Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7234
Peer-review started: August 16, 2023
First decision: September 4, 2023
Revised: September 10, 2023
Accepted: September 19, 2023
Article in press: September 19, 2023
Published online: October 16, 2023
Processing time: 58 Days and 11 Hours
The occurrence of long-term bilioenteric anastomotic stenosis can readily induce liver atrophy and hyperplasia, thereby causing significant alterations in the anatomical and morphological aspects of the liver. This condition significantly hampers the accuracy of preoperative imaging diagnosis, while also exacerbating the complexity of surgical procedures and the likelihood of complications.
A 60-year-old female patient was admitted to the hospital presenting with recurring epigastric pain accompanied by a high fever. The patient had a history of cholecystectomy, although the surgical records were not accessible. Based on preoperative imaging and laboratory examination, the initial diagnosis indicated the presence of intrahepatic calculi, abnormal right liver morphology, and acute cholangitis. However, during the surgical procedure, it was observed that both the left and right liver lobes exhibited evident atrophy and thinness. Additionally, there was a noticeable increase in the volume of the hepatic caudate lobe, and the original bilioenteric anastomosis was narrowed. The anastomosis underwent enlargement subsequent to hepatectomy. As a consequence of the presence of remaining stones in the caudate lobe, the second stage was effectively executed utilizing ultrasound-guided percutaneous transhepatic catheter drainage. Following the puncture, three days elapsed before the drain tip inadvertently perforated the liver, leading to the development of biliary panperitonitis, subsequently followed by pulmonary infection. The patient and her family strongly refused operation, and she died.
The hepatic atrophy-hypertrophy complex induces notable alterations in the anatomical structure, thereby posing a substantial challenge in terms of imaging diagnosis and surgical procedures. Additionally, the long-term presence of hepatic fibrosis changes heightens the likelihood of complications arising from puncture procedures.
Core Tip: This case report presents a recurrent occurrence of acute cholangitis caused by persistent stenosis following a bilioenterostomy, leading to the development of the hepatic atrophic hyperplasia complex. This complex is characterized by notable atrophy in the left and right hepatic lobes and significant hyperplasia in the caudate lobe. The preoperative imaging failed to accurately detect these anatomical alterations, and the subsequent delayed puncturing of the liver envelope through puncture drainage resulted in biliary peritonitis and ultimately, mortality. Therefore, a comprehensive preoperative surgical history, precise comprehension of anatomical anomalies and pathological changes are crucial for effective management in such cases.
- Citation: Liang SY, Lu JG, Wang ZD. Imaging misdiagnosis and clinical analysis of significant hepatic atrophy after bilioenteric anastomosis: A case report. World J Clin Cases 2023; 11(29): 7234-7241
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7234.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7234
The liver is the largest substantial digestive organ in the human body, involved in complex physiological functions, including digestion, metabolism, detoxification, excretion and so on[1]. The normal liver has a dual supply of blood and oxygen from the hepatic artery and portal vein, as well as biliary tract and nerve innervation in the hilum hepatis[2,3]. According to the published literature, the damage of these structures can lead to abnormal liver structure and physiological function[4,5]. Hepatic artery occlusion caused by hepatic artery injury may lead to liver insufficiency and liver abscess[6]. Acute complete occlusion of blood flow caused by portal vein injury can lead to acute liver failure[7].
Whereas, the liver has a powerful regenerative capacity. After partial liver resection, the remnant liver can rapidly proliferate to restore liver volume and function[8]. However, chronic injury can lead to the accumulation of inflammatory cells in the liver, chronic fibrosis, and atrophy, which is accompanied by compensatory proliferation of normal liver tissue to achieve normal liver morphology and function. This pathological change leads to normal morphological and anatomical changes in the liver[9]. Clinically, it is called hepatic atrophy-hypertrophy complex, which brings challenges to preoperative imaging diagnosis and surgery[10].
In addition, the abnormal liver morphology and function caused by bile duct injury are permanent and complex, and different surgical methods may lead to inconsistent results. Complete bile duct rupture due to iatrogenic injury was often treated with choledochoduodenostomy decades ago, which often resulted in recurrent acute cholangitis due to severe reflux[10,11]. Therefore, recurrent episodes of acute cholangitis led to abnormal liver morphology and function. Furthermore, the persistent inflammatory stimulation of bilioenteric anastomosis can lead to anastomotic stenosis, which also delays the improvement of acute cholangitis. These patients are prone to elevated liver enzymes, dyspepsia, liver abscess, and liver atrophy and deformation, some of these patients may develop cholangiocarcinoma[12].
Imaging findings play an increasingly important role in medicine, especially for surgeons. The commonly used preoperative examinations of the liver include ultrasound, computed tomography (CT), and Magnetic resonance imaging (MRI), which provide important guidance for surgical preparation. In particular, contrast-enhanced CT and MRI can clearly show the vascular structure in the liver, including biliary strictures, biliary dilatation, and abnormal space-occupying lesions in the biliary tract[13-15]. However, in some cases, complex nonneoplastic liver lesions may interfere with the accurate interpretation of imaging results, which requires a review of the patient's detailed symptoms, signs, and the details of surgery history. Herein, we report a case of severe atrophy of all liver lobes except for caudate lobe hyperplasia, which was misdiagnosed by preoperative imaging.
A female patient, aged 60, was hospitalized mainly due to recurrent epigastric pain for 30 years, which was accompanied by chills and high fever for half a year. The patient did not have nausea, vomiting, black stool, hematemesis, or obvious jaundice. Intermittent symptoms of slight upper abdominal pain occurred after surgery without other discomfort.
She described a slight loss of appetite and normal bowel and bladder function. The patient did not receive any medications prior to admission.
She underwent open cholecystectomy for gallstones more than 30 years ago, and the surgical records were not found. Adhesiolysis was performed 20 years ago because of adhesive intestinal obstruction.
She denied alcohol assumption and smoke, and she took no medications. She denied any other personal medical history or a family history.
The patient's body mass index (BMI) was 18.5. On admission, physical examination revealed mild jaundice of bilateral sclera, surgical incision scar in the abdomen, tenderness in the right upper abdomen, no rebound pain or muscle tension, positive tapping pain in the hepatic region, and no abnormal bowel sounds.
The laboratory test results are shown in Table 1.
| Index | Test results |
| White blood cell count | 3.85 × 109/L |
| Hemoglobin | 121.0 g/dL |
| Platelet count | 117.0 × 109/L |
| Glucose | 4.79 mmol/L |
| International normalized ratio | 0.99 |
| Blood urea nitrogen | 5.40 mmol/L |
| Total bilirubin | 31.7 μmol/L |
| Direct bilirubin | 25.3 μmol/L |
| Glutamylaminotransferase | 832 U/L |
| Alkaline phosphatase | 722 U/L |
| Serum albumin | 39.1 g/dL |
| Procalcitonin | 0.12 ng/mL |
| Interleukin-6 | 13.4 pg/mL |
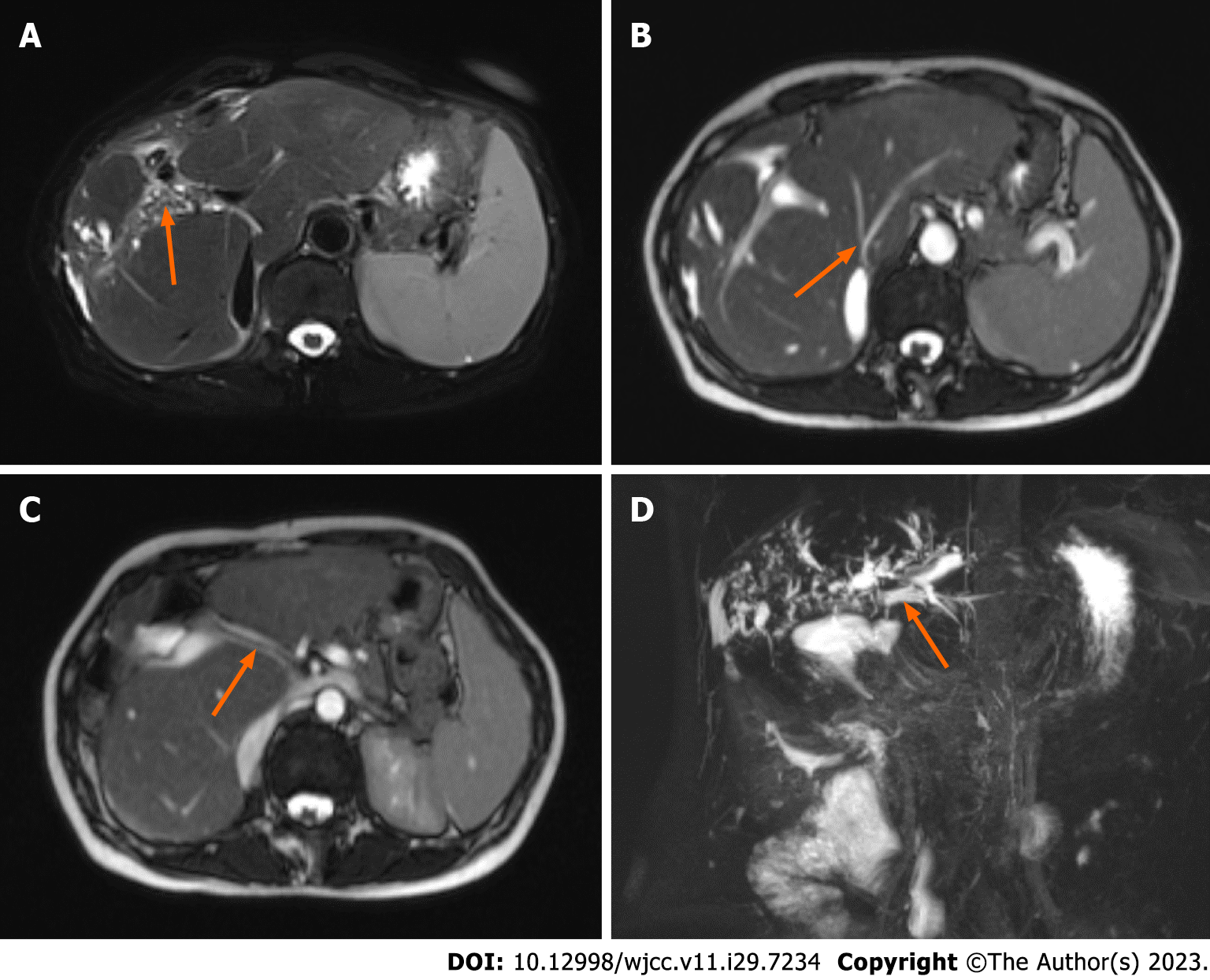
The results of abdominal enhanced CT examination showed that there was no gallbladder, the edge of the liver was not smooth, intrahepatic bile duct stones were accompanied by bile duct dilatation, the morphology of the right lobe of the liver was abnormal, the left and right hepatic veins were narrowed, and the middle hepatic vein was not shown. The size of the spleen and pancreas was normal (Figure 1). Magnetic resonance cholangiopancreatography (MRCP) showed intrahepatic bile duct dilatation with stones, no gallbladder was shown, the common bile duct and hepatic duct were not clearly visualized, the shape of the right lobe of the liver was abnormal with stasis of hepatic lymph nodes, and the pancreatic duct was well developed without dilatation or stenosis (Figure 2).
An initial diagnosis of hepatolithiasis associated with acute cholangitis was considered.
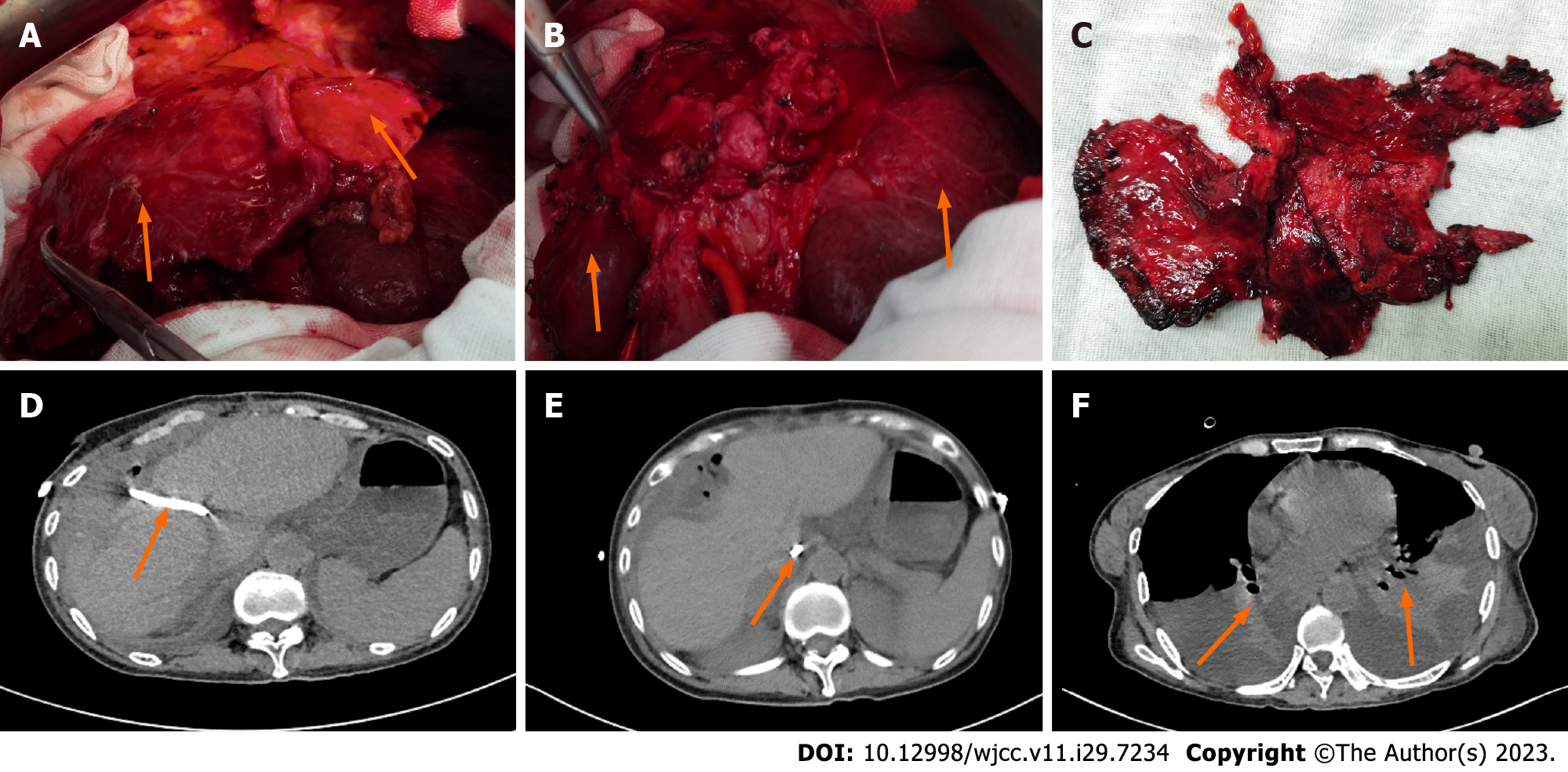
Under general anesthesia, exploratory laparotomy revealed obvious adhesion in the right upper quadrant of the abdomen. After separation of the adhesion, obvious fibrosis of the left and right liver lobes was observed, and the volume was significantly reduced and thinner, namely liver atrophy. The caudate lobe of the liver significantly increased in volume and became hard in texture, and the portal vein and hepatic artery were moved in front of it (Figure 3A-C). Intraoperative ultrasound examination revealed bile duct stones in the caudate lobe. The gallbladder was not found, and an end-to-side anastomosis between the common bile duct and the duodenum was seen at the first hepatic hilum. After the resection of the atrophic liver lobe, the stenotic anastomosis was enlarged, reanastomosed, and a T-tube was placed. The patient recovered well after surgery, and the fever and jaundice gradually relieved.
On postoperative day 25, abdominal pain, distention, and jaundice recurred. After local anesthesia, percutaneous transhepatic catheter drainage (PTCD) was performed under ultrasound guidance. Three days after surgery, she had a sudden onset of severe abdominal pain and distention after ambulation. On abdominal examination, the patients had distention of the abdomen, whole abdominal tenderness, rebound pain and muscle tension, especially in the right upper quadrant, and weakened bowel sounds. Reexamination of abdominal and chest CT showed that the caudate lobe of the liver was consistent with that before operation, the intrahepatic bile duct had drainage tube shadow, the right intestinal wall was edema and thickening, and a small amount of peritoneal effusion. Chest CT also showed atelectasis in both lower lobes with pleural effusion (Figure 3D-F).
Reexamination of the laboratory results indicated significant signs of infection. white blood cell count was 12.12 × 109/L, hemoglobin was 97.0 g/dL, total bilirubin was 54.0 μmol/L, direct bilirubin was 46.3 μmol/L, Glutamylaminotransferase was 762 U/L, alkaline phosphatase was 683 U/L, PCT was assessed 8.68 ng/mL, interleukin-6 was 60.60 pg/mL, The tip of the intrahepatic drainage tube was displaced outside the liver and biliary chemical peritonitis was diagnosed. We withdrew the drainage tube 2 cm. The patient strongly refused reoperation, and we had to only perform anti-infection, liver function protection, water and electrolyte balance maintenance, and nutritional support treatment. Unfortunately, the patient's physical condition gradually deteriorated and she died 10 d after the puncture. The patient's family did not consent to her autopsy.
Imaging examination can provide an important basis for preoperative evaluation of liver surgery[15,16]. However, in this patient, preoperative abdominal CT failed to accurately identify severely atrophic and malshaped liver anatomy, and MRCP did not show normal biliary system architecture. To some extent, this increases the difficulty of preoperative evaluation for surgeons. In addition, this patient's hepatic atrophy-hypertrophy complex was closely related to an open cholecystectomy she had undergone 30 years earlier, with possible common bile duct injury estimated and a presumed choledochoduodenostomy performed. Unfortunately, the patient's previous medical records were not available. Without surgical intervention, it is unlikely that her anastomotic stenosis and recurrent acute cholangitis can be effectively alleviated or cured. Additionally, the shrunken left and right liver is susceptible to cancer, necessitating surgical removal. However, in cases where only intrahepatic bile duct drainage is performed, it has been found to effectively alleviate acute cholangitis. Palliative measures such as percutaneous transhepatic puncture sinus lithotripsy and lithotomy can address intrahepatic bile duct stones, but surgical resection remains the only viable option for removing the atrophied liver.
The abnormal anatomical structure of the patient's liver increases the difficulty and risk of surgery, while intraoperative bleeding increases the anesthesia time, and long-term anesthesia also has a great challenge to liver function and limits the operation time[17]. Therefore, after resection of the atrophic liver lobe, anastomosis was performed after plastic enlargement of the anastomotic stricture, and a second-stage operation was performed for the caudate lobe stone. In the early postoperative period, the patient had transient abnormal liver function, and the liver enzymes gradually decreased after liver protective drugs were given. However, intrahepatic cholangitis recurred about 3 wk later, PTCD was performed under local anesthesia, and percutaneous transhepatic sinus lithotomy was planned. However, the patient developed peritonitis symptoms 3 d after PTCD, and reexamination of CT suggested that the tip of the drainage tube may have penetrated the liver. The patient did not have abdominal pain within 3 d, which may be related to the caudate lobe of intrahepatic bile duct filled with stones, the change of drainage tube position after walking, and then bile leakage.
Due to the dilation of the bile duct in the caudate lobe, accompanied by the presence of stones and fibrotic changes in the liver tissue, the act of piercing becomes more challenging and risky. Ultrasound-guided puncture images reveal that the catheter tip has been successfully inserted into the intrahepatic bile duct. However, it is important to note that the catheter may shift due to increased activity or coughing, potentially resulting in perforation of the bile duct and hepatic envelope. Consequently, it is advisable to exercise caution and avoid inserting the catheter too deeply in such cases, as this can mitigate the occurrence of chemical peritonitis to a certain extent. So far, no similar cases have been reported.
This patient's marked hepatic atrophy was closely related to a previous anastomotic stricture after a choledochoduodenostomy. In China 30 years ago, minilaparotomy cholecystectomy was the mainstream method for the treatment of gallstones. When severe inflammation occurs in the gallbladder, surgery is easy to cause iatrogenic injury to the biliary system[18,19]. The operation of choledochoduodenostomy is simple, However, the disadvantage is that patients are prone to recurrent intrahepatic cholangitis, anastomotic stenosis and other complications[20,21]. Because the patient did not receive any treatment for recurrent abdominal pain after surgery, it eventually led to severe atrophy and deformation of the liver. Therefore, detailed surgical records are very important for later surgical evaluation and surgery.
In our case, the abdominal enhanced CT and MRI before the first operation failed to accurately diagnose the anatomical structure of the hepatobiliary system. The main reasons are as follows: First, the patient had severe atrophy of the left and right liver, completely lost the normal liver shape, and the caudate lobe had compensatory hyperplasia, which significantly exceeded the original volume; At the same time, the hepatic vein in the caudate lobe was also significantly enlarged, which caused the radiologist to mistakenly diagnose it as hepatic vein. Secondly, the patient's hepatic atrophy-hypertrophy complex was closely related to the previous surgical history. However, detailed medical records were not obtained and no significant evidence or guidance was provided to the diagnosing physician. Thirdly, MRCP imaging is based on water distribution; in this patient, anastomotic stenosis compromised visualization, and the presence of filled bile duct stones obscured visualization of the intrahepatic biliary system.
Repeated episodes of intrahepatic cholangitis after choledochojejunostomy resulted in severe left and right liver atrophy and deformation, obvious compensatory hyperplasia of the caudate lobe, and loss of normal anatomical structure, which affected the accurate diagnosis and preoperative evaluation of imaging. Hepatectomy for severe hepatic atrophy-hypertrophy complex should be performed with minimal surgical trauma.
The authors thank the radiologists of Fengjie County People's Hospital for reviewing the imaging results of the case, as well as the ultrasound-guided puncture provided by the ultrasound physicians.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ren W; Saito H, Japan S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Xing B, Lan H, Li H. HBO1 as an Important Target for the Treatment of CCL4-Induced Liver Fibrosis and Aged-Related Liver Aging and Fibrosis. Oxid Med Cell Longev. 2022;2022:1881519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16:6046-6057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 339] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (2)] |
| 3. | Balemba OB, Salter MJ, Mawe GM. Innervation of the extrahepatic biliary tract. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Nardo B, Montalti R, Puviani L, Pacilè V, Beltempo P, Bertelli R, Licursi M, Neri F, Prezzi D, Tsivian M, Pariali M, Cianciavicchia D. Portal vein oxygen supply through a liver extracorporeal device to treat acute liver failure in Swine induced by subtotal hepatectomy: preliminary data. Transplant Proc. 2006;38:1190-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Rappaport AM, Schneiderman JH. The function of the hepatic artery. Rev Physiol Biochem Pharmacol. 1976;76:129-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Sato A, Yamada T, Takase K, Matsuhashi T, Higano S, Kaneda T, Egawa S, Takeda K, Ishibashi T, Takahashi S. The fatal risk in hepatic artery embolization for hemostasis after pancreatic and hepatic surgery: importance of collateral arterial pathways. J Vasc Interv Radiol. 2011;22:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Obed A, Jarrad A, Bashir A. First Left Hepatic Trisectionectomy Including Segment One with New Associated Liver Partition and Portal Vein Ligation with Staged Hepatectomy (ALPPS) Modification: How To Do It? Am J Case Rep. 2016;17:759-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Starlinger P, Luyendyk JP, Groeneveld DJ. Hemostasis and Liver Regeneration. Semin Thromb Hemost. 2020;46:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 783] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 10. | Kim RD, Kim JS, Watanabe G, Mohuczy D, Behrns KE. Liver regeneration and the atrophy-hypertrophy complex. Semin Intervent Radiol. 2008;25:92-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Oliverius M, Gürlich R. Surgery of iatrogenic bile duct injuries. Rozhl Chir. 2022;101:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Zhu JQ, Li XL, Kou JT, Dong HM, Liu HY, Bai C, Ma J, He Q. Bilioenteric anastomotic stricture in patients with benign and malignant tumors: prevalence, risk factors and treatment. Hepatobiliary Pancreat Dis Int. 2017;16:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Reitz S, Slam K, Chambers LW. Biliary, pancreatic, and hepatic imaging for the general surgeon. Surg Clin North Am. 2011;91:59-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Wigham A, Alexander Grant L. Preoperative hepatobiliary imaging: what does the radiologist need to know? Semin Ultrasound CT MR. 2013;34:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Cannella R, Taibbi A, Pardo S, Lo Re G, La Grutta L, Bartolotta TV. Communicating with the hepatobiliary surgeon through structured report. BJR Open. 2019;1:20190012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Venkatesh SK, Chandan V, Roberts LR. Liver masses: a clinical, radiologic, and pathologic perspective. Clin Gastroenterol Hepatol. 2014;12:1414-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Krige A, Kelliher LJS. Anaesthesia for Hepatic Resection Surgery. Anesthesiol Clin. 2022;40:91-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | al-Tameem MM. Minilaparotomy cholecystectomy. J R Coll Surg Edinb. 1993;38:154-157. [PubMed] |
| 19. | Zhao JJ, Syn NL, Chong C, Tan HL, Ng JYX, Yap A, Kabir T, Goh BKP. Comparative outcomes of needlescopic, single-incision laparoscopic, standard laparoscopic, mini-laparotomy, and open cholecystectomy: A systematic review and network meta-analysis of 96 randomized controlled trials with 11,083 patients. Surgery. 2021;170:994-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Schreuder AM, Franken LC, van Dieren S, Besselink MG, Busch OR, van Gulik TM. Choledochoduodenostomy versus hepaticojejunostomy - a matched case-control analysis. HPB (Oxford). 2021;23:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Escudero-Fabre A, Escallon A Jr, Sack J, Halpern NB, Aldrete JS. Choledochoduodenostomy. Analysis of 71 cases followed for 5 to 15 years. Ann Surg. 1991;213:635-42; discussion 643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |