, Sun B. Acinar cell cystadenoma of the pancreas: A retrospective analysis of ten-year experience from a single academic institution. Pancreatology. 2016;16:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Mitra S, Kalra M, Purkait S, Mishra P, Mohanty PK, Som TK, Adhya AK, Das P. Congenital Acinar Cystic Transformation of the Pancreas with Proximal Jejunal Atresia and Hepatic Iron Overload: An Autopsy Case. Fetal Pediatr Pathol. 2022;41:828-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |