Published online Aug 16, 2025. doi: 10.12998/wjcc.v13.i23.106886
Revised: March 31, 2025
Accepted: May 7, 2025
Published online: August 16, 2025
Processing time: 86 Days and 15.7 Hours
In open heart surgery requiring cardiopulmonary bypass (CPB), ventricular fibrillation (VF) is common, but refractory recurrent VF is uncommon but perilous.
This article reports a 58-year-old male patient with an ascending aortic aneurysm who presented for a Bentall procedure and subsequently experienced multiple occurrences of unexplained VF after weaning from CPB. The recurrent episodes of VF in this case were felt to be related to coronary insufficiency after reconstruction of the aortic root. Coronary artery bypass grafting (CABG) of the proximal right coronary artery and the left anterior descending artery successfully resolved VF. Finally, this patient was safely transferred to the postoperative intensive care unit, and was discharged successfully after subsequent supportive treatment.
In aortic root replacement, coronary insufficiency is a potential cause of VF episodes and should be considered in the differential diagnosis. CABG is the sole effective treatment for VF caused by coronary insufficiency.
Core Tip: Refractory recurrent ventricular fibrillation (VF) is a severe ventricular arrhythmia in cardiac surgery. We present a rare case of refractory VF caused by coronary insufficiency during Bentall procedure. This report underscores the rarity and severity of coronary insufficiency following aortic root reconstruction. Additionally, we review causes and treatment of refractory VF in cardiac surgery and provide a clinical management protocol for refractory VF during cardiac surgery.
- Citation: Zhu M, Tang MM, Zhou RH. Refractory ventricular fibrillation caused by coronary insufficiency after Bentall procedure: A case report. World J Clin Cases 2025; 13(23): 106886
- URL: https://www.wjgnet.com/2307-8960/full/v13/i23/106886.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i23.106886
Ventricular fibrillation, particularly when occurring following the release of the aortic cross-clamp (ACC) in heart surgery, has been frequently reported and studied concerning its underlying mechanisms as well as strategies for prevention and treatment. Nonetheless, the mechanisms, causes, and conditions of ventricular fibrillation (VF) episodes during cardiac surgery are too complex to be broadly generalized. This highlights the intricacy of ruling out the causes of VF and underscores the importance of individualized treatment plans in reversing VF.
The Bentall procedure is a classic aortic root replacement surgery, suitable for aortic root aneurysms, aortic dissections, and severe aortic valve pathologies. Coronary insufficiency due to malposition of the coronary buttons is uncommon surgical complication of aortic root replacement surgery. It can lead to myocardial ischemia, ventricular arrhythmias, myocardial infarction, and pump failure. Emergency coronary artery bypass grafting (CABG) is the only effective therapeutic option[1,2]. Here, this article presents a severe case of multiple occurrences of VF after weaning off cardiopulmonary bypass in a Bentall procedure. Based on intraoperative transesophageal echocardiography, electrocardiogram (ECG), clinical manifestation, and operation way, refractory VF was attributed to coronary insufficiency. Coronary artery bypass grafting of the proximal right coronary artery and the left anterior descending artery was performed in com
A 58-year-old male was diagnosed with cardiac disease during routine physical examination 3 weeks ago.
The patient (height: 176 cm; weight: 66 kg) was admitted with an ascending aortic aneurysm. Upon admission to the hospital, the patient exhibited no signs of heart failure or myocardial infarction and demonstrated stable respiratory and circulatory functions. The patient was categorized as NYHA functional class II.
His past medical history included hypertension managed with antihypertensives, and coronary heart disease treated with nicorandil and atorvastatin calcium tablets. According to coronary angiography, he was not a candidate for coronary stent placement.
No clinically relevant family history was identified.
An ECG was conducted on admission and revealed junctional ectopic tachycardia, a heart rate of 81 beats per minute. A pre-operative echocardiogram showed left cardiac enlargement, mild right atrial enlargement, left ventricular (LV) wall hypertrophy, normal left ventricular size, aortic root aneurysmal dilatation, ascending aorta dilatation, and no regional LV wall motion abnormalities.
Preoperative laboratory tests revealed no significant abnormalities.
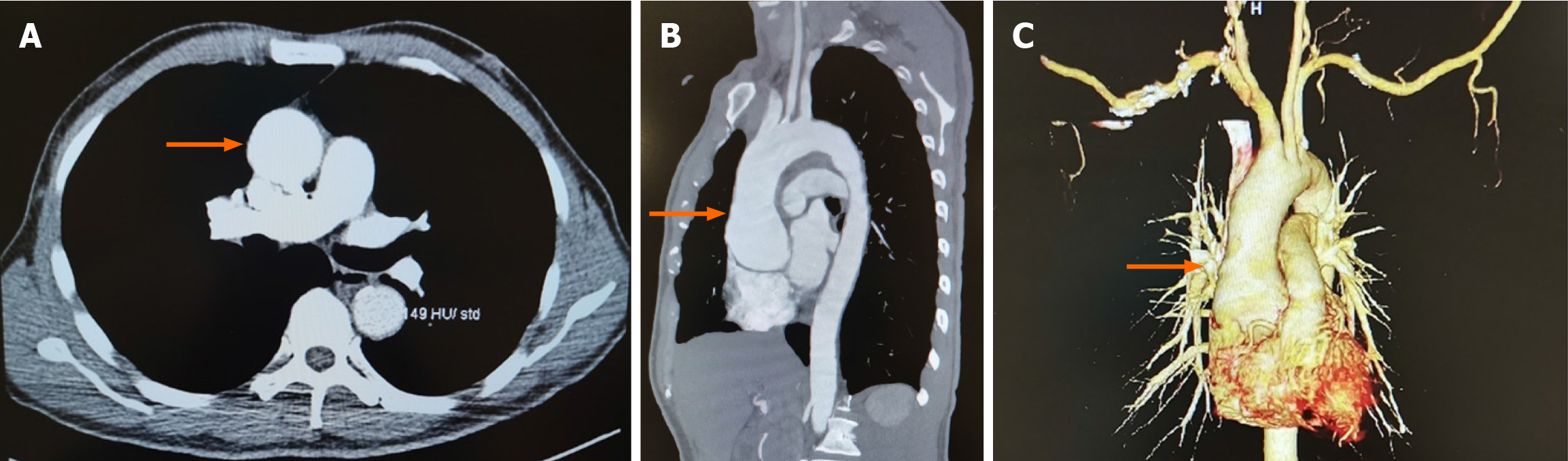
Computed tomography angiography demonstrated aortic root aneurysmal dilatation (maximum cross-sectional area of approximately 6.2 cm × 5.7 cm) (Figure 1).
He was diagnosed as ascending aortic aneurysm, coronary heart disease, hypertension, and junctional ectopic ta
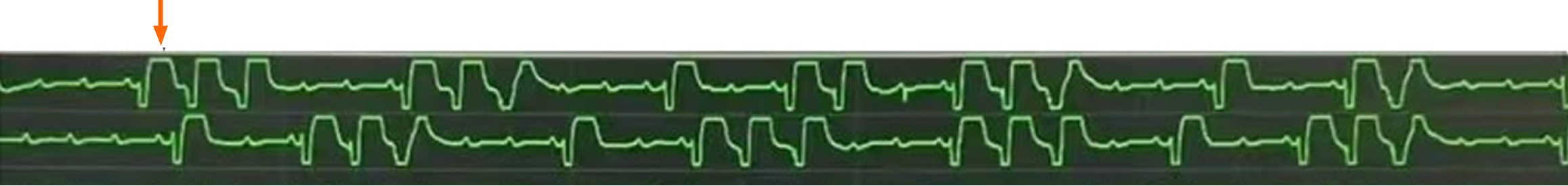
The patient was scheduled to undergo a Bentall procedure. Extracorporeal circulation was established via aortic and bicaval cannulation after chest opening. After aortic clamping, cardiac arrest was induced using cold cardioplegia administered via right and left coronary ostium. The cardioplegia solution consisted of a high-potassium formulation with added magnesium and lidocaine to enhance myocardial protection. The aorta was trimmed to the proximal to the opening of the innominate artery. The ostia of the left and right coronary arteries were isolated and trimmed into a button shape, the aortic valve leaflets were removed, and an artificial blood vessel with a valve was placed and sutured. Following aortic cross-clamp release, the patient resumed sinus rhythm and was successfully weaned off CPB. Transoesophageal echocardiography (TEE) showed normally functioning prosthetic aortic valve, mild mitral regurgitation, mild tricuspid regurgitation, and a patent blood stream in the ostia of the left and right coronary artery. Five minutes later, the patient suddenly developed episodes of VF. CPB was initiated again, and the patient was immediately defibrillated to sinus rhythm. CPB was removed after achieving a relatively stable condition. The procedure was resumed uneventfully until sternum closing. However, the patient developed VF again and cardiac arrest followed. Spontaneous circulation was restored after 10 minutes through external defibrillation, cardiopulmonary resuscitation (CPR), administration of epinephrine and lidocaine, and hypothermic cerebral protection. At this point, TEE demonstrated the absence of significant bubbles within both the atrial and ventricular chambers, normal functioning of prosthetic valves, and uncoordinated as well as weak myocardial contraction. Arterial blood gas analysis indicated pH 7.296, PCO2 42.3 mmHg, PO2 278.9 mmHg, K+ 3.35 mmol/L, Ca2+ 1.054 mmol/L, total hemoglobin concentrations 137.7 g/L, lactate 3.6 mmol/L, and base excess -6.04. Potassium and calcium were supplemented to ameliorate the internal environment, and a continuous transfusion of epinephrine and noradrenaline was administered to increase myocardial contractility and cardiac afterload, improve cardiac function, and maintain stable circulation. Meanwhile, cardiac surgeons reopened the chest for further exploration to identify the underlying causes of VF. Because VF recurred during sternum closure, suggesting a possible mechanical or ischemic trigger related to chest closure. However, no mechanical obstruction of the coronary artery was detected, and intra-coronary artery air embolus was ruled out based on continuous TEE monitoring results. After determining that the patient's vital signs were stable, the surgeons closed the sternum again. But an electrical storm of ventricular fibrillation recurred, which was not reversed by external direct current shocks. Sudden cardiac arrested ensued once more and CPR was administered. The chest was re-opened for internal defibrillation. Subsequently, CPB was promptly established. Immediate TEE results demonstrated absence of significant bubbles in the atrial and ventricular chambers, no myocardial edema, but weak myocardial contractility with regional wall motion abnormalities (Video). An ECG showed ST-segment elevation (Figure 2). Additionally, at the moment of sternum closing, arterial blood gas analysis revealed normal parameters. Considering the ECG, TEE results, operation way, and the timing of VF episodes, cardiologists and cardiac surgeons concluded that coronary insufficiency was the most likely cause of episodes of repeated VF in this patient. Therefore, CABG (proximal right coronary artery and the left anterior descending artery) was determined as the next step of treatment.
Following the completion of CABG, the patient was successfully weaned from CPB with the support of IABP and then transferred to the intensive care unit (ICU) for further postoperative management. On postoperative day 2, an echocardiogram demonstrated LV hypertrophy, normal LV wall amplitude, undercoordinated overall motion, normal LV systolic function, and decreased right ventricular systolic function. The IABP was evacuated on the 5th postoperative day. Two days later, the patient was transferred to the general ward. On postoperative day 10, the echocardiogram showed normally function in prosthetic valves, normal blood flow in the artificial ascending aorta, and normal LV and RV systolic function, with a left ventricular ejection fraction of 71%. Finally, the patient was discharged safely after subsequent treatment.
VF is relatively common during intracardiac surgery under extracorporeal circulation, with electrical defibrillation being the first-line therapy for VF. However, recurrent VF and repeated defibrillation can increase myocardial oxygen consumption, damage cardiomyocytes, and reduce the postoperative prognosis of patients. Thus, the causes of refractory VF should be identified and treated accordingly.
In open heart operation, the causes of VF include ischemic reperfusion injury, internal environment disturbance, and coronary artery diseases (air embolism, thromboembolism, spasm, stenosis, and so on). While coronary insufficiency can lead to VF, post-Bentall coronary insufficiency is rare and difficult to distinguish from ischemia caused by other factors in a short time. Coronary insufficiency after Bentall procedure is caused by coronary obstruction due to excessive tension, buckling, or distortion of the coronary arteries. This condition typically presents with ECG evidence of ischemia (e.g., ST-segment elevation), regional wall motion abnormalities on TEE, ventricular arrhythmias (e.g., ventricular fibrillation), and failure to wean from CPB. Preventive strategies involve identifying coronary artery alignment, standard valve rotation practices, and ensuring sufficient mobilization of coronary buttons. Shahriari et al[1] reviewed 139 patients undergoing aortic root replacement and found that 3 patients (2.2%) required emergency CABG for coronary insufficiency. The first patient developed severe hemodynamic instability and ventricular arrhythmias upon chest closure. The second patient experienced VF after surgery in ICU. The third patient showed regional wall motion abnormalities, accompanied by a deterioration in hemodynamic status. Notably, they promptly performed rescue CABG after suspecting coronary insufficiency, and successfully saved the patients. In this case, the patient developed recurrent VF after weaning from CPB, with episodes occurring both after cross-clamp release and during sternum closure. The most likely cause of refractory VF in this patient was acute myocardial ischemia due to coronary insufficiency, as evidenced by ST-segment elevation on ECG and regional wall motion abnormalities on TEE. The successful resolution of VF following CABG further supports this hypothesis. Additionally, reperfusion injury following aortic cross-clamp release may have contributed to the initial episode of VF, while mechanical stimulation during sternum closure likely increased compression of coronary artery, exacerbating ischemia and thus triggering subsequent episodes. Although electrolyte disturbances and acidosis were present, they were promptly corrected and are less likely to be the primary cause. TEE findings excluded air embolism and damage or obstruction of other great vessels or myocardial tissue as potential causes.
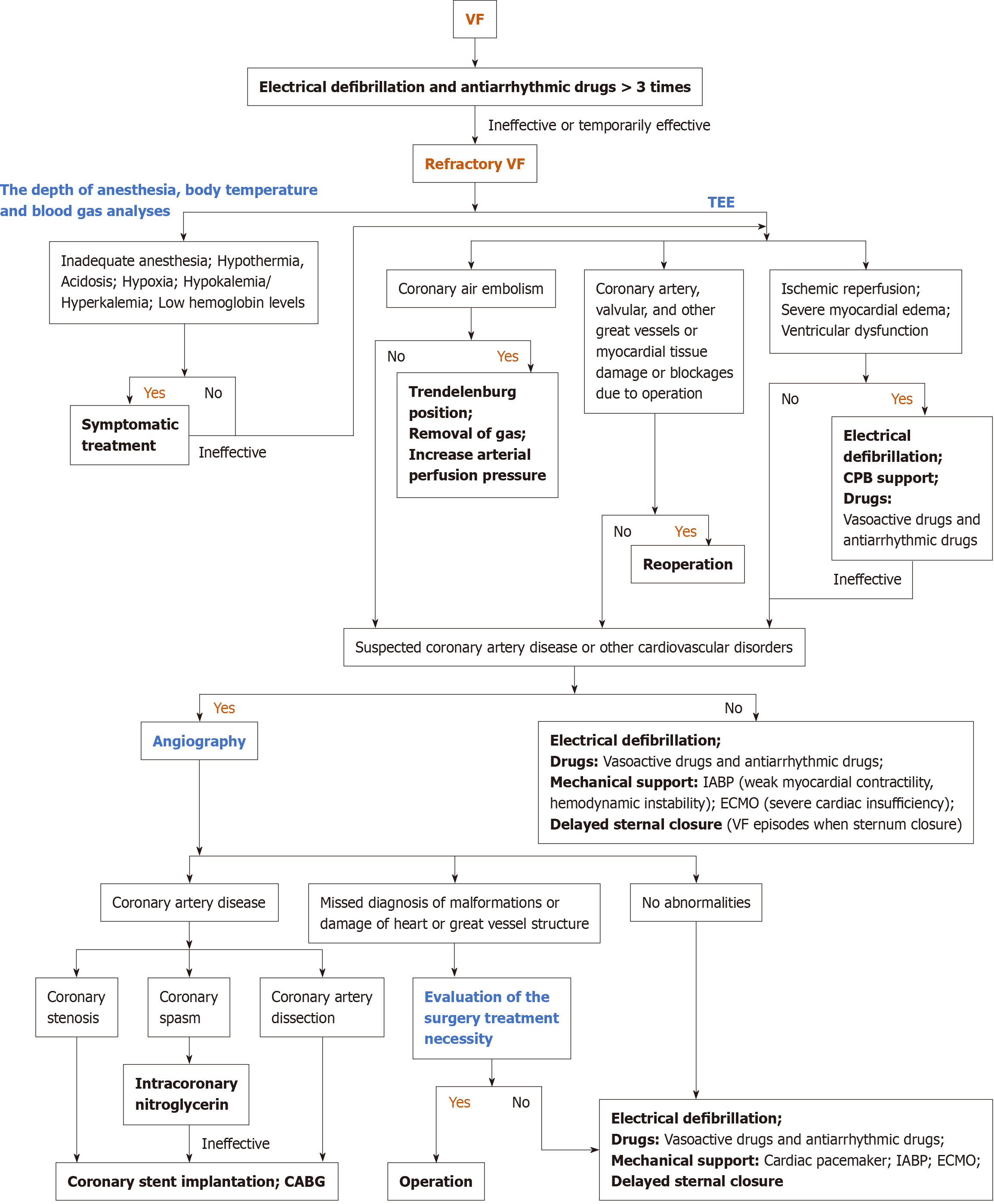
In fact, reperfusion VF is the most frequently reported and studied arrhythmia, occurring following the release of the ACC, with incidence rates ranging from 74% to 96%[3]. This complication has been attributed to the heterogeneous recovery of myocardial cells, ischemia-induced increases in reentry and automaticity, as well as the potential for reperfusion injury[4]. Preoperative severe myocardial damage and ventricular hypertrophy could increase the risk of reperfusion VF. Usually, electrical defibrillation, CPB support, antiarrhythmic drugs like lidocaine and amiodarone, and elevated coronary perfusion pressure assist in terminating reperfusion ventricular fibrillation. Interestingly, the efficacy of amiodarone or lidocaine in preventing reperfusion VF remains controversial[5,6]. In contrast, VF due to acidosis, hypoxia, potassium level, and coronary air embolism could be easily prevented, diagnosed, and treated. However, no clear expert consensus has been reached concerning the diagnostic and therapeutic strategies for refractory VF. Moreover, the research and reports of refractory VF are few in open heart surgery, and the treatment options for reference are limited accordingly. Jacob et al[7] reported a rare case of refractory VF induced by right coronary artery dissection after aortic valve replacement; CABG was undoubtedly the only effective method to treat radically refractory VF. Barr et al[8] employed delayed sternal closure to successfully resolve the repetitive VF that occurred upon chest closure in a 49-year-old male who underwent surgical repair of right coronary aneurysms. In addition, Extracorporeal membrane oxygenation (ECMO) is a viable therapeutic option for unexplained intractable VF, providing an opportunity for restoring the patient's cardiac function and figuring out the causes of VF[9]. In cardiac surgery, coronary artery spasm is rare but fatal and also serves as a cause of refractory VF[10]. Several predisposing factors have been proposed, including elevated catecholamine levels, local trauma, platelet release of vasoconstrictor mediators, respiratory alkalosis, hypothermia, and sympathoadrenergic stimulation[11]. Rahmouni and Ahmad et al[11,12] reported successful cases of post-cardiac valve coronary artery spasm treated with intracoronary nitroglycerin, implantable cardioverter-defibrillator (ICD), coronary stent implantation, and CABG, which are also therapeutic options for coronary spasm ICD[13,14]. Here, we summarize the diagnostic and therapeutic strategies for refractory VF during cardiac surgery based on previous studies and reports (Figure 3).
In summary, identifying the causes is crucial to treat refractory VF. In addition, once coronary insufficiency is confirmed or suspected in aortic root replacement surgery, CABG should be promptly performed.
This report presents a case of refractory VF induced by coronary insufficiency following the cessation of CPB during Bentall surgery. This case highlights the need to consider the possibility of coronary insufficiency in patients undergoing aortic root replacement if they develop refractory VF, severe myocardial ischemia, or pump failure, and stresses the comprehensive understanding of the occurrence mechanisms for refractory VF in open heart operation to explore further therapeutic options.
We thank the patient for consenting to the publication of this case report.
| 1. | Shahriari A, Eng M, Tranquilli M, Elefteriades JA. Rescue coronary artery bypass grafting (CABG) after aortic composite graft replacement. J Card Surg. 2009;24:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Kincaid EH, Cordell AR, Hammon JW, Adair SM, Kon ND. Coronary insufficiency after stentless aortic root replacement: risk factors and solutions. Ann Thorac Surg. 2007;83:964-8; discussion 968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Baraka A, Kawkabani N, Dabbous A, Nawfal M. Lidocaine for prevention of reperfusion ventricular fibrillation after release of aortic cross-clamping. J Cardiothorac Vasc Anesth. 2000;14:531-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Zhou N, Gong J, Liang X, Liu W, Li H, Li W. Preoperative Risk Prediction Score for and In-Hospital Clinical Outcomes of Reperfusion Ventricular Fibrillation After Release of Aortic Cross-Clamps: A Retrospective Study. J Cardiothorac Vasc Anesth. 2023;37:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Zheng Y, Gu Q, Chen HW, Peng HM, Jia DY, Zhou Y, Xiang MX. Efficacy of amiodarone and lidocaine for preventing ventricular fibrillation after aortic cross-clamp release in open heart surgery: a meta-analysis of randomized controlled trials. J Zhejiang Univ Sci B. 2017;18:1113-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Mauermann WJ, Pulido JN, Barbara DW, Abel MD, Li Z, Meade LA, Schaff HV, White RD. Amiodarone versus lidocaine and placebo for the prevention of ventricular fibrillation after aortic crossclamping: a randomized, double-blind, placebo-controlled trial. J Thorac Cardiovasc Surg. 2012;144:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Jacob A, Hara N, Goli G, Lall K. Right coronary artery dissection after aortic valve replacement presenting with refractory ventricular fibrillation. J Surg Case Rep. 2024;2024:rjad717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Barr J, Acharya MN, Kourliouros A, Raja SG. Technical Considerations of Giant Right Coronary Artery Aneurysm Exclusion. Case Rep Surg. 2016;2016:3795640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Soynov I, Kornilov I, Zubritskiy A, Ponomarev D, Nichay N, Gorbatykh A, Voitov A, Karaskov A. Postcardiotomy refractory ventricular fibrillation: rescue using veno-arterial extracorporeal membrane oxygenation. Perfusion. 2018;33:401-403. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Chen C, Chen Y, Zhang X, Zou L. Perioperative multivessel coronary artery spasm and cardiac arrest after cardiac surgery. Interdiscip Cardiovasc Thorac Surg. 2024;38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Rahmouni El Idrissi K, Chauvette V, Lamarche Y, El-Hamamsy I. Recurrent Coronary Vasospasm After Cardiac Surgery. Ann Thorac Surg. 2020;110:e481-e483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Ahmad T, Kishore KS, Maheshwarappa NN, Pasarad AK. Postoperative diffuse coronary spasm after two valve surgery - a rare phenomenon. Indian Heart J. 2015;67:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Gülşen K, Ayça B, Cerit L, Okuyan E. Coronary vasospasm-induced periodic ventricular fibrillation and successful ablation through coronary stenting. Postepy Kardiol Interwencyjnej. 2015;11:337-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Sugiyama K, Fujimoto M, Watanuki H, Matsuyama K. Surgical revascularization for severe spasm in the left main coronary artery. Clin Case Rep. 2023;11:e6815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |