Published online Feb 26, 2024. doi: 10.12998/wjcc.v12.i6.1094
Peer-review started: December 8, 2023
First decision: December 20, 2023
Revised: January 3, 2024
Accepted: January 19, 2024
Article in press: January 19, 2024
Published online: February 26, 2024
Processing time: 73 Days and 19.4 Hours
Accumulating evidence suggests that the gut microbiome is involved in the pathogenesis of insulin resistance (IR). However, the link between two of the most prevalent bowel disorders, chronic diarrhea and constipation, and the triglyceride glucose (TyG) index, a marker of IR, has not yet been investigated.
To investigate the potential association between TyG and the incidence of chronic diarrhea and constipation.
This cross-sectional study enrolled 2400 participants from the National Health and Nutrition Examination Survey database from 2009-2010. TyG was used as an exposure variable, with chronic diarrhea and constipation as determined by the Bristol Stool Form Scale used as the outcome variables. A demographic investigation based on TyG quartile subgroups was performed. The application of multivariate logistic regression models and weighted generalized additive models revealed potential correlations between TyG, chronic diarrhea, and constipation. Subgroup analyses were performed to examine the stability of any potential associations.
In the chosen sample, chronic diarrhea had a prevalence of 8.00%, while chronic constipation had a prevalence of 8.04%. In multiple logistic regression, a more prominent positive association was found between TyG and chronic diarrhea, particularly in model 1 (OR = 1.45; 95%CI: 1.17-1.79, P = 0.0007) and model 2 (OR = 1.40; 95%CI: 1.12-1.76, P = 0.0033). No definite association was observed bet
Higher TyG levels were positively associated with abnormal bowel health.
Core Tip: Chronic diarrhea and constipation are two common conditions that interfere with daily life. Herein, we identified a positive association between the triglyceride glucose index, a marker of insulin resistance (IR), and chronic diarrhea in the National Health and Nutrition Examination Survey database. These results suggest that early and comprehensive management of IR may be beneficial for maintaining normal bowel health. Further investigations should be conducted on the underlying pathological mechanisms.
- Citation: Zhu JY, Liu MY, Sun C. Assessment of the triglyceride glucose index in adult patients with chronic diarrhea and constipation. World J Clin Cases 2024; 12(6): 1094-1103
- URL: https://www.wjgnet.com/2307-8960/full/v12/i6/1094.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i6.1094
Chronic diarrhea and chronic constipation are prevalent disorders that can severely impact a patient's quality of life[1,2]. Incomplete statistics have estimated that functional bowel disorders, as defined by the Rome Standard IV, result in more than four million medical visits per year in the United States[3]. Abnormal stool consistency is assessed as a part of the evaluation metrics in clinical practice[4,5]. The Bristol Stool Form Scale (BSFS)[6] was used to quantify these symptoms in the National Health and Nutrition Examination Survey (NHANES). Extensive research has been conducted using epidemiological data based on these criteria[7-10]. In the NHANES 2005-2010 sample population, the frequency of chronic diarrhea was higher in patients diagnosed with metabolic syndrome and nonalcoholic fatty liver disease than in patients with chronic constipation, or in the normal population[9]. Patients with chronic diarrhea and constipation have an increased prevalence of selected cancers, cardiovascular diseases, and risk of all-cause mortality[8]. Additionally, chronic diarrhea is more common in diabetic patients than non-diabetic patients, and the two are strongly inter-correlated[10]. According to epidemiological evidence, abnormal bowel habits are closely associated with chronic and metabolic diseases.
Insulin resistance (IR) is a metabolic condition believed to be a precursor of type 2 diabetes[11], and a manifestation of metabolic syndrome involving pathophysiological mechanisms[12,13]. Metagenomic research tools and animal expe
Participants for this cross-sectional study were selected from the NHANES 2009-2010 database, for which informed written consent was obtained from all participants prior to engagement, and which contained no personal patient information. The dataset utilized a complex multistage probability sampling design that included, but was not limited to demographics, dietary habits, and test examinations.
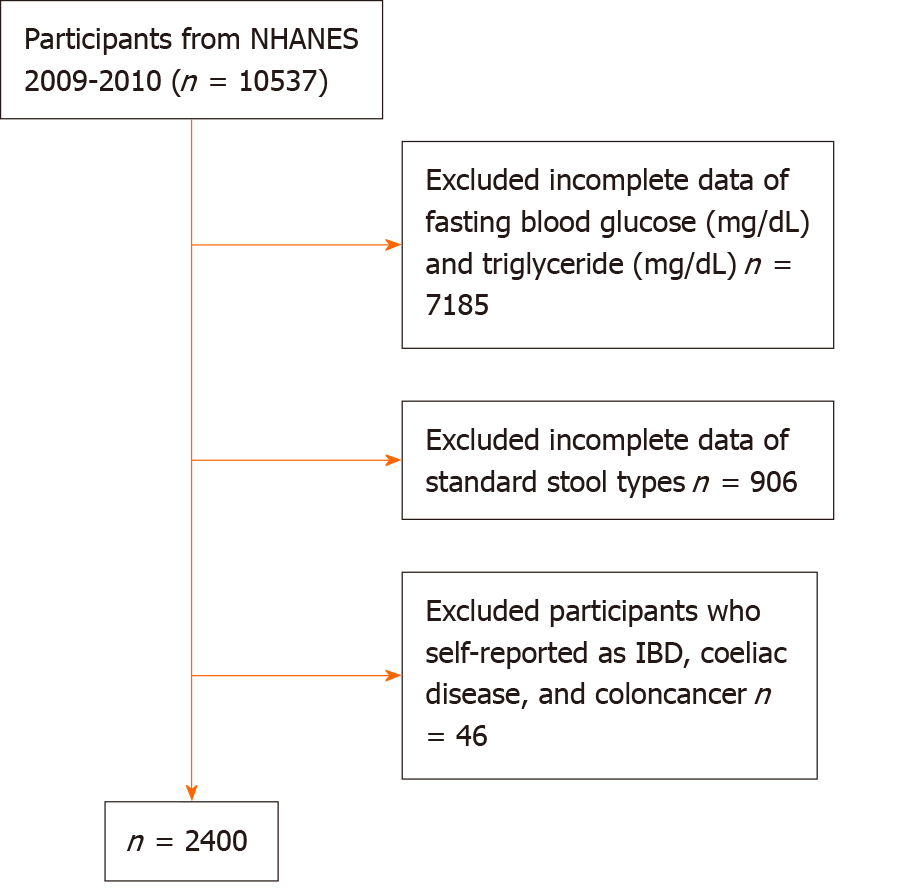
All selected participants responded to the Bowel Health Questionnaire, which investigated standard stool types, and provided data on their fasting blood glucose and triglyceride levels. We further excluded participants who self-reported as having inflammatory bowel disease, celiac disease, or colon cancer. Ultimately, the study included 2400 individuals aged 20 years or older. Figure 1 depicts the sample selection process.
Responses to a general question about stool type were provided in the Bowel Health Questionnaire of the NHANES 2009-2010 database. In this system, stool types 1-7 are classified based on the BSFS criteria, which primarily assess the shape and consistency of the stools; these criteria are widely implemented in clinical practice[7]. Specifically, stools were described as changing in shape and consistency in a stepwise manner from type 1 (separate hard lumps resembling nuts) to type 7 (watery, no solid pieces). Chronic diarrhea was defined as type 6 or 7; chronic constipation as type 1 or type 2; and the remaining types were considered to indicate healthy bowels.
In this study, TyG, which comprises fasting blood glucose and triglycerides, was chosen as the exposure variable. The calculation to obtain Ln [fasting triglyceride (mg/dL) fasting glucose (mg/dL)/2] is straightforward and rapid to implement.
Based on similar previous studies, the following covariates were considered and included: Age, sex, race, education (adults 20+), ratio of family income to poverty, body mass index (BMI), laxatives, alcohol, self-reported hypertension, diabetes mellitus, and hypercholesterolemia. All the above covariates were considered in the fully adjusted model. Table 1 presents the breakdown conditions for each covariate.
| Q1, n = 599 (6.89-8.24) | Q2, n = 601 (8.25-8.62) | Q3, n = 600 (8.62-9.04) | Q4, n = 600 (9.04-12.34) | P value | |
| Age (yr), mean ± SD | 43.47 ± 16.99 | 49.03 ± 17.81 | 51.93 ± 17.77 | 52.95 ± 16.24 | < 0.001c |
| Triglyceride (mg/dL), mean ± SD | 62.00 ± 13.32 | 94.21 ± 15.25 | 131.68 ± 22.99 | 246.43 ± 186.73 | < 0.001c |
| Fasting glucose (mg/dL), mean ± SD | 92.59 ± 10.28 | 100.66 ± 16.57 | 106.19 ± 18.46 | 129.96 ± 51.53 | < 0.001c |
| Gender, n (%) | < 0.001c | ||||
| Male | 219 (36.56) | 277 (46.09) | 327 (54.50) | 334 (55.67) | |
| Female | 380 (63.44) | 324 (53.91) | 273 (45.50) | 266 (44.33) | |
| Race, n (%) | < 0.001c | ||||
| Mexican American | 77 (12.85) | 108 (17.97) | 116 (19.33) | 160 (26.67) | |
| Other Hispanic | 67 (11.19) | 60 (9.98) | 58 (9.67) | 83 (13.83) | |
| Non-Hispanic white | 291 (48.58) | 286 (47.59) | 313 (52.17) | 276 (46.00) | |
| Non-Hispanic black | 135 (22.54) | 124 (20.63) | 74 (12.33) | 60 (10.00) | |
| Other races | 29 (4.84) | 23 (3.83) | 39 (6.50) | 21 (3.50) | |
| Levels of education, n (%) | < 0.001c | ||||
| ≤ High school | 108 (18.03) | 156 (25.96) | 172 (28.76) | 237 (39.83) | |
| > High school | 491 (81.97) | 445 (74.04) | 426 (71.24) | 358 (60.17) | |
| Ratio of family income to poverty, n (%) | 0.008b | ||||
| ≤ 1 | 97 (17.86) | 113 (20.14) | 128 (23.23) | 137 (25.95) | |
| > 1 | 446 (82.14) | 448 (79.86) | 423 (76.77) | 391 (74.05) | |
| Body mass index, n (%) | < 0.001c | ||||
| Under/normal weight | 294 (49.33) | 178 (29.82) | 125 (20.94) | 71 (11.97) | |
| Overweight | 176 (29.53) | 218 (36.52) | 212 (35.51) | 208 (35.08) | |
| Obese | 126 (21.14) | 201 (33.67) | 260 (43.55) | 314 (52.95) | |
| Diabetes, n (%) | < 0.001c | ||||
| Yes | 20 (3.37) | 48 (8.18) | 65 (11.05) | 145 (25.09) | |
| No | 573 (96.63) | 539 (91.82) | 523 (88.95) | 433 (74.91) | |
| Hypertension, n (%) | < 0.001c | ||||
| Yes | 128 (21.37) | 204 (33.94) | 237 (39.50) | 283 (47.17) | |
| No | 471 (78.63) | 397 (66.06) | 363 (60.50) | 317 (52.83) | |
| High cholesterol level, n (%) | < 0.001c | ||||
| Yes | 114 (28.22) | 162 (39.71) | 183 (41.78) | 276 (61.47) | |
| No | 290 (71.78) | 246 (60.29) | 255 (58.22) | 173 (38.53) | |
| Alcohol, n (%) | < 0.001c | ||||
| ≥ 1, < 8 | 419 (96.54) | 418 (94.36) | 385 (92.55) | 331 (88.74) | |
| ≥ 8 | 15 (3.46) | 25 (5.64) | 31 (7.45) | 42 (11.26) | |
| Chronic diarrhea, n (%) | 0.004b | ||||
| Yes | 37 (6.18) | 37 (6.16) | 52 (8.67) | 66 (11.00) | |
| No | 562 (93.82) | 564 (93.84) | 548 (91.33) | 534 (89.00) | |
| Chronic constipation, n (%) | 0.806 | ||||
| Yes | 53 (8.85) | 49 (8.15) | 44 (7.33) | 47 (7.83) | |
| No | 546 (91.15) | 552 (91.85) | 556 (92.67) | 553 (92.17) |
The population was segmented according to TyG quartiles ranging from low to high, and discrepancies in demographic information were measured. Three generalized linear regression models, adjusted for covariates, were used to explore the relationship between TyG and chronic diarrhea or constipation. The non-linear relationship was analyzed using smooth curve fitting and generalized additivity models, and the presence and importance of the inflection points were investigated by applying two-stage linear models and log-likelihood ratios. Subgroup analyses were conducted to assess the consistency of this association across varying age groups, sexes, and BMI ranges, and among individuals with hypertension and diabetes. All preceding research stages were conducted using Empower software and R version 3.4.3.
Table 1 presents the primary demographic features of the cohort of 2400 patients, which comprised 48.21% males and 51.79% females enrolled in the study. The mean age and TyG index values were 49.35 ± 17.60 and 8.68 ± 0.64 respectively. For assessment purposes, participants were categorized into four groups. The overall incidence of chronic constipation was 8.04% among all participants, while the incidences of chronic constipation in the population stratified by TyG quartiles were as follows: Quartile 1 (Q1) (6.89-8.24): 8.85%; Q2 (8.25-8.62), 8.15%; Q3 (8.62-9.04), 7.33%, and Q4 (9.04-12.34), 7.83%, with a P value of 0.806. The prevalence of chronic diarrhea was 8.00% in all participants, and the prevalence of chronic diarrhea in the population grouped by TyG quartiles was Q1, 6.00%; Q2, 6.16%; Q3, 7.33%; and Q4, 7.83%, (P = 0.004). Compared to individuals in Q1-3, Q4 exhibited the highest range of TyG indices, including a higher proportion of males, an increase in the percentage of low-educated and poor people, an indication of overweight and obesity based on BMI, and a significant increase in the percentage of self-reported hypertension, diabetes mellitus, and hypercholesterolemia.
Table 2 presents the association between TyG index and bowel health. Our findings indicated that elevated levels of TyG were positively correlated with the risk of chronic diarrhea, particularly in the crude model (OR = 1.45; 95%CI: 1.17-1.79, P = 0.0007) and partly adjusted model 2 (OR = 1.40; 95%CI: 1.12-1.76, P = 0.0033). This relationship became less significant in model 3 after full variable control (OR = 1.35; 95%CI: 0.85-2.13, P = 0.2071). Nevertheless, no correlation between the TyG index and chronic constipation were found, with ORs (95%CIs) of 0.96 (0.76-1.21), 1.10 (0.86-1.40), and 1.50 (0.95-2.37) for models 1, 2, and 3, respectively.
| TyG | OR (95%CI) | ||
| Chronic constipation | Chronic diarrhea | ||
| Model 1 continuous | 0.96 (0.76, 1.21) | 1.45 (1.17, 1.79) | |
| Q1 | 6.89-8.24 | 1.0 (reference) | 1.0 (reference) |
| Q2 | 8.25-8.62 | 0.91 (0.61, 1.37) | 1.00 (0.62, 1.59) |
| Q3 | 8.62-9.04 | 0.82 (0.54, 1.24) | 1.44 (0.93, 2.23) |
| Q4 | 9.04-12.34 | 0.88 (0.58, 1.32) | 1.88 (1.23, 2.86) |
| P for trend | 0.4591 | 0.0007a | |
| Model 2 continuous | 1.10 (0.86, 1.40) | 1.40 (1.12, 1.76) | |
| Q1 | 6.89-8.24 | 1.0 (reference) | 1.0 (reference) |
| Q2 | 8.25-8.62 | 1.00 (0.66, 1.52) | 0.95 (0.59, 1.53) |
| Q3 | 8.62-9.04 | 1.00 (0.65, 1.55) | 1.38 (0.88, 2.18) |
| Q4 | 9.04-12.34 | 1.06 (0.68, 1.64) | 1.71 (1.10, 2.65) |
| P for trend | 0.8026 | 0.0044b | |
| Model 3 continuous | 1.50 (0.95, 2.37) | 1.35 (0.85, 2.13) | |
| Q1 | 6.89-8.24 | 1.0 (reference) | 1.0 (reference) |
| Q2 | 8.25-8.62 | 1.07 (0.50, 2.28) | 0.92 (0.39, 2.14) |
| Q3 | 8.62-9.04 | 1.86 (0.89, 3.90) | 1.18 (0.52, 2.66) |
| Q4 | 9.04-12.34 | 1.76 (0.76, 4.07) | 1.52 (0.65, 3.56) |
| P for trend | 0.0999 | 0.2268 | |
TyG levels were subsequently split into quartiles to further examine changes in the tendency of relationships. In models 1 and 2, positive correlation patterns emerged for chronic diarrhea. In a crude model, for example, when the TyG index increased by one standard deviation, participants in the top TyG quartile exhibited a 1.88-fold greater likelihood of suffering from chronic diarrhea than those in the bottom quartile (OR = 1.88; 95%CI: 1.23-2.86, P for trend = 0.0007). In model 3, individuals in the upper quartile of TyG were more likely to experience chronic diarrhea than those in the lower quartile, although statistical difference was not reached (OR = 1.52; 95%CI: 0.65-3.56, P for trend = 0.2268). None of the trend tests between TyG and chronic constipation showed statistical significance (P > 0.05) in models 1-3.
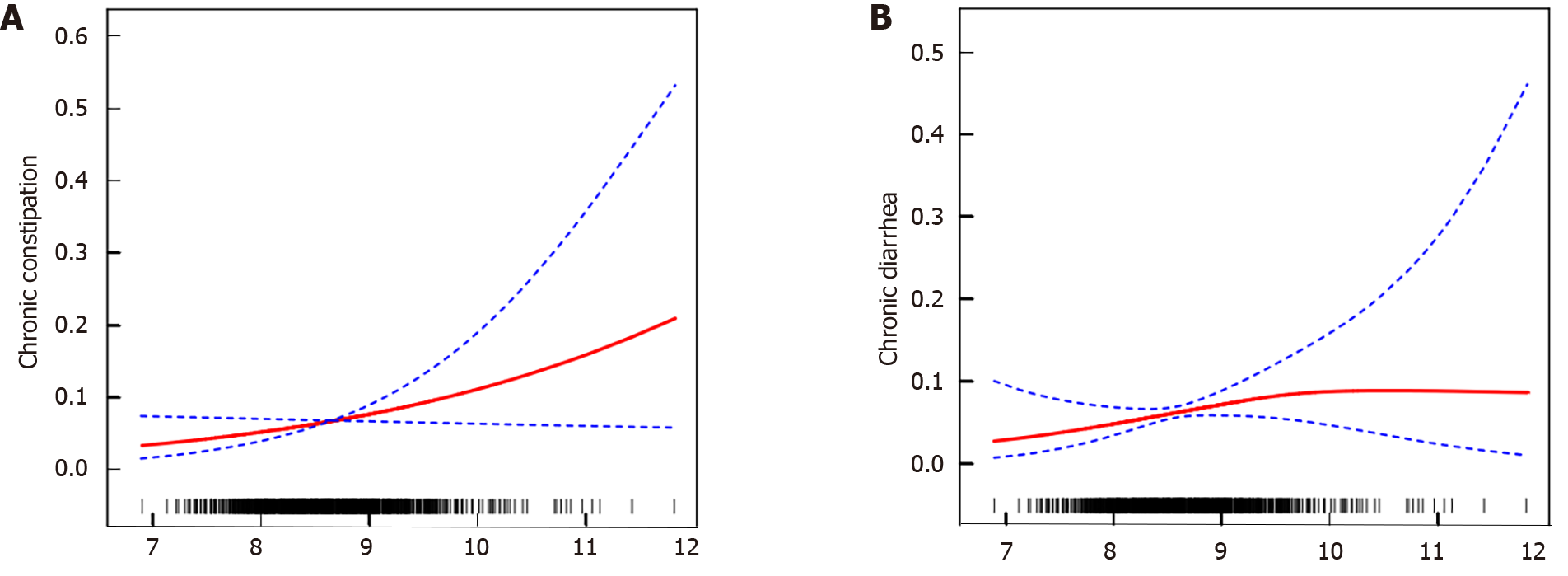
After considering all the covariates, smooth curve fitting and a generalized additivity model were used (Figure 2). Regarding chronic diarrhea, a two-stage linear model was applied, resulting in an inflection point of 9.63, which showed statistical significance on the log-likelihood ratio test (P = 0.047). When the TyG index fell below 9.63, the chances of suffering from chronic diarrhea rose by 89% with each one-SD increase in the TyG index (OR = 1.89; 95%CI: 1.05-3.41, P = 0.0344). Conversely, no association was seen above 9.63 (OR = 0.24; 95%CI: 0.03-2.22, P = 0.2080), and the curve tended to flatten. Regarding chronic constipation, a positive correlation was found between TyG and chronic constipation only when the TyG value exceeded 8.2 (OR = 1.74; 95%CI: 1.02-2.95, P = 0.0415), but the P value of the log-likelihood ratio did not meet the required significance (P = 0.321).
Initially, we aimed to investigate the impact of a range of factors on the risk of chronic diarrhea. First, in models 1 and 3, we examined the subgroups categorized by age, sex, BMI, diabetes, and hypertension. Despite an intermittent lack of positive correlation between TyG and chronic diarrhea in some subgroups in the crude model, the interaction test confirmed that the association remained unaffected by these factors. Furthermore, this positive correlation was consistent across different age groups and hypertensive conditions. In summary, model 1 demonstrated that the variables mentioned above did not affect the occurrence of chronic diarrhea. For the subgroup analysis of model 3, the P value of the interaction test was greater than 0.05, supporting the inference that the connection between TyG and chronic diarrhea was similar across populations.
Subsequently, further subgroup analyses were performed using model 3 to check the robustness of the relationship between TyG levels and chronic constipation. It is worth noting that higher TyG scores were found to be correlated with an increased risk of chronic constipation in the hypertensive population (OR = 2.53; 95%CI: 1.19-5.37, P = 0.0159), but not in the non-hypertensive population, indicating that this association may be stronger in hypertensive individuals. However, no connections with P values for interactions were found to fulfill the statistically significant interaction criteria, emphasizing that the association between TyG and chronic constipation is dependent.
In this cross-sectional study encompassing 2400 participants, our findings demonstrated a heightened risk of chronic diarrhea with elevated TyG levels. This non-linear connection demonstrated that TyG was positively correlated with chronic diarrhea and constipation at distinct value bands. Subgroup analysis further indicated that this relationship persisted irrespective of sex, age, BMI, hypertension, or diabetes status.
To our knowledge, this is the first study to evaluate the correlation between TyG index and abnormal gut health. The TyG formula indicated that an elevated value reflected anomalies in glucose and lipid levels. The gut microbiota is a primary regulator of the host's metabolic energy and substrate metabolism[18,19]. Bäckhed et al[20] previously showed that hyperglycemia directly and specifically shaped intestinal barrier failure and increased the susceptibility to intestinal infections. They also discovered that hyperglycemia affects intestinal epithelial cells via the bidirectional glucose transporter receptor GLU2, causing the intracellular recording of metabolism-related genes. Disturbances in the composition of the gut microbiota can disrupt the immune system, leading to inflammation, oxidative stress, and IR. Certain prebiotics and probiotics have further been proven to regulate fat metabolism, enhance insulin sensitivity, and control intestinal inflammation and oxidative stress in mice, as evidenced by animal models. Cranberry extracts enriched with phenolic compounds, green tea powder, and Lactobacillus plantarum have also demonstrated positive effects on metabolic phenotypes. Specifically, these substances have been observed to increase the proportion of gut bacteria belonging to the genus Akkermansia. Additionally, the expression of various modulators of inflammation was found to be lowered following their administration[21,22]. Similarly, the probiotic Lactobacillus acidophilus has also been demonstrated to alter gut microbial abundance and diversity; suppress the TLR4/NF-κB signaling pathway; and improve energy, glucose, and lipid metabolism[23]. Dysbiosis of gut microbes, in turn, facilitates the pathology of a variety of intestinal disorders, including chronic diarrhea and chronic constipation[24], through mechanisms that primarily include the production of large amounts of toxins by certain opportunistic pathogenic bacteria[25], altered metabolic function of bile acids[26,27], and involvement in the regulation of gastrointestinal motility through the production and uptake of 5-hydroxytryptamine[28,29]. Overall, gut microbes seem to play a joint role in the development of IR and abnormal gut health, but it has not been directly established whether IR causally mediates chronic diarrhea through modulation of the gut microbes. Smoothed curve-fitting results have indicated that TyG impacts chronic diarrhea and constipation at two relatively separate intervals, with chronic diarrhea in the antecedent half of the curve, and chronic constipation in the subsequent half. This indicates that the pathogenic mechanisms underlying TyG, chronic diarrhea, and constipation may differ. Additionally, the results of this research could provide further insights into subsequent basic experiments investigating the influence of metabolic factors on the pathological mechanisms of abnormal gut health.
In prior studies, abnormal gut health and several chronic diseases have been associated with the dietary inflammation index and C-reactive protein levels. The inflammatory response and oxidative stress are undoubtedly involved in the intrinsic evolution of a variety of disease states. However, this study was unable to provide further evidence of the precise mechanisms by which TyG may mediate chronic diarrhea or constipation. In addition to IR, higher TyG indices are indicative of a poor health status and have been implicated in cardiovascular disease[30], obesity[31], diabetes[32], hypertension[33], metabolic syndrome[34], and lipid metabolism[35]. In the present study, the positive link between TyG and diarrhea remained after controlling for basic demographic characteristics, but disappeared in the fully adjusted model, indicating that TyG may be inextricably linked to physical conditions and personal aggregates. However, the interaction reached statistical significance in the subgroup analyses for models 1 and 3, which included sex, age, BMI, hypertension, and diabetes. TyG levels are closely correlated with constipation in individuals with hypertension. To the best of our knowledge, only one study has reported that hypertension (22%) is the most frequent comorbidity in patients with chronic constipation[36].
Overall, the present study contributes to our understanding of the relationship between IR and chronic diarrhea, indicating that timely co-management may be critical. Similar to previous studies on abnormal gut health and type 2 diabetes, chronic diarrhea seems to be more strongly linked to other diseases than chronic constipation[37]. It is also worth noting that while the results for TyG and chronic constipation lacked statistical significance, this did not rule out the role of TyG in chronic constipation. There is a current pressing need for a reliable indicator of intestinal dysfunction for the co-treatment of chronic illnesses. Given the lack of more detailed data on disease progression in the NHANES database, such as the temporal relationship between elevated TyG levels and the emergence of abnormal gut health. Thus, a well-designed randomized controlled trial is necessary to determine whether TyG could be applied as a reliable predictor of chronic diarrhea and constipation, as well as to assess its potential use in practice.
This study has several shortcomings. Firstly, the definitions of persistent constipation and diarrhea did not follow the most recent Rome criteria. As this was only a cross-sectional study, it is important to consider that the causal relationships and mechanisms underlying the association between TyG and chronic diarrhea and constipation require further investigation through prospective studies with larger sample sizes and basic experiments. Such further investigation will aid in the future application of TyG in clinical practice.
Overall, the present analysis of subjects enrolled in the NHANES 2009-2010 database indicated a correlation between a higher TyG index and an increased likelihood of chronic diarrhea. Further studies are required to understand the pathological mechanisms underlying TyG and abnormal gut health. Improving the treatment and management of IR may reduce the incidence of abnormal bowel health.
Triglyceride glucose (TyG) was associated with a variety of chronic diseases. However, there is currently a lack of research regarding their association with abnormal gut health.
The National Health and Nutrition Examination Survey (NHANES) provides national-level data on the health and nutritional status of the United States population. The gut microbiome and pathogenesis of insulin resistance (IR) has been intensively studies using this data. As TyG as a marker of IR, we decided to explore the association between TyG and abnormal gut health using the NHANES database.
To study the association between TyG and the incidence of chronic diarrhea and constipation in United States adults.
This cross-sectional study was conducted among adults with complete data on TyG, chronic diarrhea, and constipation included in the 2009-2010 NHANES. TyG was calculated using the following equation: Ln [fasting triglyceride (mg/dL) fasting glucose (mg/dL)/2]. Chronic diarrhea and constipation were assessed using the Bristol Stool Form Scale. Weighted multivariate regression and subgroup analyses were conducted to explore the independent relationship between TyG, chronic diarrhea, and constipation.
In this cross-sectional study encompassing 2400 participants, our findings demonstrated a heightened risk of chronic diarrhea with elevated TyG levels. The non-linear connection demonstrated that TyG positively correlated with chronic diarrhea and constipation at distinct value bands. Subgroup analysis indicated that this relationship persisted irrespective of sex, age, BMI, hypertension, or diabetes status.
A total of 2400 participants were included in this cross-sectional study, which revealed a correlation between elevated TyG levels and a heightened risk of chronic diarrhea.
Further research is required to establish the exact causal relationship between TyG and abnormal gut health, which will contribute to the prediction, co-management, and treatment of subsequent diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Herrero-Fresneda I, Spain S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
| 1. | Araki M, Shinzaki S, Yamada T, Arimitsu S, Komori M, Shibukawa N, Mukai A, Nakajima S, Kinoshita K, Kitamura S, Murayama Y, Ogawa H, Yasunaga Y, Oshita M, Fukui H, Masuda E, Tsujii M, Kawai S, Hiyama S, Inoue T, Tanimukai H, Iijima H, Takehara T. Psychologic stress and disease activity in patients with inflammatory bowel disease: A multicenter cross-sectional study. PLoS One. 2020;15:e0233365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Gîlc-Blanariu GE, Ștefnescu G, Trifan AV, Moscalu M, Dimofte MG, Ștefnescu C, Drug VL, Afrsnie VA, Ciocoiu M. Sleep Impairment and Psychological Distress among Patients with Inflammatory Bowel Disease-beyond the Obvious. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Ma C, Congly SE, Novak KL, Belletrutti PJ, Raman M, Woo M, Andrews CN, Nasser Y. Epidemiologic Burden and Treatment of Chronic Symptomatic Functional Bowel Disorders in the United States: A Nationwide Analysis. Gastroenterology. 2021;160:88-98.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Koyama T, Nagata N, Nishiura K, Miura N, Kawai T, Yamamoto H. Prune Juice Containing Sorbitol, Pectin, and Polyphenol Ameliorates Subjective Complaints and Hard Feces While Normalizing Stool in Chronic Constipation: A Randomized Placebo-Controlled Trial. Am J Gastroenterol. 2022;117:1714-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Hamad A, Fragkos KC, Forbes A. A systematic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300:439-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 383] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | Ballou S, Katon J, Singh P, Rangan V, Lee HN, McMahon C, Iturrino J, Lembo A, Nee J. Chronic Diarrhea and Constipation Are More Common in Depressed Individuals. Clin Gastroenterol Hepatol. 2019;17:2696-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 8. | Peng Y, Liu F, Qiao Y, Wang P, Ma B, Li L, Si C, Wang X, Zhang M, Song F. Association of abnormal bowel health with major chronic diseases and risk of mortality. Ann Epidemiol. 2022;75:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 9. | Shin A, Xu H, Imperiale TF. Associations of chronic diarrhoea with non-alcoholic fatty liver disease and obesity-related disorders among US adults. BMJ Open Gastroenterol. 2019;6:e000322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Sommers T, Mitsuhashi S, Singh P, Hirsch W, Katon J, Ballou S, Rangan V, Cheng V, Friedlander D, Iturrino J, Lembo A, Nee J. Prevalence of Chronic Constipation and Chronic Diarrhea in Diabetic Individuals in the United States. Am J Gastroenterol. 2019;114:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3146] [Cited by in RCA: 3638] [Article Influence: 202.1] [Reference Citation Analysis (0)] |
| 12. | Brown AE, Walker M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr Cardiol Rep. 2016;18:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 13. | da Silva AA, do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of Hyperinsulinemia and Insulin Resistance in Hypertension: Metabolic Syndrome Revisited. Can J Cardiol. 2020;36:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 14. | Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 687] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 15. | Cao GT, Dai B, Wang KL, Yan Y, Xu YL, Wang YX, Yang CM. Bacillus licheniformis, a potential probiotic, inhibits obesity by modulating colonic microflora in C57BL/6J mice model. J Appl Microbiol. 2019;127:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Liu J, Yue S, Yang Z, Feng W, Meng X, Wang A, Peng C, Wang C, Yan D. Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol Res. 2018;134:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 17. | Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, Soewondo P. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16:102581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 261] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 18. | Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L; MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1311] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 19. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4406] [Article Influence: 209.8] [Reference Citation Analysis (4)] |
| 20. | Saad MJ, Santos A, Prada PO. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology (Bethesda). 2016;31:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 21. | Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64:872-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 849] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 22. | Axling U, Olsson C, Xu J, Fernandez C, Larsson S, Ström K, Ahrné S, Holm C, Molin G, Berger K. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond). 2012;9:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Kang Y, Kang X, Yang H, Liu H, Yang X, Liu Q, Tian H, Xue Y, Ren P, Kuang X, Cai Y, Tong M, Li L, Fan W. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Pharmacol Res. 2022;175:106020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 24. | Altomare A, Di Rosa C, Imperia E, Emerenziani S, Cicala M, Guarino MPL. Diarrhea Predominant-Irritable Bowel Syndrome (IBS-D): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Zhong W, Lu X, Shi H, Zhao G, Song Y, Wang Y, Zhang J, Jin Y, Wang S. Distinct Microbial Populations Exist in the Mucosa-associated Microbiota of Diarrhea Predominant Irritable Bowel Syndrome and Ulcerative Colitis. J Clin Gastroenterol. 2019;53:660-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Dior M, Delagrèverie H, Duboc H, Jouet P, Coffin B, Brot L, Humbert L, Trugnan G, Seksik P, Sokol H, Rainteau D, Sabate JM. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2016;28:1330-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Cao H, Liu X, An Y, Zhou G, Liu Y, Xu M, Dong W, Wang S, Yan F, Jiang K, Wang B. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep. 2017;7:10322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 29. | Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 1799] [Article Influence: 299.8] [Reference Citation Analysis (1)] |
| 30. | Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 482] [Cited by in RCA: 494] [Article Influence: 164.7] [Reference Citation Analysis (0)] |
| 31. | Sheng G, Lu S, Xie Q, Peng N, Kuang M, Zou Y. The usefulness of obesity and lipid-related indices to predict the presence of Non-alcoholic fatty liver disease. Lipids Health Dis. 2021;20:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 32. | Park B, Lee HS, Lee YJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean Genome and Epidemiology Study cohort. Transl Res. 2021;228:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 33. | Huang Z, Ding X, Yue Q, Wang X, Chen Z, Cai Z, Li W, Chen G, Lan Y, Wu W, Wu S, Chen Y. Triglyceride-glucose index trajectory and stroke incidence in patients with hypertension: a prospective cohort study. Cardiovasc Diabetol. 2022;21:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 34. | Mirr M, Skrypnik D, Bogdański P, Owecki M. Newly proposed insulin resistance indexes called TyG-NC and TyG-NHtR show efficacy in diagnosing the metabolic syndrome. J Endocrinol Invest. 2021;44:2831-2843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Zhao J, Fan H, Wang T, Yu B, Mao S, Wang X, Zhang W, Wang L, Zhang Y, Ren Z, Liang B. TyG index is positively associated with risk of CHD and coronary atherosclerosis severity among NAFLD patients. Cardiovasc Diabetol. 2022;21:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 36. | Bruce Wirta S, Hodgkins P, Joseph A. Economic burden associated with chronic constipation in Sweden: a retrospective cohort study. Clinicoecon Outcomes Res. 2014;6:369-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Fagherazzi G, Gusto G, Balkau B, Boutron-Ruault MC, Clavel-Chapelon F, Bonnet F. Functional gastrointestinal disorders and incidence of type 2 diabetes: Evidence from the E3N-EPIC cohort study. Diabetes Metab. 2016;42:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |