Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1712
Peer-review started: December 14, 2022
First decision: January 3, 2023
Revised: January 14, 2023
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 16, 2023
Processing time: 82 Days and 16.6 Hours
Postoperative complications of phacoemulsification, such as corneal edema caused by human corneal endothelial cell (CEC) injury, are still a matter of concern. Although several factors are known to cause CEC damage, the influence of ultrasound on the formation of free radicals during surgery should be considered. Ultrasound in aqueous humor induces cavitation and promotes the formation of hydroxyl radicals or reactive oxygen species (ROS). ROS-induced apoptosis and autophagy in phacoemulsification have been suggested to significantly promote CEC injury. CEC cannot regenerate after injury, and measures must be taken to prevent the loss of CEC after phacoemulsification or other CEC injuries. Antioxidants can reduce the oxidative stress injury of CEC during phacoemulsification. Evidence from rabbit eye studies shows that ascorbic acid infusion during operation or local application of ascorbic acid during phacoemulsification has a protective effect by scavenging free radicals or reducing oxidative stress. Both in experiments and clinical practice, hydrogen dissolved in the irrigating solution can also prevent CEC damage during phacoemulsification surgery. Astaxanthin (AST) can inhibit oxidative damage, thereby protecting different cells from most pathological conditions, such as myocardial cells, luteinized granulosa cells of the ovary, umbilical vascular endothelial cells, and human retina pigment epithelium cell line (ARPE-19). However, existing research has not focused on the application of AST to prevent oxidative stress during phacoemulsification, and the related mechanisms need to be studied. The Rho related helical coil kinase inhibitor Y-27632 can inhibit CEC apoptosis after phacoemulsification. Rigorous experiments are required to confirm whether its effect is realized through improving the ROS clearance ability of CEC.
Core Tip: Ultrasound in aqueous solution induces cavitation and promotes the formation of hydroxyl radicals or reactive oxygen species (ROS). ROS-induced apoptosis and autophagy in phacoemulsification have been suggested to significantly promote corneal endothelial cell (CEC) injury. Antioxidants can reduce the oxidative stress-induced injury to CECs during phacoemulsification.
- Citation: Chen K, Xu WY, Sun SS, Zhou HW. Corneal endothelial cells and acoustic cavitation in phacoemulsification. World J Clin Cases 2023; 11(8): 1712-1718
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1712.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1712
Phacoemulsification has recently become an increasingly popular surgical procedure for cataracts, with improvements in devices and surgical techniques. However, postoperative complications are still a problem worthy of concern, such as infective endophthalmitis, retinal detachment, and corneal edema due to damage to human corneal endothelial cells (CECs). Although several factors are known to cause CEC damage, the effects of free radical formation by ultrasound waves during the procedure should be considered. Ultrasound in aqueous humor induces acoustic cavitation[1] and promotes the formation of reactive oxygen species (ROS)[2]. ROS-induced corneal endothelial damage has been demonstrated to significantly promote apoptosis and autophagy during phacoemulsification[3]. CECs cannot regenerate after injuries[4], and strategies must be taken to prevent CEC loss after phacoemulsification or other endothelial injuries. This paper discusses corneal endothelial injury caused by oxidative stress secondary to acoustic cavitation during phacoemulsification and also related protective measures and implications for related fields.
Phacoemulsification surgery is the most commonly used surgery to treat cataracts. The complications of phacoemulsification, which affect the prognosis of visual acuity, are still a matter of concern. These complications include macular cystoid edema, infectious endophthalmitis, retinal detachment, and corneal edema due to loss of CEC. Corneal edema caused by decompensation of CEC is one of the most serious complications of phacoemulsification and can lead to severe visual loss and pain. CECs play a crucial role in regulating the constant dehydration of the corneal stroma and transparency[5]. CECs play a role mainly through active fluid pump and barrier function[6]. Both clinical and experimental studies have shown that the proliferation ability of human CECs is limited[7]. CEC density (ECD) usually starts from 4000 cells/mm2 at birth and gradually decreases with age. The average value for adults is approximately 2500 cells/mm2, while the average value for elderly individuals is less than 2000 cells/mm2. Corneas with an ECD less than 1000 cells/mm2 may not be able to tolerate intraocular surgery. When ECD is less than 500 cells/mm2, corneal edema and compensatory imbalance usually occur[8]. In previous studies, uneventful cataract surgery has been proven to induce CEC loss ranging from 12% to 20%[9,10]. At present, the only effective option to treat corneal endothelial dysfunction is corneal transplantation (e.g., full thickness penetrating keratoplasty or lamellar endothelial keratoplasty)[11]. In phacoemulsification, CEC damage is caused by ultrasonic mechanical damage, such as turbulence of the anterior chamber fluid and mechanical collision of bubbles and crystal fragments[12]. The application of viscoelastic materials in ophthalmology can limit the CEC damage caused by this aspect. Studies have confirmed that endogenous damage caused by oxidative stress plays a major role in CEC damage caused by phacoemulsification[3,13]. Therefore, how to maximize the prevention of CEC loss and injury during and after phacoemulsification and how to find the best effective safety strategy to protect CECs is a research priority.
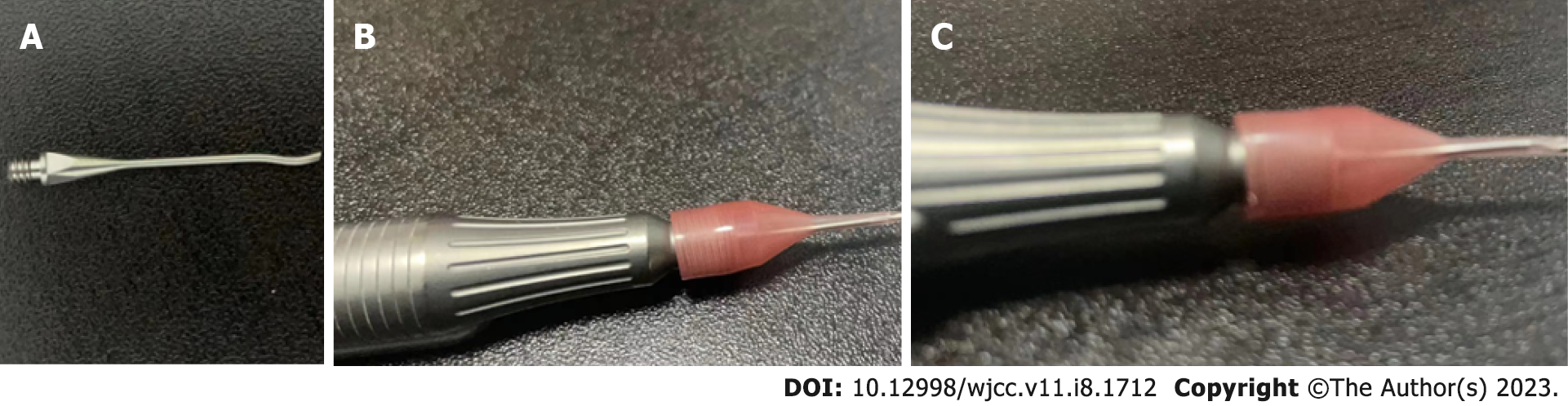
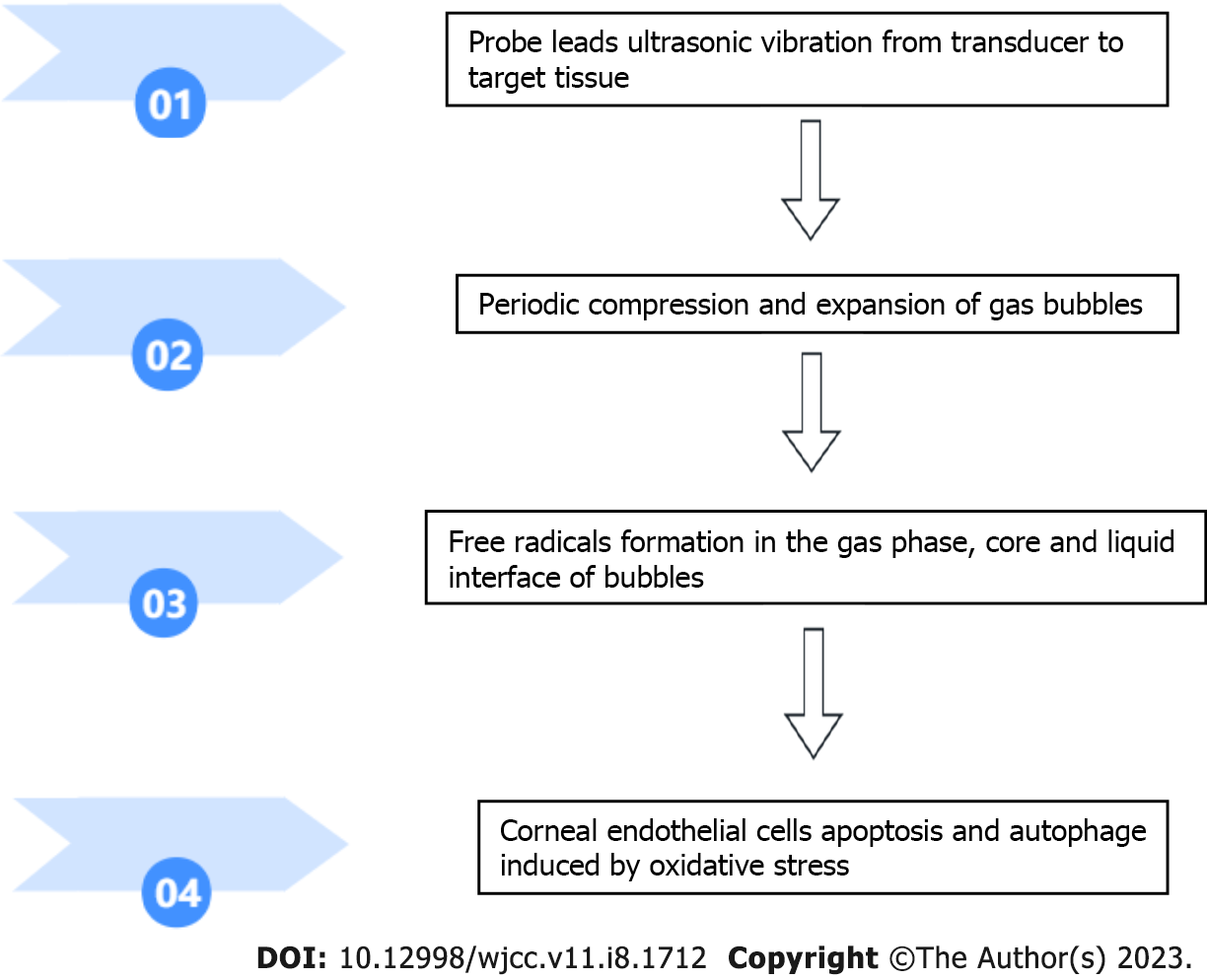
Ultrasonic energy is used in various surgical operations and is generated by the conversion of electrical energy by piezoelectric crystals located in the casing head. The probe is a hollow or solid metal conduit that directs ultrasonic vibration energy from the transducer to the target tissue (Figure 1). The low-frequency and high-energy ultrasonic radiation from the transducer can lead to the periodic compression and expansion of bubbles suspended in the liquid medium. Acoustic cavitation (that is, bubble expansion and rapid adiabatic collapse in solution) can generate enough energy to generate free radicals, sonoluminescence, high pressure and temperature rise in the bubble gas phase, bubble core and liquid interface[14]. In a water medium, water molecules decompose to form H+ and OH- radicals as the main primary radicals. The generation of acoustic cavitation in a water medium is a random, nonlinear, complex and multifactor-related phenomenon.
Bond[14] proposed a mechanical model to describe the interaction between phacoemulsification or other ultrasonic operations and various tissues. In the process of phacoemulsification, the direct mechanical vibration effect between the probe and the tissue will produce a large amount of heat accumulation, so continuous synchronous irrigation can be used to reduce the heat effect. The application of irrigating fluid in ophthalmic surgery can reduce friction and thermal effects; however, the gas nuclei in these aqueous solutions may produce acoustic cavitation. Acoustic cavitation may interact with tissues through chemical, electromagnetic radiation, thermal and mechanical effects and reportedly cause cell and intracellular damage. The combined effects of acoustic cavitation on the survival of damaged cells (that is, the generation of radiation, ultraviolet radiation, high temperature and high pressure leading to acoustic perforation) may lead to ultrasonic energy damage and may explain immediate cell damage, apoptosis and death[15,16].
In phacoemulsification, because monolayer endothelial cells cannot regenerate, the damage and destruction of CECs should be minimized or eliminated. Aqueous humor contains natural antioxidants. In phacoemulsification, the irrigating solution circulates through the anterior chamber of the eye at a rate of approximately 20-30 mL/min to prevent heat accumulation. This effectively irrigates all aqueous humor within a few seconds, thus removing all natural antioxidants that usually protect the anterior chamber from oxidative damage. Therefore, ultrasonic emulsification in the irrigating solution environment can produce acoustic cavitation, but it depletes the natural antioxidants of aqueous humor.
The main expected effect of phacoemulsification is to chop, emulsify and empty the cataractous lens through the small clear corneal incision. To reduce heat accumulation and help lens fragmentation and emulsification, as well as the removal of debris, synchronous irrigation must be carried out continuously. However, for the generation of free radicals and sonoluminescence, cavitation may be considered an undesirable byproduct of phacoemulsification. Free radicals have been shown to cause toxic ocular oxidative damage, such as in the retina, lens and cornea[17]. The cytotoxicity of ROS to photosensitizer-treated and light-treated bovine CEC has been proven to be mediated by a parallel pathway leading to apoptosis and necrosis[18].
Studies have confirmed that endogenous damage caused by oxidative stress plays an important role in CEC damage caused by phacoemulsification[3,16]. Therefore, some antioxidants can reduce the damage to CECs in phacoemulsification.
Holst et al[18] determined the formation of free radicals by adding luminol to the buffer solution and measuring the chemiluminescence in vitro and in rabbit eyes during phacoemulsification. They found that free radicals were formed during the process of phacoemulsification, and the number of free radicals was related to the power of ultrasound. In addition, the free radical scavenger superoxide dismutase (SOD) can inhibit the formation of free radicals, indicating that adding SOD to the irrigating solution during phacoemulsification can reduce the damage to CECs. Rubowitz et al[19] performed long-term phacoemulsification in the anterior chamber on 17 rabbit eyes. In 9 eyes, balanced salt eye solution was used in the phacoemulsification, and in 8 eyes, 0.001 M ascorbic acid was added to the solution. All other parameters were the same between the two groups. They found that there was no significant difference between the two groups in the number of CECs before the operation, but the ECD of the ascorbic acid treated group was higher compared to the other group after the operation (P = 0.011). They believed that adding ascorbic acid in the irrigating solution could significantly reduce the loss of CEC during phacoemulsification by approximately 70% and that it was due to the free radical scavenging properties of ascorbic acid. M Padua et al[12] showed that during phacoemulsification surgery in dogs, the application of ascorbic acid at a final concentration of 0.001 M in the irrigating solution significantly reduced the loss of CEC, the loss of hexagonal cells, and the coefficient of variation of CEC. Lee et al[20] reported the clinical application of ascorbic acid to prevent CEC damage related to phacoemulsification surgery in 2 patients during the perioperative period. In addition to cataracts, 2 patients suffered from Fuchs corneal endothelial dystrophy and corneal endotheliitis. Nakamura et al[21] emphasized the protective effect of the antioxidant glutathione on CEC caused by ROS and found that oxidized glutathione has a better effect. Both oxidized glutathione and reduced glutathione can protect CECs.
The most relevant studies on acoustic cavitation and its effects on CECs are summarized in Table 1. A figure describing acoustic cavitation and its effects on CECs is included (Figure 2).
| Ref. | Journal | Key points |
| Hsueh et al[3] | Cells | ROS-induced CECs damage has been demonstrated to significantly promote apoptosis and autophagy during phacoemulsification |
| M Padua et al[12] | Vet Ophthalmol | Oxidative stress plays a major role in CECs damage and ascorbic acid significantly protected CECs during phacoemulsification |
| Ashush et al[13] | Cancer Res | Acoustic cavitation can generate free radicals, sonoluminescence, high pressure and temperature rise |
| Holst et al[18] | Curr Eye Res | SOD can inhibit the formation of free radicals during phacoemulsification |
| Rubowitz et al[19] | Invest Ophthalmol Vis Sci | The number of postoperative CECs in the study group treated with ascorbic acid was significantly more |
| Lee et al[20] | World J Clin Cases | Clinical application of ascorbic acid can prevent CECs damage related to phacoemulsification |
Endogenous injury of CECs induced by oxidative stress is the main cause of CEC injury induced by phacoemulsification. Reducing the damage to CECs during cataract surgery has been a hot topic in the field of ophthalmology.
Astaxanthin (AST) is an orange red carotenoid pigment that is the strongest antioxidant in nature. It has a variety of biological activities, including anticancer, anti-inflammatory, antiaging, antidiabetic, immune regulation and neuroprotective activities. However, it is unclear whether AST can protect CECs from endogenous damage caused by oxidative stress during phacoemulsification.
AST does not exist in human eyes and aqueous humor. At present, there are some studies on AST in ophthalmology. Otsuka et al[22] found that AST can prevent retinal ischemic damage through its antioxidant effect in a retinal ischemia‒reperfusion model. Dong et al[23] found that in the diabetes db/db mouse model, AST can reduce the apoptosis of retinal ganglion cells, reduce the oxidative stress pressure of retinal tissue in db/db mice, reduce superoxide anion, reduce malondialdehyde, reduce 8-hydroxy-2-deoxyguanosine (8-OHdG) and increase manganese superoxide dismutase (Mn SOD) activity. Hashimoto et al[24] conducted a clinical experiment to evaluate the antioxidant effect of AST through changes in superoxide scavenging activity, hydrogen peroxide level and total organic peroxide in human aqueous humor. The subjects were 35 patients who underwent bilateral cataract surgery before and after taking AST (6 mg/day for 2 wk), and aqueous humor was taken during the surgery. After AST intake, the superoxide scavenging activity increased significantly, and the total organic peroxide level decreased significantly. The superoxide scavenging activity was significantly, negatively correlated with the total organic peroxide level (R = -0.485, P < 0.01), indicating that AST intake obviously enhanced the superoxide scavenging activity of human aqueous humor and inhibited the production of total organic peroxide in aqueous humor.
Many scholars have conducted in-depth research on the mechanism of AST in various cells. In a recent study, an ultraviolet (UV) B-induced oxidative stress model was used to evaluate the antioxidant effect of AST on a human retina pigment epithelium cell line (ARPE-19). The results showed that 20 μM and 40 μM AST can increase cell viability and have synergistic effects with ascorbic acid[25,26]. Increasing evidence shows that ROS production stimulates Phosphatidylinositol-3-kinase-serine/threonine kinase Akt (PI3K-Akt) mediated autophagy, while AST improves cell survival under oxidative inflammation conditions by activating the Akt signaling pathway[27]. Li Z and colleagues have proven that AST plays a protective role in H2O2-induced oxidative damage by activating the PI3K-Akt pathway, and then PI3K-Akt further activates several downstream signal transduction media, such as mammalian target of rapamycin (mTOR) and the Nuclear factor erythroid 2-related factor 2-antioxidant response elements (Nrf2-ARE) pathway[28].
However, no previous studies have focused on the role of AST in the oxidative stress injury of CEC during phacoemulsification and its related mechanisms. In fact, in addition to phacoemulsification, there are many risk factors for the increase in CEC, such as solar ultraviolet radiation, aging and malnutrition. Therefore, local application of AST may contribute to the protection of patients with fragile CEC.
Previous papers have suggested that Rho related helical coil kinase (ROCK) plays important roles in cell cycle control because ROCK inhibits the premature separation of two centrioles in G1 period and is indispensable for the contraction of the cleavage groove (a necessary step for the completion of cytokinesis)[29]. The ROCK inhibitor Y-27632 has been proven to increase proliferation and even immortalize primary keratinocytes in the presence of feeder cells[30]. Achiron et al[30] divided the corneal ring of a human donor into fragments, stored them in commercial storage medium with or without 10 mmol/L ROCK inhibitor, and then exposed them to phacoemulsification. The sample was separated into single cells by trypsin digestion. CEC was labeled with anti-CD166 antibodies to evaluate the early and late apoptosis rate of survival of CEC by flow cytometry analysis of annexin V and propidium iodide (PI) double staining. Six corneal and scleral rings from 4 donors were studied. After phacoemulsification, compared with the control group, the CEC exposed to Y-27632 showed that the early apoptosis rate decreased by 37.06%, and the late apoptosis rate decreased by 45.27%. The authors believe that ROCK inhibitors can be used before cataract surgery, especially in high-risk patients. This may be a promising new method to prevent pseudophakic bullous keratopathy. However, the author has not clarified the mechanism behind the effect. Zhou et al[31] found that the primary limbal epithelial cells of rabbits treated with Y-27632 also showed improved colony formation efficiency by enhancing the expansion of stem/progenitor cells. They proved that Y-27632 improved the cloning efficiency of rabbit limbal stem/progenitor cells by improving their adhesion and ROS clearance ability. Therefore, rigorous experiments are required to confirm whether the effect of Y-27632 on inhibiting the apoptosis of CECs after phacoemulsification is realized through the mechanism of improving the ROS clearance ability of CECs.
Ultrasound in aqueous humor can induce cavitation and promote the formation of ROS. ROS-induced apoptosis and autophagy have been suggested to significantly promote CEC injury during phacoemulsification. AST can inhibit oxidative damage, thus protecting different cells from most pathological conditions. However, existing research has not focused on the application of AST to prevent oxidative stress during phacoemulsification. Y-27632 inhibited CEC apoptosis after phacoemulsification. Further experiments are required to confirm whether the effect is realized by improving the ROS clearance ability of CECs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cheraqpour K, Iran; Dragonieri S, Italy S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Topaz M, Motiei M, Assia E, Meyerstein D, Meyerstein N, Gedanken A. Acoustic cavitation in phacoemulsification: chemical effects, modes of action and cavitation index. Ultrasound Med Biol. 2002;28:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Augustin AJ, Dick HB. Oxidative tissue damage after phacoemulsification: influence of ophthalmic viscosurgical devices. J Cataract Refract Surg. 2004;30:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Hsueh YJ, Meir YJ, Yeh LK, Wang TK, Huang CC, Lu TT, Cheng CM, Wu WC, Chen HC. Topical Ascorbic Acid Ameliorates Oxidative Stress-Induced Corneal Endothelial Damage via Suppression of Apoptosis and Autophagic Flux Blockage. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Català P, Thuret G, Skottman H, Mehta JS, Parekh M, Ní Dhubhghaill S, Collin RWJ, Nuijts RMMA, Ferrari S, LaPointe VLS, Dickman MM. Approaches for corneal endothelium regenerative medicine. Prog Retin Eye Res. 2022;87:100987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Sahu PK, Das GK, Agrawal S, Kumar S. Comparative Evaluation of Corneal Endothelium in Patients with Diabetes Undergoing Phacoemulsification. Middle East Afr J Ophthalmol. 2017;24:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Hsueh YJ, Chen HC, Wu SE, Wang TK, Chen JK, Ma DH. Lysophosphatidic acid induces YAP-promoted proliferation of human corneal endothelial cells via PI3K and ROCK pathways. Mol Ther Methods Clin Dev. 2015;2:15014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Rouhbakhshzaeri M, Rabiee B, Azar N, Ghahari E, Putra I, Eslani M, Djalilian AR. New ex vivo model of corneal endothelial phacoemulsification injury and rescue therapy with mesenchymal stromal cell secretome. J Cataract Refract Surg. 2019;45:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 8. | Choi JY, Han YK. Long-term (≥10 years) results of corneal endothelial cell loss after cataract surgery. Can J Ophthalmol. 2019;54:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Al-Mohtaseb Z, He X, Yesilirmak N, Waren D, Donaldson KE. Comparison of Corneal Endothelial Cell Loss Between Two Femtosecond Laser Platforms and Standard Phacoemulsification. J Refract Surg. 2017;33:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Shen L, Sun P, Zhang C, Yang L, Du L, Wu X. Therapy of corneal endothelial dysfunction with corneal endothelial cell-like cells derived from skin-derived precursors. Sci Rep. 2017;7:13400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Wilkinson SW, Park SSE, Ungricht EL, Trapnell M, Nydegger J, Cardenas IA, Brintz BJ, Mamalis N, Olson RJ, Werner L. Effect of simulated lenticular debris on corneal endothelial cells: experimental study in rabbit eyes. J Cataract Refract Surg. 2022;48:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 12. | M Padua IR, P Valdetaro G, B Lima T, K Kobashigawa K, E S Silva P, Aldrovani M, M Padua PP, Laus JL. Effects of intracameral ascorbic acid on the corneal endothelium of dogs undergoing phacoemulsification. Vet Ophthalmol. 2018;21:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Ashush H, Rozenszajn LA, Blass M, Barda-Saad M, Azimov D, Radnay J, Zipori D, Rosenschein U. Apoptosis induction of human myeloid leukemic cells by ultrasound exposure. Cancer Res. 2000;60:1014-1020. [PubMed] |
| 14. | Bond LJ, Cimino WW. Physics of ultrasonic surgery using tissue fragmentation. Ultrasonics. 1996;34:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Murano N, Ishizaki M, Sato S, Fukuda Y, Takahashi H. Corneal endothelial cell damage by free radicals associated with ultrasound oscillation. Arch Ophthalmol. 2008;126:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Giblin FJ, McCready JP. The effect of inhibition of glutathione reductase on the detoxification of H2O2 by rabbit lens. Invest Ophthalmol Vis Sci. 1983;24:113-118. [PubMed] |
| 17. | Cho KS, Lee EH, Choi JS, Joo CK. Reactive oxygen species-induced apoptosis and necrosis in bovine corneal endothelial cells. Invest Ophthalmol Vis Sci. 1999;40:911-919. [PubMed] |
| 18. | Holst A, Rolfsen W, Svensson B, Ollinger K, Lundgren B. Formation of free radicals during phacoemulsification. Curr Eye Res. 1993;12:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Rubowitz A, Assia EI, Rosner M, Topaz M. Antioxidant protection against corneal damage by free radicals during phacoemulsification. Invest Ophthalmol Vis Sci. 2003;44:1866-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Lee CY, Chen HT, Hsueh YJ, Chen HC, Huang CC, Meir YJ, Cheng CM, Wu WC. Perioperative topical ascorbic acid for the prevention of phacoemulsification-related corneal endothelial damage: Two case reports and review of literature. World J Clin Cases. 2019;7:642-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Nakamura M, Nakano T, Hikida M. Effects of oxidized glutathione and reduced glutathione on the barrier function of the corneal endothelium. Cornea. 1994;13:493-495. [PubMed] |
| 22. | Otsuka T, Shimazawa M, Inoue Y, Nakano Y, Ojino K, Izawa H, Tsuruma K, Ishibashi T, Hara H. Astaxanthin Protects Against Retinal Damage: Evidence from In Vivo and In Vitro Retinal Ischemia and Reperfusion Models. Curr Eye Res. 2016;41:1465-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Dong LY, Jin J, Lu G, Kang XL. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar Drugs. 2013;11:960-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Hashimoto H, Arai K, Hayashi S, Okamoto H, Takahashi J, Chikuda M, Obara Y. Effects of astaxanthin on antioxidation in human aqueous humor. J Clin Biochem Nutr. 2013;53:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Oh S, Kim YJ, Lee EK, Park SW, Yu HG. Antioxidative Effects of Ascorbic Acid and Astaxanthin on ARPE-19 Cells in an Oxidative Stress Model. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Zhang J, Ding C, Zhang S, Xu Y. Neuroprotective effects of astaxanthin against oxygen and glucose deprivation damage via the PI3K/Akt/GSK3β/Nrf2 signalling pathway in vitro. J Cell Mol Med. 2020;24:8977-8985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y, Hou D, Zhang X. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis. 2013;19:1656-1666. [PubMed] |
| 28. | Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1515] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 29. | Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 601] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 30. | Achiron A, Feldman A, Karmona L, Avizemer H, Barequet IS, Rosner M, Knyazer B, Bartov E, Burgansky Z, Vishnevskia-Dai V. Prophylactic exposure of human corneal endothelial cells to Rho-associated kinase inhibitor reduced apoptosis rate after phacoemulsification: Ex vivo study. J Cataract Refract Surg. 2018;44:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Zhou Q, Duan H, Wang Y, Qu M, Yang L, Xie L. ROCK inhibitor Y-27632 increases the cloning efficiency of limbal stem/progenitor cells by improving their adherence and ROS-scavenging capacity. Tissue Eng Part C Methods. 2013;19:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |