Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.136
Peer-review started: August 25, 2023
First decision: December 7, 2023
Revised: December 12, 2023
Accepted: December 19, 2023
Article in press: December 19, 2023
Published online: January 6, 2024
Processing time: 130 Days and 3.7 Hours
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, highly invasive malignant neoplasm. There is no universally accepted standard of care because of its rarity and the dearth of prospective research. It is still challenging for some patients to achieve persistent clinical remission or cure, despite the success of allogeneic hematopoietic stem cell transplantation (allo-HSCT), indicating that there is still a significant recurrence rate. We report a case of prevention of BPDCN allograft recurrence by azacitidine maintenance therapy and review the relevant literature.
We report a 41-year-old man with BPDCN who was admitted to hospital due to skin sclerosis for > 5 mo’ duration. BPDCN was diagnosed by combined clinical assessment and laboratory examinations. Following diagnosis, the patients underwent induction consolidation chemotherapy to achieve the first complete remission, followed by bridging allo-HSCT. Post-transplantation, azacitidine (75 mg/m2 for 7 d) was administered as maintenance therapy, with repeat administration every 4–6 wk and appropriate extension of the chemotherapy cycle. After 10 cycles, the patient has been disease free for 26 mo after transplantation. Regular assessments of bone marrow morphology, minimal residual disease, full donor chimerism, Epstein–Barr virus, and cytomegalovirus all yielded normal results with no abnormalities detected.
Azacitidine may be a safe and effective maintenance treatment for BPDCN following transplantation because there were no overt adverse events during the course of treatment.
Core Tip: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematological tumor with cutaneous invasion as the first clinical manifestation, as well as lymph nodes, soft tissues, peripheral blood, and bone marrow involvement. The first recommended treatment option is allogeneic hematopoietic stem cell transplantation, but there is still a high recurrence rate after transplantation. We present a case of azacitidine maintenance treatment to prevent BPDCN allograft relapse in our center.
- Citation: Tao LL, Wen HT, Wang ZY, Cheng J, Zhao L. Azacitidine maintenance therapy for blastic plasmacytoid dendritic cell neoplasm allograft: A case report. World J Clin Cases 2024; 12(1): 136-141
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/136.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.136
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematological malignancy that develops from a precursor of plasmacytoid dendritic cells. The median age of BPDCN patients at diagnosis is 61–67 years, and the median overall survival (OS) is 12–14 mo[1,2].
Pathomorphological and immunophenotypic characteristics are the key factors used to diagnose BPDCN. The 2022 5th edition of the World Health Organization Classification of Myeloid and Histiocytic/Dendritic Neoplasms[3] places BPDCN after myeloid neoplasms, which are thought to be derived from common myeloid progenitors that give rise to monocytes/histiocytes/dendritic cells and are morphologically easily confused with other more common myeloid malignancies. The classification focuses on immunophenotypic diagnosis[3]. Our patient expressed CD4, CD56, CD123, CD303 and CD304, and none of the expected negative markers were present. Bridging allogeneic hematopoietic stem cell transplantation (allo-HSCT) after the first complete remission (CR1) with induction consolidation chemotherapy is currently the best option for treating BPDCN. Patients can undergo maintenance therapy after transplantation to reduce recurrence and lengthen survival. We present a case of a patient diagnosed with BPDCN at our center who was treated with azacitidine maintenance therapy after allo-HSCT and had good outcomes.
In September 2020, a 41-year-old man was admitted to our hospital for skin sclerosis for > 5 mo’ duration.
Symptoms started 2 wk before presentation with purplish subcutaneous nodules on the skin of the head, trunk and limbs.
The patient was previously healthy. There was no disease history in other systems.
The patient denied any family history of malignant tumors.
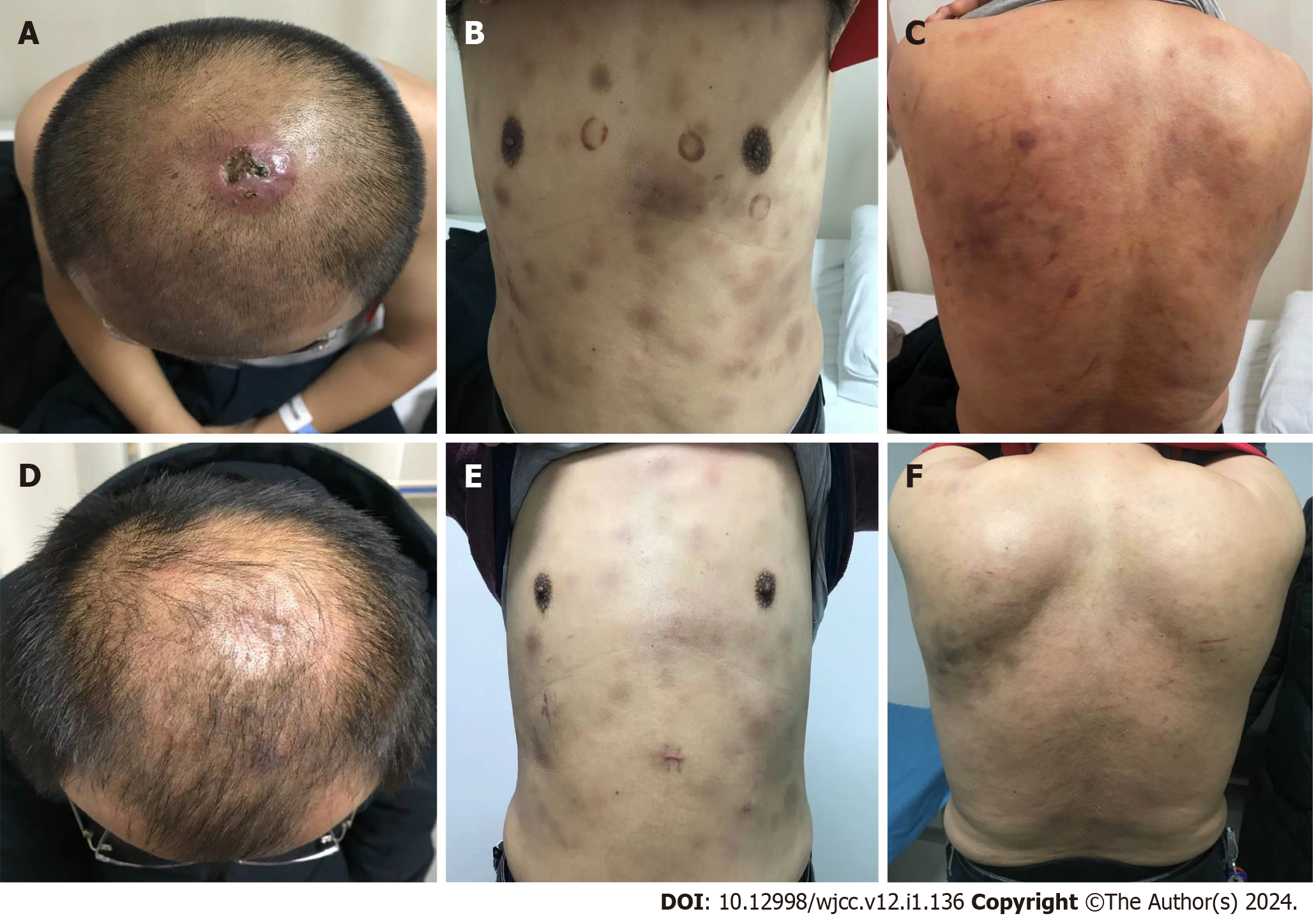
Physical examination revealed scattered cyanotic subcutaneous nodules on the skin of the head, trunk and extremities, higher than the skin surface, with clear boundaries, no tenderness and poor mobility. The head induration and ulceration formed dark purple scabs, and no abnormalities were found in the remaining physical examination (Figure 1A-C).
No abnormality was found in routine blood and urine analyses.
Positron emission tomography–computed tomography (PET-CT) revealed a 28-mm-long thickening of the subcutaneous soft tissue behind the left scapula, as well as mild fluorodeoxyglucose uptake (maximum standard uptake value 1.26).
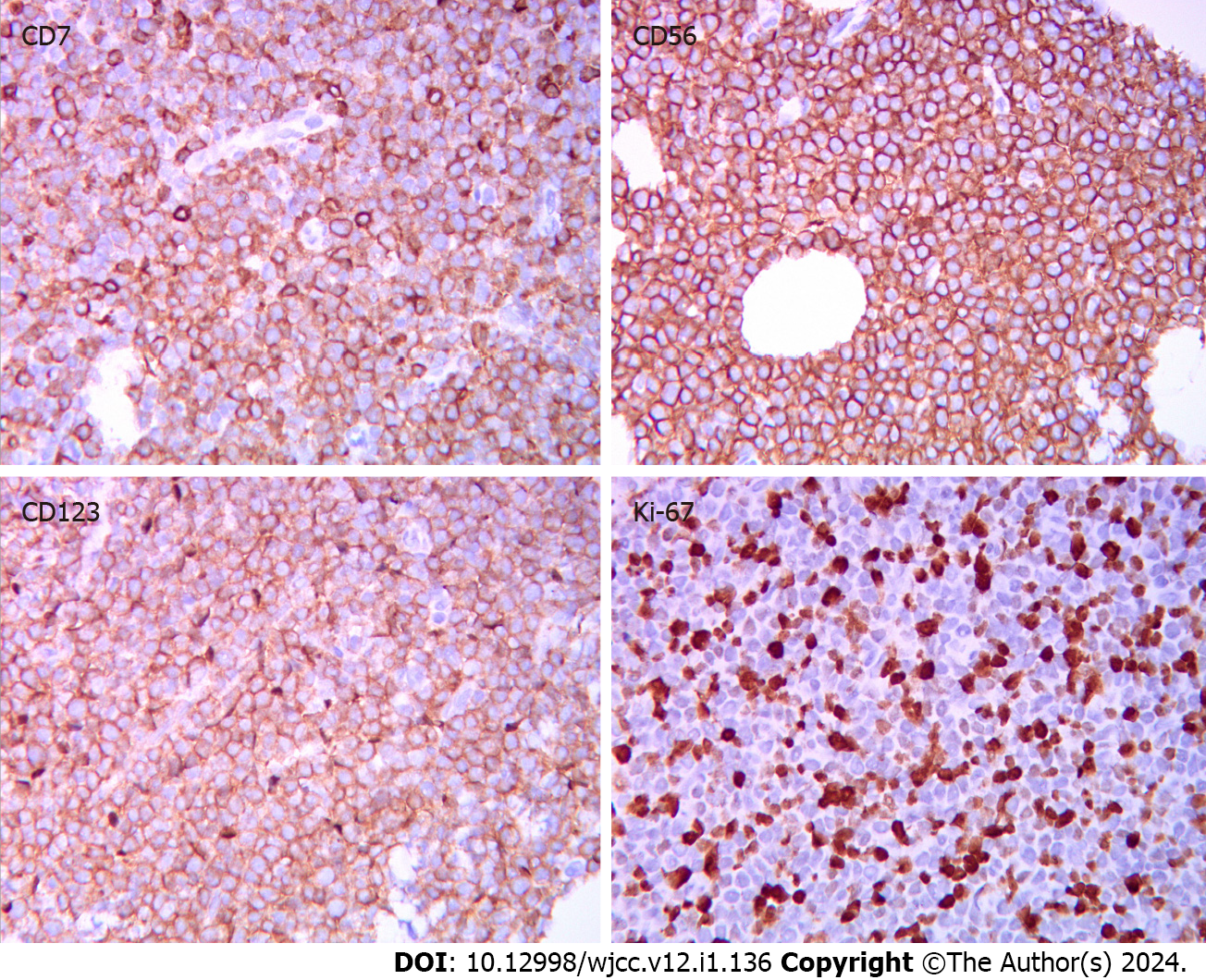
Bone marrow cytology showed active granulopoiesis megakaryocytes were visible, platelets were scattered in clusters, and the lymphocyte ratio was normal. Pathological biopsy showed malignant infiltration of hematopoietic tissue in the abdominal skin. Immunohistochemistry showed (Figure 2): CD4 (+), CD56 (+), CD123 (+), CD43 (+), CD45 (+), CD7 (+), CD99 (+), CD2 (+/-, poor signal localization), CD5 (-), CD20 (-), CD30 (-), CD34 (-), CD117 (-), CD138 (-), myeloperoxidase (-), terminal deoxynucleotidyl transferase (less +), TIA-1 (-), Ki-67 (+, 80%–90%). We considered the diagnosis of BPDCN. Flow cytometry of bone marrow cells revealed that 0.49% of cells (occupying nuclei) expressed CD123, Human leukocyte antigen (HLA)-DR (a subtype of Major histocompatibility complex class II), CD56, CD123, CD303, CD304, CD4, and CD7dim, but not CD34, CD117, CD33, CD13, CD11b, CD14, CD64, CD5, CD3, CD4, CD8, or CD2. Epstein–Barr encoding region in situ hybridization was negative, as was TCR gene rearrangement. Karyotype: 46, XY[20]. Bone marrow biopsy revealed a small amount of juvenile cell hyperplasia.
Given the immunohistochemistry results and clinical history, bone marrow infiltration of BPDCN was considered.
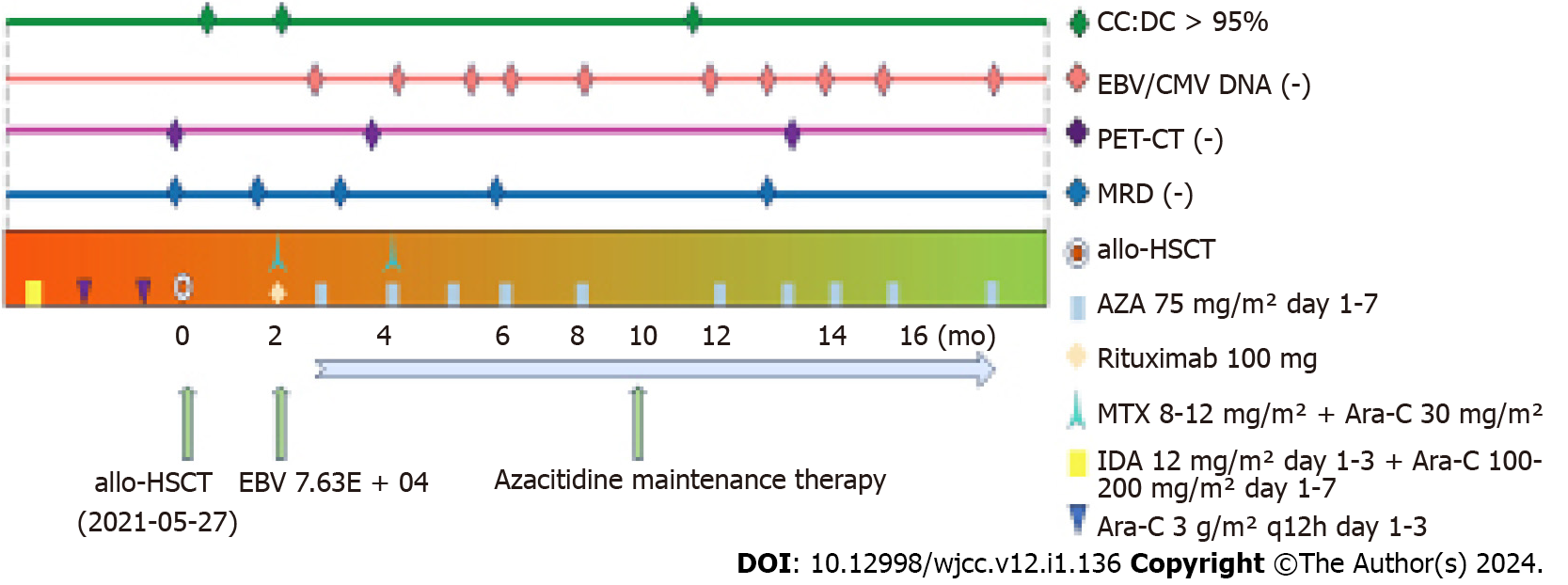
The treatment was as follows (Figure 3). From February 2021, he was given three cycles of induction chemotherapy with the idarubicin/cytarabine regimen and the high-dose cytarabine regimen, the patient's damaged skin healed (Figure 1). In April 2021, review of minimal residual disease (MRD) results less than 10-4; PET-CT showed that uptake was not abnormally high on systemic imaging. In May 2021, allo-HSCT was performed. The donor’s son had an HLA compatibility of 6/12. Pretreatment scheme: Cotysine arabinoside + busulfan/cyclophosphamide + anti-thymocyte globulin; Neutrophil engraftment appeared at day + 12 post-transplantation, and platelet engraftment showed up at + 15. Review of the quantitative results of Epstein–Barr virus (EBV) DNA in July 2021: 7.63 × 104. EBV results turned negative after a single dose of rituximab 100 mg. In September 2021, azacitidine (75 mg/m2 for 7 d) maintenance treatment was administered, which was repeated every 4–6 wk, and the chemotherapy cycle was appropriately extended.
At 26 mo after transplantation, the patient had been treated for 10 cycles, and no serious adverse events occurred. Regular review detected MRD, bone marrow morphology, EBV, chimerism, and cytomegalovirus.
The diagnosis of BPDCN was confirmed after the pathomorphological findings were considered. BPDCN is extremely rare worldwide, and there is no standard unified treatment protocol. Local radiotherapy, systemic chemotherapy, and HSCT are common clinical treatment regimens[4]. The cyclophosphamide, doxorubicin, vincristine, prednisone, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, acute lymphoblastic leukemia-like, acute myeloid leukemia (AML)-like, and NK/T-like regimens, among others, are commonly used chemotherapeutic regimens. Clinical trials using 5-azacitidine to treat BPDCN have also yielded positive results[5]. However, the number of cases so treated was small, and the long-term therapeutic effect requires confirmation in large clinical trials. Some cases of BPDCN can be transformed into AML or may coexist with myelodysplastic syndromes. TET2 gene mutations associated with DNA methylation have been identified in BPDCN patients. The demethylating agent azacitidine has been approved for the treatment of moderate to high-risk myelodysplastic syndromes and AML. Given that BPDCN is also a myeloid malignancy, azacitidine may be used for its treatment as well. Pagano et al[6] assessed the efficacy in 43 patients of various chemotherapeutic regimens, concluding that ALL-like regimens were superior to AML-like regimens. The first CD123-targeted drug, tagraxofusp (SL-401), was approved in 2018 for patients aged ≥ 2 years with BPDCN. SL-401, a recombinant fusion protein that directly targets the interleukin 3 receptor on the surface of BPDCN cells, has shown moderate efficacy in this disease, but the number of cases is limited, and more convincing prospective randomized controlled trials are needed to validate this finding[7,8]. Bridging allo-HSCT after CR1 with induction consolidation chemotherapy is currently the best option for treating AML and BPDCN[9,10]. By analyzing 15 patients with BPDCN who received allo-HSCT and underwent marrow clearance, Lu et al[9] found an OS rate of 73.3% ± 10.5%. However, the risk of relapse after transplantation remains high, with 40%–70% of AML patients relapsing within the first year of treatment, and the 1-year OS rate for relapsed patients is only 23%[11,12]. Patients who receive maintenance therapy after transplantation may experience fewer relapses and live longer.
Azacitidine has been recognized as one of the best agents for post-transplant maintenance therapy in AML patients, but there is still disagreement about when to begin maintenance therapy after transplantation, the duration of therapy, the optimal maintenance dose, and whether a combination of drugs is needed[13,14]. Azacitidine administration soon after transplantation was found to improve the graft-versus-leukemia (GVL) effect by increasing CD8+ T-cell responses induced by epigenetic-silencing-related oncogenes. Azacitidine increased the GVL effect but not the incidence of graft-versus-host disease (GVHD), which may be related to the increase in T regulatory cell numbers[15]. It also has no effect on neutrophil implantation, and similar immunomodulatory effects of another methylating drug, decitabine, have not been reported[13,16,17]. There have been cases of exploratory use of azacitidine or decitabine in the maintenance treatment of AML or myelodysplastic syndromein in China and elsewhere[16], with good efficacy, but the azacitidine dose is typically 32 mg/m2 for 5 d. Azacitidine has less cytotoxicity and milder myelosuppressive effects than decitabine. Azacitidine is better tolerated by patients as a post-transplant maintenance therapy for AML (32/36 mg/m2, 5 d) and significantly prolongs OS and disease-free interval (DFS), according to phase 1 and 2 clinical studies[18,19]. Lou et al[15] assessed the efficacy of azacitidine (75 mg/m2, 7 d) maintenance therapy in 30 AML transplant patients. They treated 10 patients who were positive for MRD prior to azacitidine treatment, and nine of the 10 MRD-positive patients, including all seven flow MRD-positive patients and two of three molecular MRD-positive patients, became negative after treatment. The median OS of the 30 patients was 33.7 ± 1.8 mo, with a 3-year OS rate of 83.2% ± 9.9% and a 3-year DFS rate of 81.3% ± 9.4%. By the end of the study, four (13.33%) patients had relapsed, for a 3-year cumulative relapse rate of 18.7% ± 9.4%. A total of 23.33% (7/30) of the treated patients had grade III/IV myelosuppression. Two patients had varying degrees of cutaneous chronic GVHD reduction, one patient had hepatic GVHD exacerbation, and no patient had new-onset GVHD. The use of azacitidine (75 mg/m2, 7 d) in maintenance therapy after allo-HSCT in AML patients reduces the relapse rate, improves OS, and is safe and well tolerated without increasing the incidence of GVHD. Patients require continuous treatment for maximum benefit because the recommended duration of azacitidine in phase 2 studies is up to 12 mo[19].
Our patient’s age at onset was 41 years, with skin and bone marrow involvement, and there were no significant adverse effects during treatment, indicating that azacitidine is safe and effective as post-transplant maintenance therapy in patients with BPDCN, but these results need to be confirmed in larger-sample prospective clinical trials.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shalaby MN, Egypt S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Trottier AM, Cerquozzi S, Owen CJ. Blastic plasmacytoid dendritic cell neoplasm: challenges and future prospects. Blood Lymphat Cancer. 2017;7:85-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Jegalian AG, Buxbaum NP, Facchetti F, Raffeld M, Pittaluga S, Wayne AS, Jaffe ES. Blastic plasmacytoid dendritic cell neoplasm in children: diagnostic features and clinical implications. Haematologica. 2010;95:1873-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703-1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 2151] [Article Influence: 717.0] [Reference Citation Analysis (0)] |
| 4. | Sweet K. Blastic plasmacytoid dendritic cell neoplasm: diagnosis, manifestations, and treatment. Curr Opin Hematol. 2020;27:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Laribi K, Denizon N, Ghnaya H, Atlassi M, Besançon A, Pineau-Vincent F, Gaulard P, Petrella T. Blastic plasmacytoid dendritic cell neoplasm: the first report of two cases treated by 5-azacytidine. Eur J Haematol. 2014;93:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Pagano L, Valentini CG, Pulsoni A, Fisogni S, Carluccio P, Mannelli F, Lunghi M, Pica G, Onida F, Cattaneo C, Piccaluga PP, Di Bona E, Todisco E, Musto P, Spadea A, D'Arco A, Pileri S, Leone G, Amadori S, Facchetti F; GIMEMA-ALWP (Gruppo Italiano Malattie EMatologiche dell'Adulto, Acute Leukemia Working Party). Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 7. | DiPippo AJ, Wilson NR, Pemmaraju N. Targeting CD123 in BPDCN: an emerging field. Expert Rev Hematol. 2021;14:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Lee SS, McCue D, Pemmaraju N. Tagraxofusp as treatment for patients with blastic plasmacytoid dendritic cell neoplasm. Expert Rev Anticancer Ther. 2020;20:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Lu Y, Sun RJ, Zhang JP, Xu F, Du ZC, Tong GL, Wang Y, Lu DP. Allogeneic hematopoietic stem cell transplantation with myeloablative conditioning regimen for blastic plasmacytoid dendritic cell neoplasm patients in complete remission: a single center study. Leuk Lymphoma. 2022;63:3092-3099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Nayak RK, Chen YB. Maintenance therapy for AML after allogeneic HCT. Front Oncol. 2022;12:895771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Ali N, Tomlinson B, Metheny L, Goldstein SC, Fu P, Cao S, Caimi P, Patel RD, Varela JC, Andrade L, Balls JW, Baer L, Smith M, Smith T, Nelson M, de Lima M, Mori S. Conditioning regimen intensity and low-dose azacitidine maintenance after allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Leuk Lymphoma. 2020;61:2839-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, Bunjes DW, Zhang MJ. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 13. | Xuan L, Liu Q. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2021;14:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Molica M, Breccia M, Foa R, Jabbour E, Kadia TM. Maintenance therapy in AML: The past, the present and the future. Am J Hematol. 2019;94:1254-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Lou D, Liu L, Qin WW. [Efficacy of maintenance therapy with azacitidine for acute myeloid leukemia after allo-geneic hematopoietic stem cell transplantation]. Zhongguo Zhongliu Linchuang. 2022;49:642-647. [DOI] [Full Text] |
| 16. | Yoshimoto G, Mori Y, Kato K, Odawara J, Kuriyama T, Ueno T, Obara T, Yurino A, Yoshida S, Ogawa R, Ohno Y, Iwasaki H, Eto T, Akashi K, Miyamoto T. Azacitidine for the treatment of patients with relapsed acute myeloid leukemia after allogeneic stem cell transplantation. Leuk Lymphoma. 2021;62:2939-2948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, Nunnick J, Khanum R, Raghavan M, Cook M, Snowden JA, Griffiths M, Russell N, Yin J, Crawley C, Cook G, Vyas P, Moss P, Malladi R, Craddock CF. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood. 2012;119:3361-3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 325] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 18. | de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, Braun TM, Nguyen HQ, Champlin R, Garcia-Manero G. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420-5431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 360] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 19. | Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, Ward J, Ferguson P, Hazlewood P, Buka R, Vyas P, Goodyear O, Tholouli E, Crawley C, Russell N, Byrne J, Malladi R, Snowden J, Dennis M. Tolerability and Clinical Activity of Post-Transplantation Azacitidine in Patients Allografted for Acute Myeloid Leukemia Treated on the RICAZA Trial. Biol Blood Marrow Transplant. 2016;22:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |