Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.196
Peer-review started: October 12, 2023
First decision: November 20, 2023
Revised: November 27, 2023
Accepted: December 20, 2023
Article in press: December 20, 2023
Published online: January 6, 2024
Processing time: 82 Days and 1.8 Hours
In the current World Health Organization classification, acinic cell carcinoma (AcCC) of the breast is considered a rare histological subtype of triple-negative breast cancer. Because of the few reports in the literature, data concerning clinical outcomes are limited. Here, we report a case of AcCC of the breast in a 48-year-old woman.
A 48-year-old woman with a mass in her right breast came to our hospital for further diagnosis. Mammography and an ultrasound (US) scan showed a mass in the upper inner side of the right breast. She then underwent surgery to resect the mass in her right breast. Postoperative pathological examination revealed that the tumor had abundant acinar-like structures formed by tumor cells with prominent eosinophilic granules in the cytoplasm, consistent with acinar cell carcinoma. The results of immunohistochemical analysis supported the diagnosis of breast acinar cell carcinoma. Two months later, she underwent breast-conserving surgery and sentinel lymph node biopsy. The pTNM stage was T2N0M0. After surgery, the patient received 30 radiotherapy sessions. The patient was followed up for a period of one year, and no recurrence was found.
AcCC of the breast is a rare type of malignant tumor. Because it is usually asym
Core Tip: Acinic cell carcinoma (AcCC) of the breast is extremely rare. It is considered a clinically low-grade malignancy. Few cases of breast AcCC have been reported. AcCC of the breast is usually asymptomatic and can be detected by imaging studies. Routine breast ultrasound or mammograms are important. Herein, a 48-year-old woman with AcCC of the breast is reported.
- Citation: Ding JS, Zhang M, Zhou FF. Primary acinic cell carcinoma of the breast: A case report and review of literature. World J Clin Cases 2024; 12(1): 196-203
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/196.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.196
Acinic cell carcinoma (AcCC) of the breast is recognized as a rare histological form of triple-negative breast cancer (TNBC) in the current World Health Organization classification[1]. It is a tumor unique to the salivary glands and can also occur in the breast[2]. It was first described in the breast in 1996 by Roncaroli et al[3]. To date, 64 cases of breast AcCC have been reported in the literature[3-34]. Here, we report a case of acinar cell carcinoma of the breast in a 48-year-old woman and review some of the literature.
A 48-year-old woman found a mass in her right breast during a routine breast ultrasound (US) 4 d prior.
The patient felt a lump in the right breast during a routine breast US examination 4 d prior, but the patient did not feel any discomfort. She had no nipple retraction, itching, pain, discharge, bleeding or ulcers. For further investigation, she came to our hospital.
The patient had no history of hypertension, heart disease, diabetes mellitus, alcohol consumption or smoking. She also had no history of surgery.
The patient denied having any relevant family history.
On examination, no palpable mass was discovered. We found no nipple discharge or palpable ipsilateral axillary or supraclavicular lymph nodes.
The results of blood biochemistry and coagulation tests were normal. Hepatitis B surface antigen was positive, and anti-human immunodeficiency virus, syphilis and anti-hepatitis C virus tests were all negative.
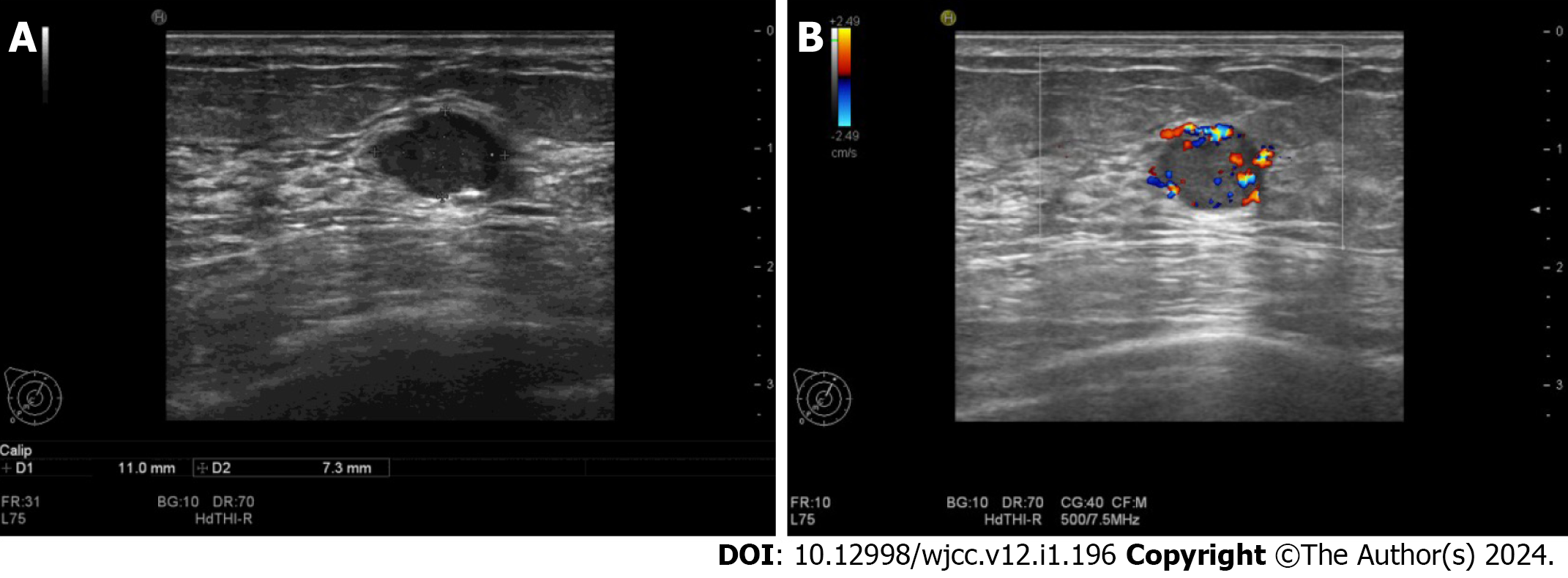
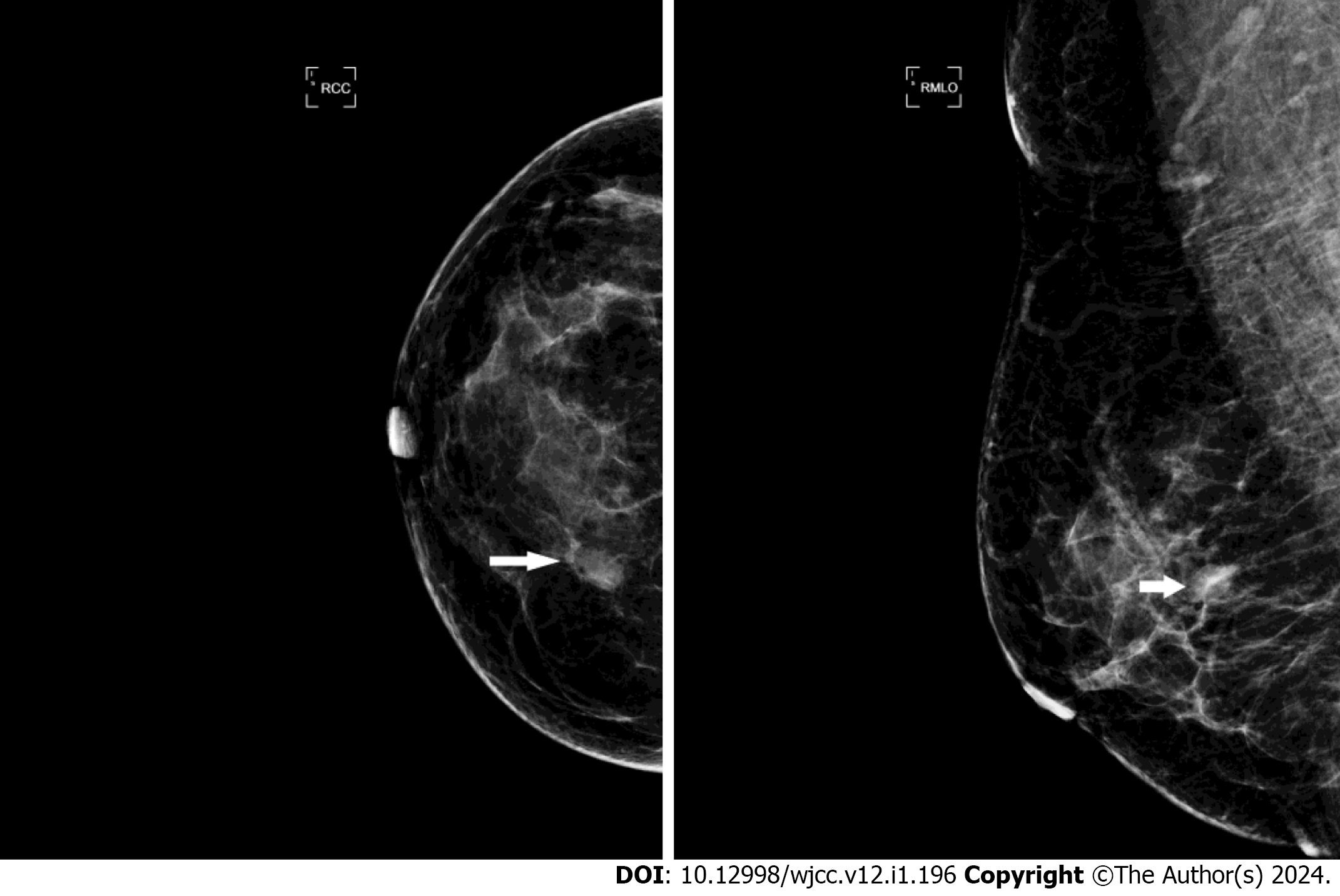
An US scan showed an 11 mm × 7 mm hypoechoic mass in the upper inner quadrant of the right breast, 30 mm away from the nipple, regular in shape, with a distinct margin. Color Doppler imaging showed hypervascularity (Figure 1). The mammogram showed a high-density mass in the upper inner quadrant of the right breast measuring 13 mm × 8 mm with indistinct margins. The subcutaneous tissue structure was clear, with no obvious calcification or thickening of the skin and no enlarged lymph nodes in the axilla (Figure 2). This case is classified as Breast Imaging Reporting and Data System (BI-RADS) 4A according to the BI-RADS category.
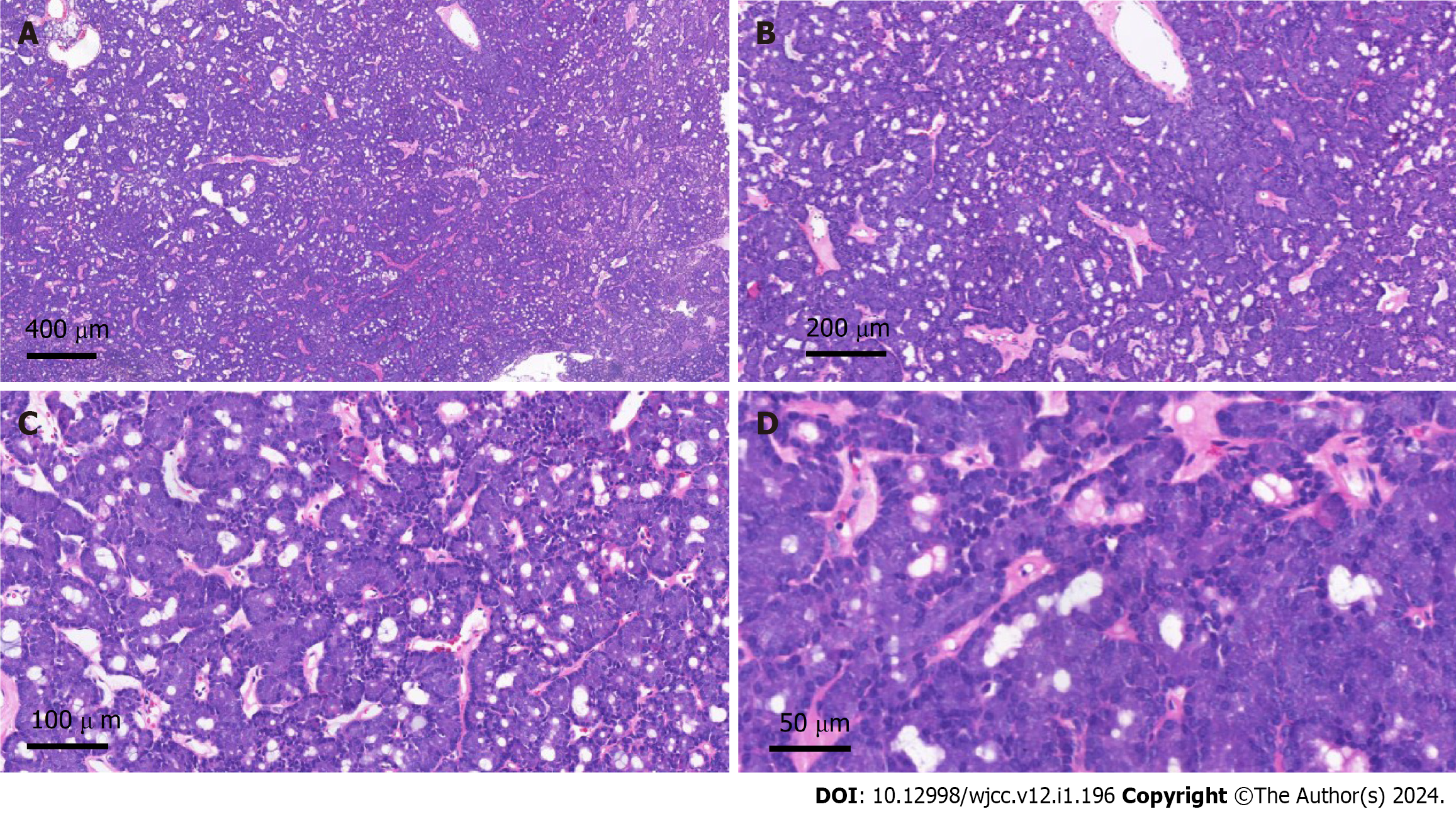
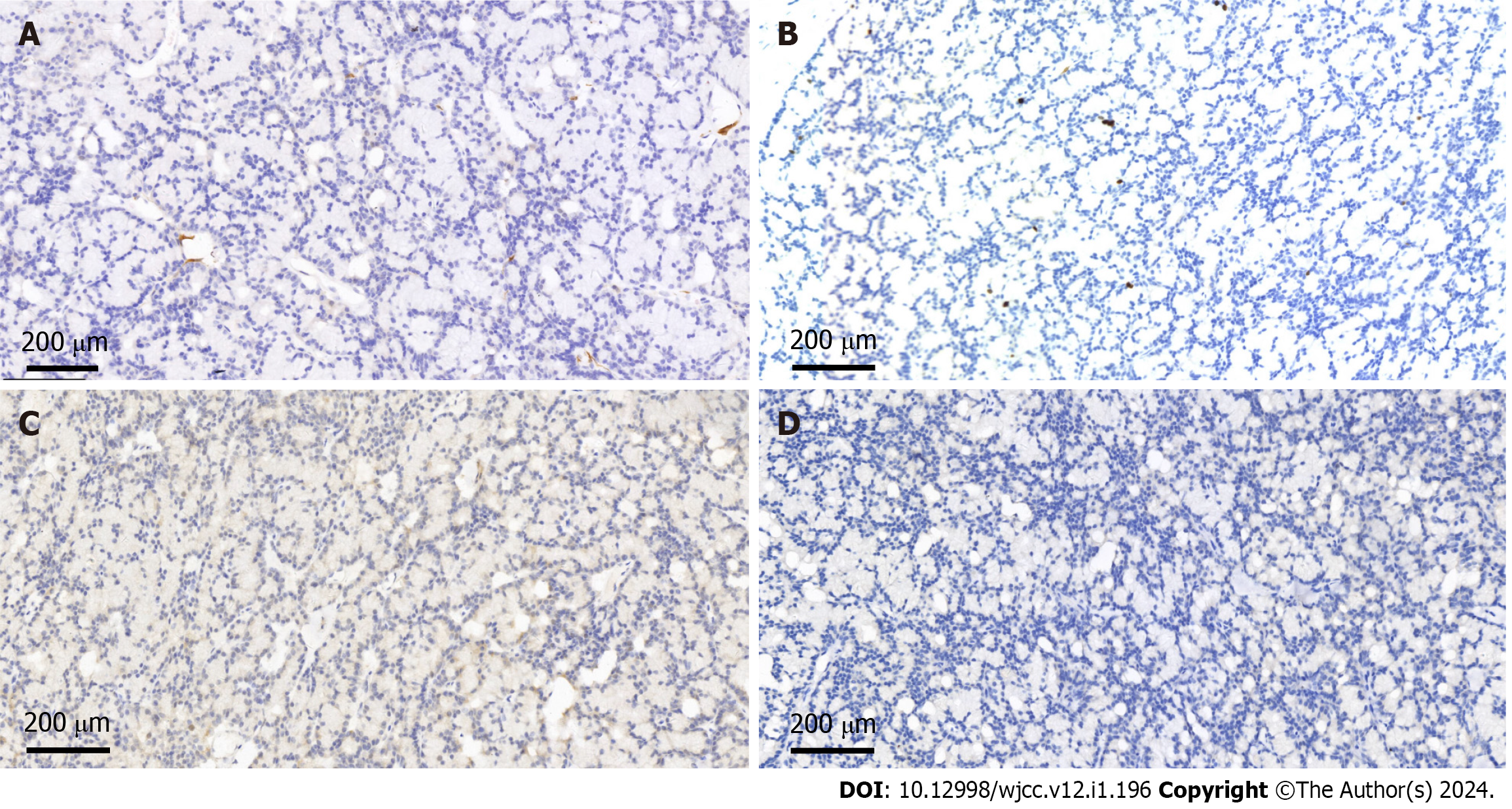
She underwent a right breast mass excision. During the surgery, a 10 mm × 10 mm solid diseased mass was detected at the upper inner side of the right breast with an intact capsule. It had a smooth surface and intermediate texture. Intraoperative frozen pathology indicated a neuroendocrine tumor, and the final diagnosis needed immunohistochemical examination. The postoperative pathological examination confirmed that the tumor revealed abundant acinar-like structures formed by tumor cells with prominent eosinophilic granules in the cytoplasm, consistent with acinar cell carcinoma (Figure 3). Immunohistochemical staining showed that the tumor cells were positive for Ki67 (1% positive) and CerbB-2 but negative for ER, PR, HER-2, CK5/6, CgA, Syn, P63 and calponin (Figure 4).
The final diagnosis was acinar cell carcinoma of the right breast.
Two months later, a right partial mastectomy was performed, and right axillary sentinel lymph node biopsy was appended. Both the surgical margins and lymph node status were negative. The pathologic stage on the synoptic report was T2N0M0. After surgery, the patient received 30 radiotherapy sessions.
No recurrence or metastasis was detected at the one-year follow-up of this patient, and the patient remains recurrence-free and metastasis-free to date.
Both breast and salivary glands are exocrine microtubule glands with similar morphological characteristics. Therefore, the same type of tumor may appear in two locations. Based on the current World Health Organization classification, it is considered a histological subtype of TNBC[1].
The first case of primary AcCC of the breast was reported in 1996, with morphological features similar to salivary glands[3]. To date, including the present case, approximately 64 cases have been reported in the literature[3-34]. The only case of male breast AcCA was reported by Shimao et al[4]. According to previous reports, the age of onset is 23 to 80 years, the average age is 49 years, the median age is 48 years, and the tumor size is 10 to 55 (average 28) mm[3-34]. The patient in this case was a 48-year-old female, which is consistent with the literature. Table 1 summarizes the clinical characteristics of the 64 previously reported cases and this case.
| Characteristic | n (%) |
| Sex | 64 |
| Male | 1 (1.6) |
| Female | 63 (98.4) |
| Age | 58 |
| ≤ 40 | 13 (22.4) |
| 40-60 | 34 (58.6) |
| ≥ 60 | 11 (19.0) |
| Tumor size (mm) | 52 |
| ≤ 20 | 12 (23.1) |
| 20-40 | 28 (53.8) |
| ≥ 40 | 12 (23.1) |
| Site | 40 |
| Left | 14 (35.0) |
| Right | 26 (65.0) |
| Lymph node metastasis | 38 |
| Yes | 9 (23.7) |
| No | 29 (76.3) |
| Surgical approach | 44 |
| MRM + ALND | 11 (25.0) |
| BCS + ALND | 11 (25.0) |
| MRM | 1 (2.2) |
| BCS | 5 (11.4) |
| MRM + SLND | 8 (18.2) |
| BCS + SLND | 8 (18.2) |
| Adjuvant therapy | 25 |
| CT | 6 (24.0) |
| Neo-CT | 3 (12.0) |
| HT | 2 (8.0) |
| RT | 4 (16.0) |
| CT + RT | 5 (20.0) |
| CT + HT | 3 (12.0) |
| CT + RT + HT | 2 (8.0) |
| Recurrences/metastasis | 36 |
| Yes | 8 (22.2) |
| No | 28 (77.8) |
| Status | 36 |
| Alive (NED) | 33 (91.7) |
| Died (DWD) | 3 (8.3) |
Regarding the diagnosis of breast AcCC, the clinical diagnosis of new breast masses is based on the “triple assessment” method, including inspection, imaging (mammogram and US) and histology (core biopsy), which together provide a comprehensive algorithm[34]. This combination of investigations is better than the diagnosis using a single investigation method. AcCC of the breast has no specific clinical manifestations. Most patients visit clinics because of the discovery of a painless breast mass. According to previous literature, none of the patients had a history of pruritus, bleeding, discharge, ulceration, skin alterations or retraction of the nipple. Routine laboratory examinations of breast AcCC have also shown no specificity.
Literature on AcCC of the breast is rare, and literature on radiological findings is even rarer. According to the literature, only 8 patients had undergone breast US examinations and mammography. Mammography imaging of breast AcCC usually presents as high-density masses and generally without skin or nipple retraction. Calcification was described for six cases, with 3 having microcalcifications and 3 having no calcifications. The typical US feature was hypoechoic. The shapes and borders of breast AcCC were oval/spiculated and circumscribed/indistinct according to the summary in this review. Three cases were circumscribed, 4 were indistinct, 3 turned into an oval shape, and 4 showed a spiculated shape. Intratumoral cystic components were not common, as there were few reports describing this. Only one male has been reported to have this disease, namely, one 23-year-old man in the study by Shimao et al[4]. Ultrasonography of the breast showed a cystic mass with clear edges, consisting of hypoechoic intracystic effusion and a hyperechoic intracystic tumor. Unfortunately, reports on breast AcCC imaging are scarce, so characteristic imaging findings cannot be summarized well.
A review of previous reports shows that the morphological appearance of breast AcCC is very consistent. It has the same morphology as tumors found in salivary glands. Most reported cases of breast AcCC have described the tumor structure, where solid and nested components are mixed with renal tubules and microglandular morphological areas[5,11,15]. In most cases, the immunohistochemical analysis of breast AcCA is negative for hormone receptors, while all specimens express lysozyme, S-100, and A1-ACT and are positive for amylase and PAS. Myoepithelial markers such as calponin and p63 are negative in breast AcCC, confirming the invasive growth pattern of the tumor[18]. In the case reported herein, the immunohistochemical results showed that the tumor cells were negative for ER, PR, HER-2, CK5/6, CgA, Syn, P63 and calcin, consistent with previous reports in the literature.
Standardized treatments for breast AcCC are lacking, perhaps due to its rarity. Treatment can involve surgery, radiotherapy, and chemotherapy. The surgical treatment options are either modified radical mastectomy (MRM) or breast-conserving surgery (BCS). The 44 patients reported in Table 1 underwent surgery, of whom 20 underwent MRM and 24 underwent BCS. Of the 38 patients treated for axillary disease, 22 underwent axillary lymph node dissection, and 16 underwent sentinel lymph node dissection (SLND). Three patients received neoadjuvant chemotherapy (CT) before surgery. According to reports, 22 patients received postoperative adjuvant therapy: 16 received CT, 7 received hormone therapy, and 11 received radiotherapy (Table 1). In our case, the patient underwent BCS and SLND, 30 radiotherapy sessions were performed after the operation, and the treatment effect was very good.
Of the 64 patients reported thus far, 36 were followed up for a period of 3 to 184.8 (mean 35.5) mo, of whom 28 (77.8%) had no recurrence, 8 (22.2%) had recurrence or distant metastasis (bone, liver, lung or leptomeningeal), and three of them died at 12, 32 and 34 mo after diagnosis. Due to the lack of early reports in the literature, it is generally believed that the prognosis of breast AcCC is good[31]. However, breast AcCC may also coexist with highly malignant TNBC, which could lead to recurrence, metastasis, and even death[7,15,32]. Therefore, more data on this rare tumor type are needed so that a clinical consensus can be reached for the treatment of this rare neoplasm.
We report an extremely rare case of AcCC of the breast. Because AcCC of the breast is usually asymptomatic and can be detected by imaging studies, routine breast US or mammograms are important. Unfortunately, there are no characteristic imaging findings or clinical manifestations of the disease, so immunohistochemical analysis is critical for an accurate diagnosis of AcCC of the breast.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moshref L, Saudi Arabia S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL
| 1. | Frank GA, Danilova NV, Andreeva IuIu, Nefedova NA. [WHO classification of tumors of the breast, 2012]. Arkh Patol. 2013;75:53-63. [PubMed] |
| 2. | Foschini MP, Krausz T. Salivary gland-type tumors of the breast: a spectrum of benign and malignant tumors including "triple negative carcinomas" of low malignant potential. Semin Diagn Pathol. 2010;27:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Roncaroli F, Lamovec J, Zidar A, Eusebi V. Acinic cell-like carcinoma of the breast. Virchows Arch. 1996;429:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Damiani S, Pasquinelli G, Lamovec J, Peterse JL, Eusebi V. Acinic cell carcinoma of the breast: an immunohistochemical and ultrastructural study. Virchows Arch. 2000;437:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Shimao K, Haga S, Shimizu T, Imamura H, Watanabe O, Kinoshita J, Nagumo H, Utada Y, Okabe T, Kajiwara T, Oshibe N, Aiba M. Acinic Cell Adenocarcinoma Arising in the Breast of a Young Male: A Clinicopathological, Immunohistochemical and Ultrastructural Study. Breast Cancer. 1998;5:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Schmitt FC, Ribeiro CA, Alvarenga S, Lopes JM. Primary acinic cell-like carcinoma of the breast--a variant with good prognosis? Histopathology. 2000;36:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Coyne JD, Dervan PA. Primary acinic cell carcinoma of the breast. J Clin Pathol. 2002;55:545-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Elster EA, Markusic J, Ball R, Soballe P, Henry M, Louie A, Clare S. Primary acinic cell carcinoma of the breast. Am Surg. 2002;68:993-995. [PubMed] |
| 9. | Hirokawa M, Sugihara K, Sai T, Monobe Y, Kudo H, Sano N, Sano T. Secretory carcinoma of the breast: a tumour analogous to salivary gland acinic cell carcinoma? Histopathology. 2002;40:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Kahn R, Holtveg H, Nissen F, Holck S. Are acinic cell carcinoma and microglandular carcinoma of the breast related lesions? Histopathology. 2003;42:195-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Peintinger F, Leibl S, Reitsamer R, Moinfar F. Primary acinic cell carcinoma of the breast: a case report with long-term follow-up and review of the literature. Histopathology. 2004;45:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Kinkor Z, Skálová A. [Acinic cell-like differentiation in invasive ductal carcinoma and in ductal hyperplasia of the breast--report of two cases]. Cesk Patol. 2005;41:29-33. [PubMed] |
| 13. | Tanahashi C, Yabuki S, Akamine N, Yatabe Y, Ichihara S. Pure acinic cell carcinoma of the breast in an 80-year-old Japanese woman. Pathol Int. 2007;57:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Reis-Filho JS, Natrajan R, Vatcheva R, Lambros MB, Marchió C, Mahler-Araújo B, Paish C, Hodi Z, Eusebi V, Ellis IO. Is acinic cell carcinoma a variant of secretory carcinoma? A FISH study using ETV6'split apart' probes. Histopathology. 2008;52:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Huo L, Bell D, Qiu H, Sahin A, Wu Y, Sneige N. Paneth cell-like eosinophilic cytoplasmic granules in breast carcinoma. Ann Diagn Pathol. 2011;15:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Chang ED, Lee EJ, Lee AW, Kim JS, Kang CS. Primary acinic cell carcinoma of the breast: a case report with an immunohistochemical and ultrastructural studies. J Breast Cancer. 2011;14:160-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Choh CT, Komar V, Courtney SP. Primary acinic cell carcinoma of the breast: a rare lesion with good prognosis. Breast J. 2012;18:610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Sakuma T, Mimura A, Tanigawa N, Takamizu R. Fine needle aspiration cytology of acinic cell carcinoma of the breast. Cytopathology. 2013;24:403-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Shingu K, Ito T, Kaneko G, Itoh N. Primary acinic cell carcinoma of the breast: a clinicopathological and immunohistochemical study. Case Rep Oncol Med. 2013;2013:372947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Osako T, Takeuchi K, Horii R, Iwase T, Akiyama F. Secretory carcinoma of the breast and its histopathological mimics: value of markers for differential diagnosis. Histopathology. 2013;63:509-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Winkler N, Morrell G, Factor RE. Invasive carcinoma with acinic cell-like features of the breast. Breast J. 2013;19:334-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Falleti J, Coletti G, Rispoli E, Scarabeo F, Cervasio M, Tornillo L, Pettinato G, Insabato L. Acinic cell carcinoma of the breast arising in microglandular adenosis. Case Rep Pathol. 2013;2013:736048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Ripamonti CB, Colombo M, Mondini P, Siranoush M, Peissel B, Bernard L, Radice P, Carcangiu ML. First description of an acinic cell carcinoma of the breast in a BRCA1 mutation carrier: a case report. BMC Cancer. 2013;13:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Limite G, Di Micco R, Esposito E, Sollazzo V, Cervotti M, Pettinato G, Varone V, Benassai G, Monda A, Luglio G, Maisto V, Izzo G, Forestieri P. The first case of acinic cell carcinoma of the breast within a fibroadenoma: case report. Int J Surg. 2014;12 Suppl 1:S232-S235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Zhao Y, Li W, Lang R, Yang Y, Gao X, Zheng Y, Zhang C, Fu X, Fu L. Primary acinic cell carcinoma of the breast: a case report and review of the literature. Int J Surg Pathol. 2014;22:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Piscuoglio S, Hodi Z, Katabi N, Guerini-Rocco E, Macedo GS, Ng CK, Edelweiss M, De Mattos-Arruda L, Wen HY, Rakha EA, Ellis IO, Rubin BP, Weigelt B, Reis-Filho JS. Are acinic cell carcinomas of the breast and salivary glands distinct diseases? Histopathology. 2015;67:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Conlon N, Sadri N, Corben AD, Tan LK. Acinic cell carcinoma of breast: morphologic and immunohistochemical review of a rare breast cancer subtype. Hum Pathol. 2016;51:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Kawai H, Sugimoto R, Iga N, Ikeda H, Yoshida R, Waki N, Ishizaki M, Nishi H, Yamashita K. [A Case of Primary Acinic Cell Carcinoma(ACC)of the Breast]. Gan To Kagaku Ryoho. 2016;43:2019-2021. [PubMed] |
| 29. | Li H, Wang F, Shen P, Zhou F. Pure acinic cell carcinoma of the breast: A case report and literature review. Medicine (Baltimore). 2017;96:e8866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Sen R, Bhutani N, Kamboj J, Dahiya S. Primary acinic cell carcinoma of the breast: A case report with a clinicopathological and immunohistochemical study of a rare breast cancer subtype. Ann Med Surg (Lond). 2018;35:137-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Agafonoff S, Sobolewski A, Braverman TS. Adenoid cystic carcinoma of the breast - Discordant size on imaging and pathology: A case report and review of literature. Ann Med Surg (Lond). 2019;43:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Sardana R, Parwani AV, Cui X, Balakrishna J. Unusual cerebrospinal fluid finding of intracytoplasmic granules in metaplastic carcinoma of the breast with acinar differentiation. Diagn Cytopathol. 2021;49:E152-E155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Makino Y, Kawamata A, Hikino H, Murata Y, Takahashi T, Miura H. [A Case of Primary Acinic Cell Carcinoma of the Breast]. Gan To Kagaku Ryoho. 2020;47:1945-1947. [PubMed] |
| 34. | Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler AJ, Orgill DP; SCARE Group. The SCARE 2018 statement: Updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg. 2018;60:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2072] [Article Influence: 296.0] [Reference Citation Analysis (0)] |