Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.645
Peer-review started: September 26, 2022
First decision: November 11, 2022
Revised: November 18, 2022
Accepted: January 3, 2023
Article in press: January 3, 2023
Published online: January 26, 2023
Processing time: 122 Days and 13.8 Hours
Seminal vesicle abscess (SVA) is the manifestation of a relatively rare urinary system infection. In response to urinary system inflammation, an abscess forms in special locations. However, acute diffuse peritonitis (ADP) induced by SVA is unusual.
We report a case of a left SVA in a male patient complicated with pelvic abscess, ADP, multiple organ dysfunction syndrome, infectious shock, bacteremia, and acute appendiceal extraserous suppurative inflammation as a result of a long-term indwelling urinary catheter. The patient received a course of morinidazole + cefminol antibiotics but showed no obvious relief, so the perineal SVA underwent puncture drainage and abdominal abscess drainage + appendectomy was performed. The operations were successful. After the operation, anti-infection, anti-shock, and nutritional support treatments were continued and various laboratory indicators were regularly reviewed. The patient was discharged from the hospital after recovery. This disease is a challenge for the clinician because of the unusual spreading path of the abscess. Moreover, appropriate intervention and adequate drainage of abdominal and pelvic lesions are necessary, especially when the primary focus cannot be determined.
The etiology of ADP varies, but acute peritonitis secondary to SVA is very rare. In this patient, the left SVA not only affected the adjacent prostate and bladder but also spread retrogradely through the vas deferens, forming a pelvic abscess in the loose tissues of the extraperitoneal fascia layer. Inflammation involving the peritoneal layer led to ascites and pus accumulation in the abdominal cavity, and appendix involvement led to extraserous suppurative inflammation. In clinical practice, surgeons need to consider the results of various laboratory tests and imaging examinations to make comprehensive judgments involving the diagnosis and treatment plan.
Core Tip: Seminal vesicle abscess (SVA) is a relatively rare urinary system infection, and acute diffuse peritonitis (ADP) induced by SVA is unusual. We report a male patient who had a left SVA induced by a long-term indwelling urinary catheter, and this condition was complicated with pelvic abscess, ADP, multiple organ dysfunction syndrome, infectious shock, bacteremia, and acute appendiceal extraserous suppurative inflammation. With no obvious relief by conservative treatment, puncture drainage of the perineal SVA and abdominal abscess drainage + appendectomy was performed. In this case, the left SVA not only affected the adjacent prostate and bladder but also spread retrogradely through the vas deferens, forming a pelvic abscess in the loose tissues of the extraperitoneal fascia layer. Inflammation involving the peritoneal layer led to ascites and pus accumulation in the abdominal cavity, and appendix involvement led to extraserous suppurative inflammation.
- Citation: Li K, Liu NB, Liu JX, Chen QN, Shi BM. Acute diffuse peritonitis secondary to a seminal vesicle abscess: A case report. World J Clin Cases 2023; 11(3): 645-654
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/645.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.645
Acute diffuse peritonitis (ADP) is a common acute abdominal complication of general surgery. It has a high risk of mortality due to its rapid onset, rapid progression, and poor prognosis. The causes of ADP vary, but most cases are due to secondary infections. However, emergency surgery is needed regardless of the cause[1]. The main function of the seminal vesicles is to secrete nutrients and dilute the fluid in which the sperm resides. An infection of the seminal vesicle most commonly causes inflammation. Seminal vesicle abscess (SVA) is relatively rare, and diseases that develop from the “SVA–pelvic abscess–ADP” pathogenesis circuit, where the associated bacteria originate from the seminal vesicles and SVA, are even rarer. In 1978, Rajfer et al[2] reported the first case of SVA. According to Rajfer et al[2], less than 100 cases of SVA have been reported worldwide. Here, we report a male patient who developed a SVA due to a long-term indwelling catheter, analyze the basic condition and outcome, and provide the clinical diagnosis and treatment ideas.
An 86-year-old man was admitted to our hospital for lower abdominal distension and pain that lasted for 5 d. The patient had a sudden high fever of 39 °C.
Five days prior to hospital admission, the patient suffered from intermittent abdominal distension and pain. He did not visit his doctor, the symptoms of lower abdominal pain gradually worsened the night prior, and the symptoms were accompanied by frequent nausea, vomiting, and loose watery stools. After symptomatic treatment in a local hospital, there was no obvious remission, so he came to our hospital for emergency treatment. He had no jaundice or anorexia symptoms.
The patient underwent surgical treatment for benign prostatic hyperplasia 2 years prior and took finasteride + tamsulosin hydrochloride intermittently after the operation. Last year, he had difficulty urinating. Ultrasound examination showed that there was an increase in the residual urine volume and urinary retention. He had an indwelling catheter. The catheter was regularly removed and replaced in the outpatient clinic, which led to dysuria recurrence. The patient also experienced hypertension for many years, which was partly controlled with medications.
No relevant family history, travel history, or animal contact was reported.
The patient’s initial vital signs at admission were as follows: Blood pressure 146/79 mmHg, pulse 62 bpm, respiratory rate 18 breaths/min, and body temperature 39 °C. He had an altered mental state, slight abdominal distension, slightly weak bowel sounds (3/min), obvious tenderness and rebound pain in the lower abdomen, slightly tense abdominal muscles, and percussive tympany abdominal sounds. He had an indwelling urinary catheter, drainage was unobstructed, urine was dark yellow, and flocculent was visible. Scrotal symmetry, bilateral testis, and epididymis tenderness were observed.
The in-hospital laboratory examination showed the following: White blood cell (WBC) count: 12.61 × 109/L (79.1% neutrophils and 11.7% lymphocytes); C-reactive protein: 82.6 mg/L. The routine urine examination showed red blood cells 85/UL and WBCs 291/UL, indicating hematuria and pyuria. However, the patient's liver and kidney function, electrolytes, myocardial enzymes, and coagulation at the first day of admission were normal.
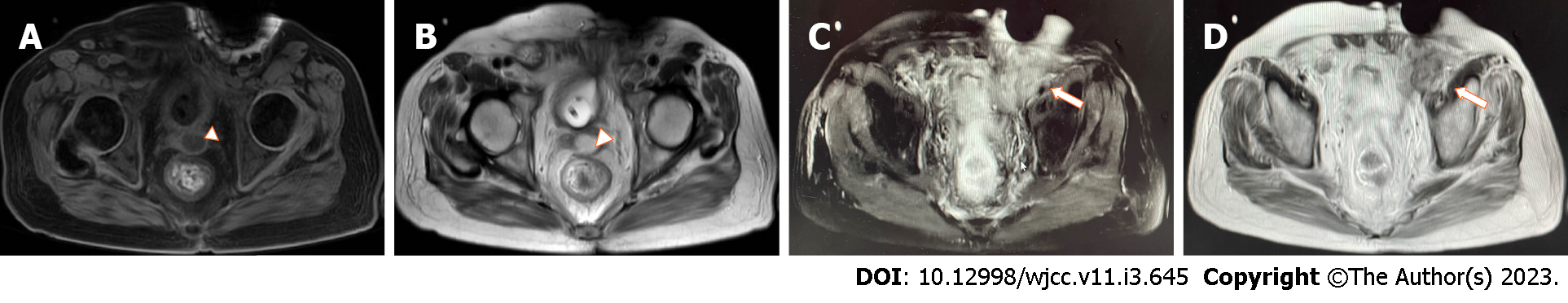
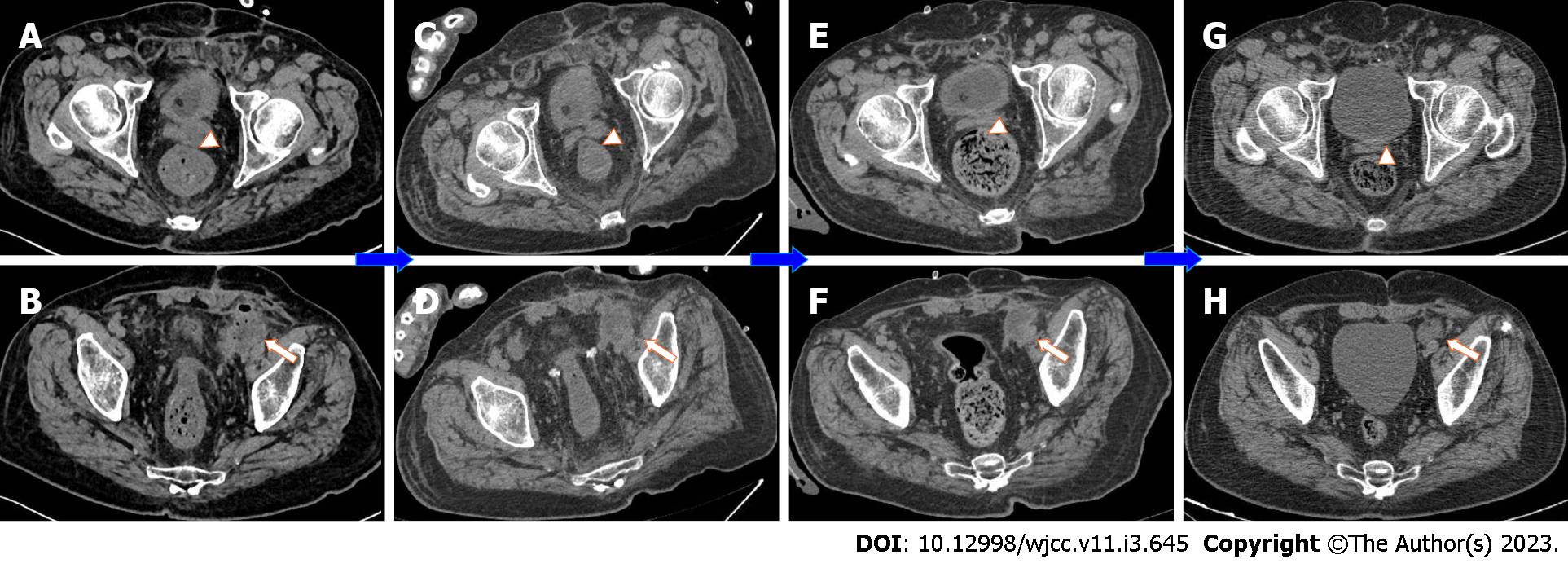
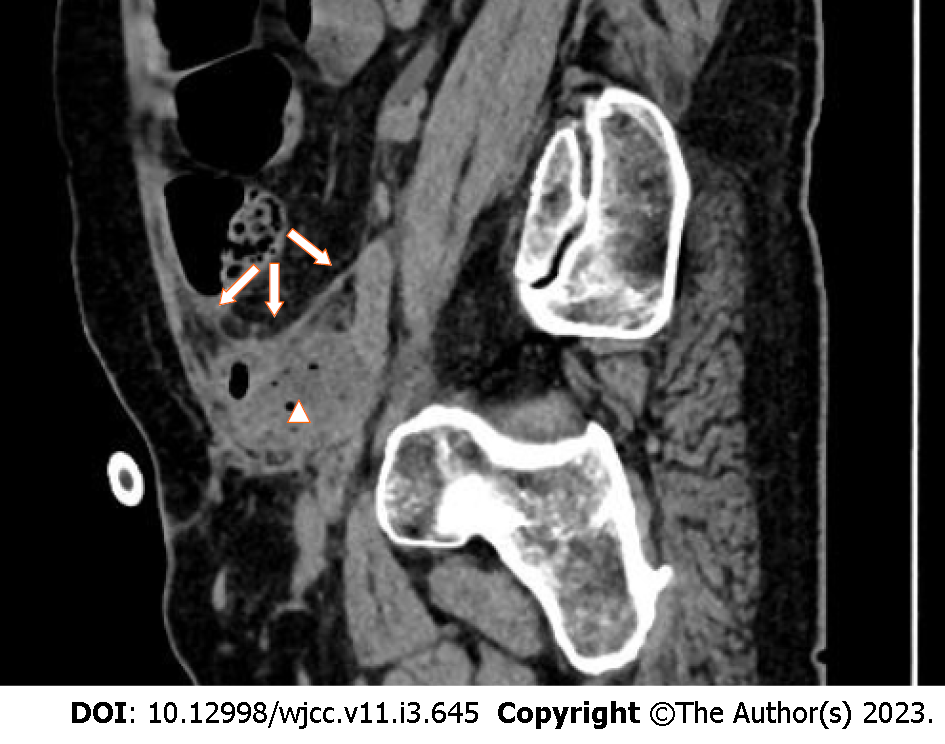
Pelvic magnetic resonance imaging (MRI) was performed 2 d after admission, which showed a lumpy and patchy left lower abdomen, with a high possibility of inflammation, a thickened sigmoid wall, blurred fatty space surrounding the pelvic cavity, and a possible left SVA (Figure 1). Combined pelvic and abdominal computed tomography (CT) showed encapsulated effusion and gas accumulation in the left lower abdomen with peripheral exudative changes, suggesting the possibility of an abscess. The left seminal vesicle was unclear with corresponding cystic foci, suggesting the possibility of an abscess (Figure 2A and B). Chest CT revealed scattered chronic inflammation in both lungs.
The discharge diagnoses were: (1) Septic shock; (2) ADP and acute extraserous suppurative appendicitis; (3) Left SVA; (4) Pelvic abscess; and (5) Hypertension grade 3 (high risk).
After admission, the patient's condition gradually worsened. Morlinidazole + cefminol was administered as anti-infection agents, and fluid resuscitation, abrosia, nutritional support, and other treatments were administered. Four days after admission, puncture drainage of the perineal SVA was performed, and a small amount of purulent fluid (bacterial culture: Klebsiella pneumoniae) was drained. Infection with Gram-positive cocci and Candida parapsilosis was detected in urine smears. However, multiple cultures were negative, so the possibility of sample contamination was considered. Abdominal pain was not obviously relieved postoperatively, and on the 5th day, blood pressure decreased and septic shock symptoms, such as weak consciousness, liver and kidney insufficiency, and abnormal coagulation function, were observed (Table 1). His vital signs at that time were as follows: Blood pressure 76/40 mmHg, pulse 118 bpm, respiratory rate 22 breaths/min, and body temperature 39 °C. The Acute Physiology and Chronic Health Evaluation II score was 15, and the Sequential Organ Failure Assessment score was 7. Considering that the patient's vital signs were not stable and after communicating with his family, it was decided to perform emergency exploratory laparotomy for active fluid replacement, volume expansion, and vital sign monitoring to identify the focus of infection and to fully drain the abscess.
| Date | Leukocytes (× 109/L) | Creatinine (μmol/L) | Albumin (g/L) | Prothrombin time (s) | C-reactive protein (mg/L) |
| DBO5 | 13 | 92 | 34.3 | 13.1 | 151.77 |
| DBO4 | 11.62 | 81 | 31.5 | 15.7 | 157.74 |
| DBO2 | 22.41 | 157 | 30.1 | 17.9 | 227.71 |
| DBO1 | 34.67 | 153 | 18.2 | 18.6 | 239.87 |
| OD | 23.85 | 173 | 25.0 | 17.7 | 177.34 |
| POD1 | 20.35 | 158 | 28.4 | 16.6 | 204.14 |
| POD2 | 14.96 | 128 | 34.2 | 16.0 | 177.26 |
| POD3 | 11.29 | 109 | 34.4 | 15.5 | 141.1 |
| POD4 | 11.41 | 104 | 35.6 | 14.1 | 99.55 |
| POD6 | 16.98 | 88 | 35.3 | 14.2 | 57.6 |
| POD8 | 15.39 | 85 | 33.5 | 15.0 | 37.0 |
| POD10 | 13.07 | 91 | 33.5 | 14.4 | 99.3 |
| POD12 | 7.3 | 87 | 30.2 | 14.3 | 118.73 |
| POD15 | 6.78 | 78 | 30.6 | 14.1 | 66.02 |
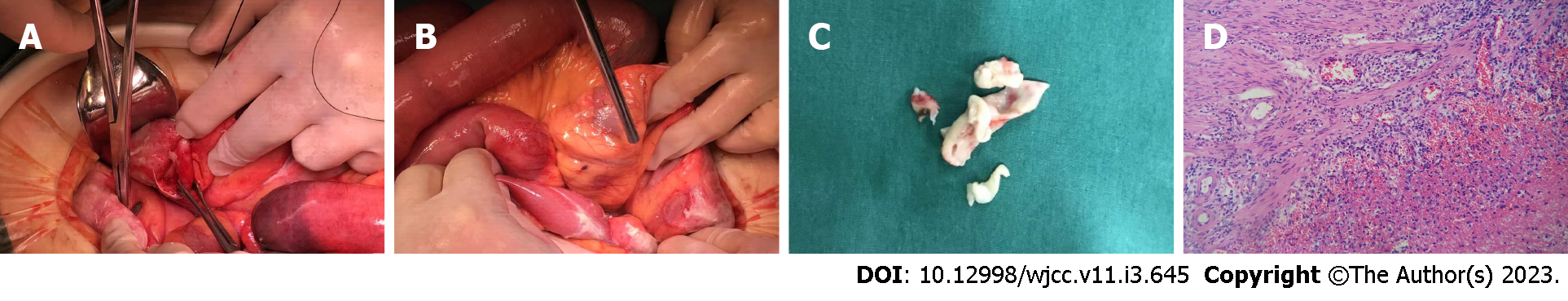
After preoperative preparation and general anesthesia, the patient underwent surgery in the supine position, and the surgery was performed by the attending physician, who had approximately 25 years of specialized training. During the operation, the intraperitoneal suppurative fluid volume was approximately 100 mL and was mainly found in the intestines and around the appendix. The head and body of the appendix were suppurated, and a large amount of pus was around the surface of the surrounding intestinal canal and sigmoid colon, without obvious intestinal perforation or tumor formation. Thus, abdominal abscess drainage + appendectomy was performed (Figure 3A-C).
After the operation, the patient was transferred to the intensive care unit due to his critical condition. He fasted and underwent electrocardiogram monitoring and continued anti-infection, anti-shock, and nutritional support treatments. The internal environment and water electrolyte balance was maintained. The function of various organs was monitored. Plasma was infused to achieve coagulation, and wound dressings were changed regularly. Various laboratory indicators were routinely checked. On the 1st day after the operation, the patient had dyspnea and atrial fibrillation with a fast ventricular rate after taking off the line. He received high flow oxygen inhalation and his heart rate was regulated with amiodarone. Treatment to resolve abdominal distension and promote gastrointestinal motility was performed. The pus culture showed Klebsiella pneumoniae infection. A combination of meropenem and linezolid was administered, and the patient's symptoms and signs were gradually resolved. On the 3rd day after the operation, the patient's heart rate returned to normal, oxygen saturation was maintained with low-flow oxygen inhalation, the infection was eradicated, and abdominal distension was relieved. Later, the patient returned to general surgery ward for further treatment. On the 4th day after the operation, a liquid diet was initiated. The amount of intravenous fluid replacement was reduced and he was encouraged to get out of bed to prevent embolization. On the 7th day after the operation, abdominal distension recurred. He continued to fast, and the wound suture was removed. On the 10th day after the operation, the patient complained of hunger but abdominal distension was relieved. Abdominal CT showed less abdominal inflammation than before (Figure 2C and D). Three days later, the patient’s diet was changed to a semiliquid diet. Two days later, the patient did not complain of any special discomfort, his body temperature returned to normal, and he was allowed to resume a normal diet. The other catheter was replaced, and the patient was discharged.
Postoperative pathology of the appendix showed extraserous suppurative inflammation, AB (+), and PAS (+) (Figure 3D). After discharge, the patient underwent a 1-year follow-up. The two inflammatory foci were managed and their size was gradually reduced after treatment and follow-up (Figure 2E-H).
The seminal vesicles are located at the bottom of the bladder and the outer side of the ampulla of the ureter. Together with the ampulla of the vas deferens and the ejaculatory duct, they form the distal structure of the seminal tract[3]. Seminal vesicle secretions account for approximately 60% of the total semen volume and include high concentrations of fructose, prostaglandin, and other substances[4]. SVA is very rare. In 2004, Sağlam et al[5] pointed out that a total of 26 related cases were reported in the literature. By searching PubMed, ScienceDirect, and other literature databases, as of August 2022, a total of 51 cases of SVA were idenfied worldwide. Table 2 shows the SVA cases reported since 2004. SVA can occur in all age groups and is more common unilaterally, and Escherichia coli is the main pathogenic bacteria. The etiology of SVA is unknown, but long-term indwelling catheters, diabetes, prostate puncture biopsy, and endoscopic operation are factors contributing to the susceptibility to this disease[6,7]. SVAs are mostly secondary to urinary tract infections such as prostatitis. Infections causing a SVA can also involve the blood, lymph, and other pathways[8]. In our case, the patient underwent surgical treatment of benign prostatic hyperplasia. He suffered difficulty urinating and had a long-term indwelling urinary catheter. We speculate that his previous medical history is an important risk factor for SVA.
| Ref. | Year | No. | Age | Complaints | Imaging | Surgery | Pathogens | Location | Underlying causes |
| Dewani et al[15] | 2006 | 1 | 35 | Infertility, hemospermia | CUS, US | Abscess incision, catheterization | Acid-fast bacilli | Unilateral | N/A |
| Chong et al[16] | 2014 | 1 | 38 | Fever, lethargy, rigor, anorexia | N/A | Percutaneous drainage | Burkholderia pseudomallei | N/A | Melioidosis, diabetes |
| Bayne et al[11] | 2013 | 1 | 67 | Myalgias, chills | MRI, IVP | Percutaneous drainage | Escherichia coli | Left | Transrectal biopsy |
| Hammad[17] | 2006 | 1 | 24 | Urinary frequency, urgency, urge incontinence | CUS, CT, IVP | Perirectal incision and drainage | N/A | Left | N/A |
| Fujinaga et al[18] | 2008 | 1 | 2 ms | Fever with leukocyturia | CUS, CT | Percutaneous transrectal aspiration | Escherichia coli | Left | Dysplastic left kidney |
| Wadei et al[19] | 2008 | 1 | 29 | Fever, dysuria, right-sided testicular pain | CT, MRI | Transrectal aspiration | Staphylococcus, Proteus mirabilis, Clostridium species | Right | Donor kidney transplant |
| Talwar et al[20] | 2021 | 1 | 20 | Fever, oliguria and uremic symptoms | MRI | Transrectal aspiration | Escherichia coli | Left | Zinner syndrome |
| Monzó et al[21] | 2005 | 1 | 38 | Dysuria, frequent urination and fever | US | Transrectal puncture drainage | Escherichia coli | Left | Bilateral orchiopexy |
| Machida et al[22] | 2008 | 1 | 81 | Swelling and left inguinal pain | CT | Inguinal canal ligation and percutaneous suprapubic vesical catheterization | Escherichia coli | Left | Diabetes |
| Bradley and Scoular[23] | 2021 | 1 | 42 | Subjective fevers and worsening fatigue | CT | Transurethral resection of the prostate | Candida parapsilosis, Candida glabrata, Bacteroides fragilis | Left | Diabetes |
| Sağlam et al[5] | 2004 | 6 | 18-47 | Rectal discomfort, left inguinal tender-swelling | N/A | Transrectal (2)/Transperineal (4) puncture and aspiration | Escherichia coli | Bilateral (4)/Unilateral (2) | 2 with rectal cancer |
| Sihra et al[12] | 2018 | 1 | 29 | Right hemiscrotal pain, swelling, and pyrexia | US, CUS | Incision, drainage, transvesical deroofing of abscess | Staphylococcus aureus | Right | Elective vasectomy |
| Saha et al[7] | 2009 | 1 | 49 | Gradually enlarging, exquisitely tender groin mass | CT, MRI, CUS, IVP | Laparoscopic drainage of the abscess | Escherichia coli | Left | Drainage of perianal abscess |
| Imperatore et al[24] | 2017 | 1 | 58 | Dysuria and fever | CT | Open abdominal pus drainage | N/A | Bilateral | N/A |
| Zheng et al[25] | 2015 | 1 | 42 | Severe dysuria abdominal pain | US, MRI | Percutaneously drainage | Staphylococcus aureus and Candida albicans | Left | Diabetes |
| Cui et al[26] | 2014 | 3 | 31-58 | N/A | US, CT | F4 single "J" catheterization | N/A | N/A | N/A |
| Kuribayashi et al[27] | 2017 | 1 | 26 | Fever and right seminal vesicle swelling | US, CT | Transperineal needle aspiration | Staphylococcus aureus | Right | Zinner syndrome |
| Somiya et al[28] | 2018 | 1 | 89 | Left lower quadrant bulge | CT, CUS | Transperineal needle aspiration | Streptococcus viridans | Right | Transurethral prostate resection |
SVA lacks specific clinical symptoms. Because the vesicles are anatomically adjacent to the bladder and other tissues, dysuria, testicular and groin pain, tenesmus, etc. are common clinical manifestations. Pandey et al[9] reviewed reports on these clinical manifestations; 74% of patients had fever, 58% had dysuria, and 32% had mild prostatitis[9]. Swollen glands can be found on digital anal examination. Ultrasound, CT, and other imaging examinations are the methods used for diagnosing SVA[6]. The typical CT manifestations include unilateral or bilateral seminal vesicle dilation, central irregular low-density areas, and thickening of adjacent organs[5]. On MRI, the abscesses are usually round, with low T1-weighted imaging and high T2-weighted imaging signals[10].
Infectious diseases can occur in the seminal vesicles, for which antibiotic anti-infective treatment is the first choice. However, once an abscess is formed, conservative treatment is often ineffective[5]. Because of the trauma and long recovery time of open surgery, abscess puncture and drainage is currently the first choice for treatment. The common puncture methods include transureth- ral/transvesical drainage under cystoscopy and ultrasound-guided transrectal drainage[11,12]. Regarding the diagnosis and treatment of this case, the patient was admitted to the hospital and due to the ineffectiveness of conservative treatment, the left SVA of the perineum was punctured and drained under local anesthesia, and a small amount of purulent fluid was aspirated out. However, the symptoms were not significantly relieved after puncture, and the infection was exacerbated. In addition, abdominal CT showed the possibility of an abdominal abscess, so an exploratory laparotomy was performed.
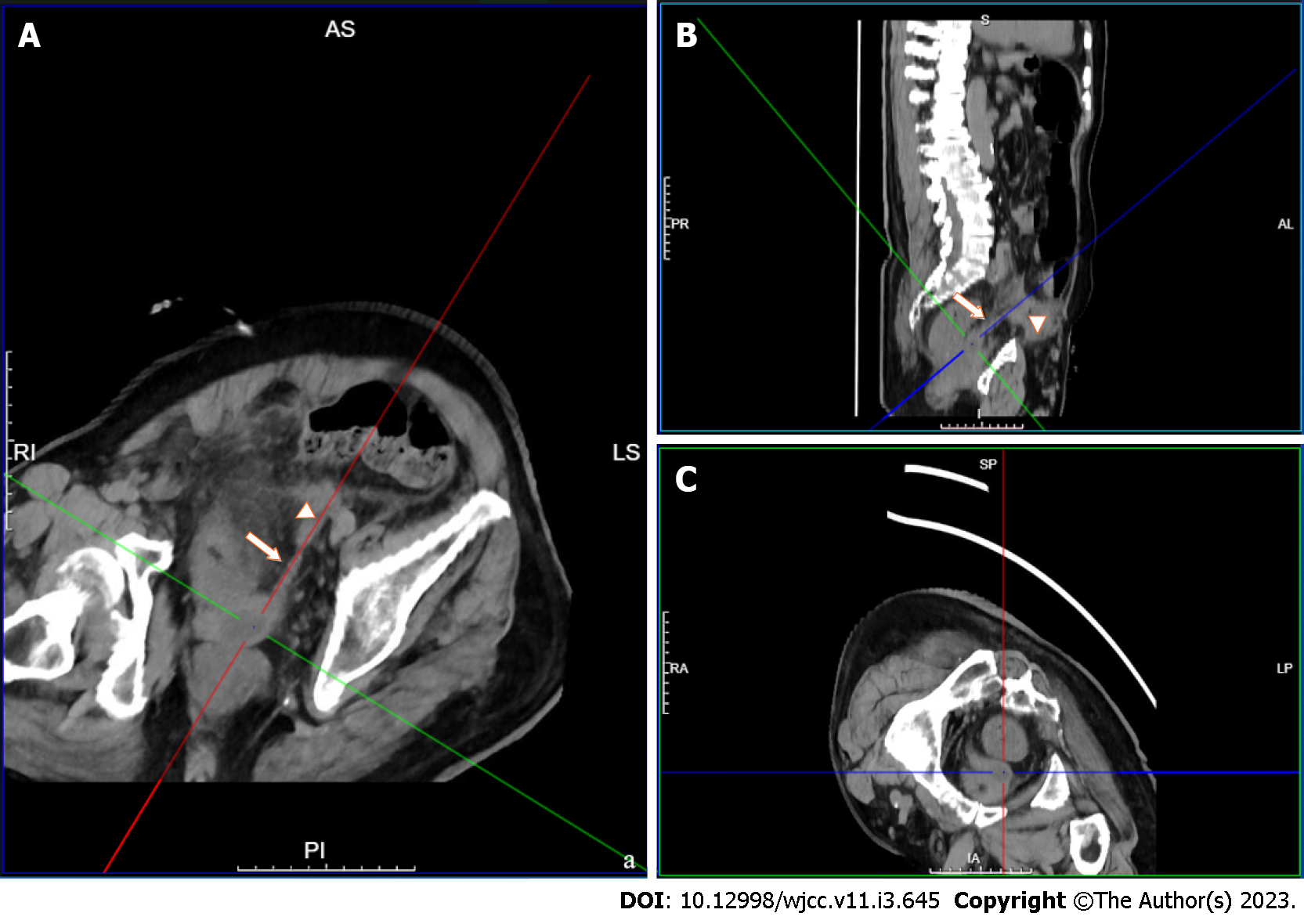
Abdominal cavity infection is a common and frequently occurring disease in general surgery and is caused by bacteria, chemical stimulation, and other factors. Intraperitoneal organ rupture, perforation, trauma, and other diseases are common causes of peritonitis. The common pathogenic bacteria of peritonitis include hemolytic Streptococcus, Pneumococcus, and Escherichia coli[13,14]. Direct diffusion is one of the main routes by which bacteria enter the abdominal cavity. In this case, neither preoperative imaging examination nor intraoperative exploration revealed perforation or rupture of any abdominal organs that may have led to secondary peritonitis. Culture of the pus showed Klebsiella pneumoniae, which is not surprising, considering that this species originates from the urinary system. By repeating the preoperative abdominal imaging examination, we found that the primary foci of the SVA spread through the ejaculatory duct-vas deferens and accumulated in the loose connective tissue of the extraperitoneal fascia to form secondary foci. The transverse abdominis muscle was anterior to the secondary abscess, and the peritoneal reflex was superior, where purulent material contacted the peritoneum, resulting in acute peritonitis and intra-abdominal inflammation (Figures 4 and 5).
By reviewing the diagnosis and treatment of this case, we summarize our experience and conclusions as follows: (1) An indwelling urinary catheter increases the risk of urinary tract infection, and the catheter indwelling time should be shortened as much as possible. For patients who must have a long-term indwelling catheter, the catheter should be replaced regularly, and high-quality nursing care should be actively performed. Elderly and frail patients with urinary tract infection should be treated in a timely manner to prevent the occurrence of complications; (2) For bacterial resistance, multiple infections, and other problems, broad-spectrum antibiotics are used for anaerobic bacteria and Gram-negative bacteria; and (3) Early primary disease and complications should be considered at the same time, including the full drainage of SVA in the early stage, as well as active surgical intervention for abdominal infection. Clinicians can use various imaging examinations to fully understand the occurrence, development, and prognosis of the disease. In this case, the general condition of the patient before surgery was poor, and the patient’s condition was complicated with sepsis. Early exploratory laparotomy, removal of the abscess, and adequate drainage were the best options.
We sincerely thank Sheng-Song Huang and Wei Le for their help in the treatment and differential diagnosis of this patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abd EL hafez A, Egypt; Tomizawa M, Japan S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Hoshino N, Endo H, Hida K, Kumamaru H, Hasegawa H, Ishigame T, Kitagawa Y, Kakeji Y, Miyata H, Sakai Y. Laparoscopic Surgery for Acute Diffuse Peritonitis Due to Gastrointestinal Perforation: A Nationwide Epidemiologic Study Using the National Clinical Database. Ann Gastroenterol Surg. 2022;6:430-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 2. | Rajfer J, Eggleston JC, Sanders RC, Walsh PC. Fever and prostatic mass in a young man. J Urol. 1978;119:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Nguyen HT, Etzell J, Turek PJ. Normal human ejaculatory duct anatomy: a study of cadaveric and surgical specimens. J Urol. 1996;155:1639-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Huang YH, Chen YH, Lin CM, Ciou YY, Kuo SP, Chen CT, Shih CM, Chang EE. Suppression effect of seminal vesicle autoantigen on platelet-activating factor-induced mouse sperm capacitation. J Cell Biochem. 2007;100:941-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Sağlam M, Uğurel S, Kilciler M, Taşar M, Somuncu I, Uçöz T. Transrectal ultrasound-guided transperineal and transrectal management of seminal vesicle abscesses. Eur J Radiol. 2004;52:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Patel B, Gujral S, Jefferson K, Evans S, Persad R. Seminal vesicle cysts and associated anomalies. BJU Int. 2002;90:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Saha S, Wright G, Arulampalam T, Corr J. An unusual groin mass. Seminal vesicle abscess: a case report. Cases J. 2009;2:6531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Zhang JQ, He CC, Yuan B, Liu R, Qi YJ, Wang ZX, He XN, Li YM. Fatal systemic emphysematous infection caused by Klebsiella pneumoniae: A case report. World J Clin Cases. 2022;10:2610-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Pandey P, Peters J, Shingleton WB. Seminal vesicle abscess: a case report and review of literature. Scand J Urol Nephrol. 1995;29:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Chen X, Wang H, Wu RP, Liang H, Mao XP, Mao CQ, Zhu HZ, Qiu SP, Wang DH. The performance of transrectal ultrasound in the diagnosis of seminal vesicle defects: a comparison with magnetic resonance imaging. Asian J Androl. 2014;16:907-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Bayne CE, Davis WA, Rothstein CP, Engel JD. Seminal vesicle abscess following prostate biopsy requiring transgluteal percutaneous drainage. Can J Urol. 2013;20:6811-6814. [PubMed] |
| 12. | Sihra N, Aboelsoud M, Oliyide A, Counsell A, Gall Z. Seminal Vesicle Abscess-An Unusual Complication Following Vasectomy. Urology. 2018;116:20-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Labricciosa FM, Sartelli M, Abbo LM, Barbadoro P, Ansaloni L, Coccolini F, Catena F. Epidemiology and Risk Factors for Isolation of Multi-Drug-Resistant Organisms in Patients with Complicated Intra-Abdominal Infections. Surg Infect (Larchmt). 2018;19:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Inoue M, Kako E, Kinugasa R, Sano F, Iguchi H, Sobue K. Necrotizing fasciitis following primary peritonitis caused by Streptococcus pyogenes with covS mutation in a healthy woman: a case report. JA Clin Rep. 2019;5:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Dewani CP, Dewani N, Bhatia D. Case report: tubercular cold abscess of seminal vesicle: minimally invasive endoscopic management. J Endourol. 2006;20:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Chong Vh VH, Sharif F, Bickle I. Urogenital melioidosis: a review of clinical presentations, characteristic and outcomes. Med J Malaysia. 2014;69:257-260. [PubMed] |
| 17. | Hammad FT. Seminal vesicle cyst forming an abscess and fistula with the rectum review of perianal drainage and treatment. Scand J Urol Nephrol. 2006;40:426-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Fujinaga S, Hirano D, Hara S, Uchida H, Kitano Y, Kobayashi K, Tada M, Someya T, Ohtomo Y, Shimizu T. Seminal vesicle abscesses associated with ipsilateral multicystic dysplastic kidney in an infant. Pediatr Nephrol. 2008;23:1551-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Wadei HM, Brumble L, Broderick GA, Gonwa TA. Polymicrobial seminal vesical abscess in a kidney transplant recipient. Urology. 2008;72:296. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Talwar HS, Mittal A, Narain TA, Panwar VK. A wide spectrum of rare clinical variants of Zinner syndrome. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Monzó JI, Garcia EL, Benavente R, Moralejo Gárate M, Cordero J, Fernández C. Absceso Primario de vesícula seminal: Diagnóstico y tratamiento mediante ecografía transrectal. ACTAS UROL ESP. 2005;29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Machida H, Ueno E, Nakazawa H, Fujimura M, Ito F. Spermatic cord abscess with concurrent prostatic abscess involving the seminal vesicle. Radiat Med. 2008;26:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Bradley ME, Scoular SK. Metastatic Klebsiella pneumoniae Invasive Liver Abscess Syndrome in Denver, Colorado. J Pharm Pract. 2021;34:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Imperatore V, Creta M, Di Meo S, Buonopane R, Spirito L, Mirone V. Seminal vesicle abscess causing unilateral hydroureteronephrosis: A case report. Arch Ital Urol Androl. 2017;89:321-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Zheng X, Wang X, Zhou J, Xiang J, Xie L. Diagnosis and treatment of community-associated methicillin-resistant Staphylococcus aureus prostatic abscess involving the seminal vesicle: A case report. Exp Ther Med. 2015;9:835-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Cui ZQ, Wang YC, Du J, Zhou HJ, Yu ZY, Gao EJ, Lu HK. [Transurethral seminal vesiculoscopy combined with finasteride for recurrent hematospermia]. Zhonghua Nan Ke Xue. 2014;20:536-538. [PubMed] |
| 27. | Kuribayashi S, Tanigawa G, Okuda Y, Kawamura M, Kishimoto N, Takezawa K, Tsutahara K, Takao T, Yamaguchi S. [Seminal Vesicle Abscess Associated with Zinner Syndrome]. Hinyokika Kiyo. 2017;63:439-443. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Somiya S, Tamaki M, Fujikawa S, Yamada Y, Kamiyama Y, Kanaoka T. [A Case of Seminal Vesicle Cyst Incidentally Diagnosed during Rupture of Abdominal Subcutaneous Abscess]. Hinyokika Kiyo. 2018;64:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |