Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6213
Peer-review started: May 14, 2023
First decision: July 17, 2023
Revised: July 29, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: September 16, 2023
Processing time: 116 Days and 23 Hours
Aggressive variant prostate cancer (AVPC) is a rare disease that progresses rapidly. The first-line treatment for AVPC is currently unknown. We examined a rare case of AVPC with rare brain and bladder metastases. A summary review of the mechanism of development, clinicopathological manifestations, associated treatments and prognosis of this disease is presented.
The patient was diagnosed with prostate cancer (PCA), and was actively treated with endocrine therapy, radiotherapy, chemotherapy, and traditional Chinese medicine. Unfortunately, he was insensitive to treatment, and the disease progressed rapidly. He died five years after being diagnosed with PCA.
We should reach consensus definitions of the AVPC and other androgen receptor-independent subtypes of PCA and develop new biomarkers to identify groups of high-risk variants. It is crucial to complete a puncture biopsy of the tumor or metastatic lesion as soon as possible in patients with advanced PCA who exhibit clinical features such as low Prostate-specific antigen levels, high carcinoembryonic antigen levels, and insensitivity to hormones to determine the patho
Core Tip: This article reviews the literature and summarizes the characteristics and research progress of the rare clinical subtype, aggressive variant prostate cancer (AVPC), especially the treatment regimen. AVPC has low prostate-specific antigen levels, is hormone refractory, is aggressive, manifests as rare brain and bladder metastases, and has a very poor clinical prognosis. The first-line regimen for AVPC is currently unclear, with platinum-based chemotherapy regimens as the mainstay of intervention at this stage. Liquid biopsy, gene detection and molecular imaging are currently hot research topics that can provide guidance for the clinical treatment of advanced prostate cancer. This report aims to serve as a reference for clinicians.
- Citation: Weng XT, Lin WL, Pan QM, Chen TF, Li SY, Gu CM. Aggressive variant prostate cancer: A case report and literature review. World J Clin Cases 2023; 11(26): 6213-6222
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6213.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6213
Prostate cancer (PCA) exhibits significant clinical biologic specificity. As a first-line treatment for advanced PCA, androgen deprivation therapy (ADT) can effectively relieve clinical symptoms and prolong the overall survival (OS) of tumor patients. However, all patients will eventually progress to castration-resistant PCA (CRPC). A very small percentage of patients with CRPC progress through mechanisms unrelated to androgen receptor (AR) signaling and eventually develop an AR independent phenotype[1]. The concept of AVPC, which is a highly lethal phenotype, was first proposed by Beltran et al[2].
Clinical features of AVPC include low prostate-specific antigen (PSA) levels, numerous visceral metastases, hormone resistance, aggressiveness, and a dismal prognosis. This subtype is relatively uncommon, and its occurrence has increased recently. The optimal treatment is not yet clear, and the mortality rate is high[3]. PCA occurs most frequently in the axial bone, pelvic lymph nodes, and lungs; PCA in the bladder and brain is extremely uncommon[4,5]. We describe a rare case of AVPC that had both uncommon brain and bladder metastases in addition to the fatal clinical presentation of the disease. Our case study and literature review present the clinical features of this disease and the available treatments to serve as a reference for clinical decision-making.
A 77-year-old Chinese male presented to a urology clinic with a complaint of obstruction during urination for 5 mo.
In July 2017, a 77-year-old male patient was admitted to a hospital in Guangzhou after experiencing trouble urinating for 4 mo. The patient was found to have prostatic hyperplasia. He underwent transurethral resection of the prostate (TURP) at this point. His total PSA (TPSA) level was 6.78 ng/mL. Multiple bladder masses were discovered during surgery, and an electrosurgical biopsy of the bladder mass was performed. The pathology results revealed prostate adenocarcinoma with bladder invasion and localized necrosis. Bone metastasis was taken into consideration when radionuclide bone emission computed tomography (ECT) revealed aberrant bone metabolism in both the pubic and sciatic bones. He was diagnosed with prostate adenocarcinoma with a pathological stage of T4N0M1b and a Gleason score of 4 + 4 = 8. On postoperative Day 5, the patient was started on ADT with a regimen of goserelin acetate and bicalutamide. At postoperative week 6, the TPSA level was 34.38 ng/mL, and testosterone (T) was less than 0.09 nmol/L. The patient was kept on ADT for 4 mo.
The patient denied any history of hepatitis, tuberculosis, malaria, hypertension, heart disease, diabetes, cerebrovascular disease, mental illness, surgery, trauma, blood transfusion, or food or drug allergy.
The patient denied any family history of malignant tumors.
On physical examination, the vital signs were as follows: Body temperature, 36.3 °C; blood pressure, 123/72 mmHg; heart rate, 80 beats per min; and respiratory rate, 18 breaths per min. No enlarged lymph nodes were palpable throughout the body. Finger examination of the prostate revealed uneven prostate, hard texture, the disappearance of the central sulcus, no tenderness, no nodules palpable, no swelling in the rectum, and no blood staining in the finger sleeve.
In October 2017, the TPSA level dropped to 0.003 ng/mL, and the T level dropped to 0.09 nmol/L.
Pelvic magnetic resonance imaging (MRI) revealed PCA with bladder metastasis, and the bilateral seminal vesicle glands were poorly visualized (Figure 1).
He was diagnosed with prostate adenocarcinoma with a pathological stage of T4N0M1b and a Gleason score of 4 + 4 = 8.
The patient entered the intermittent endocrine therapy phase, during which normal levels of TPSA and T were observed. He had a biochemical recurrence three months later, with an elevated TPSA level of 0.21 ng/mL and a T level of 5.17 nmol/L. Combined androgen blocking treatment was for resumed for 10 mo, but it was ineffective. TPSA slowly increased to 2.3 ng/mL. T levels fell to normal and remained normal throughout subsequent disease progression. In November 2018, a pelvic MRI revealed a significantly larger metastasis in the bladder cavity than the previous MRI. The patient was diagnosed with metastatic CRPC (mCRPC).
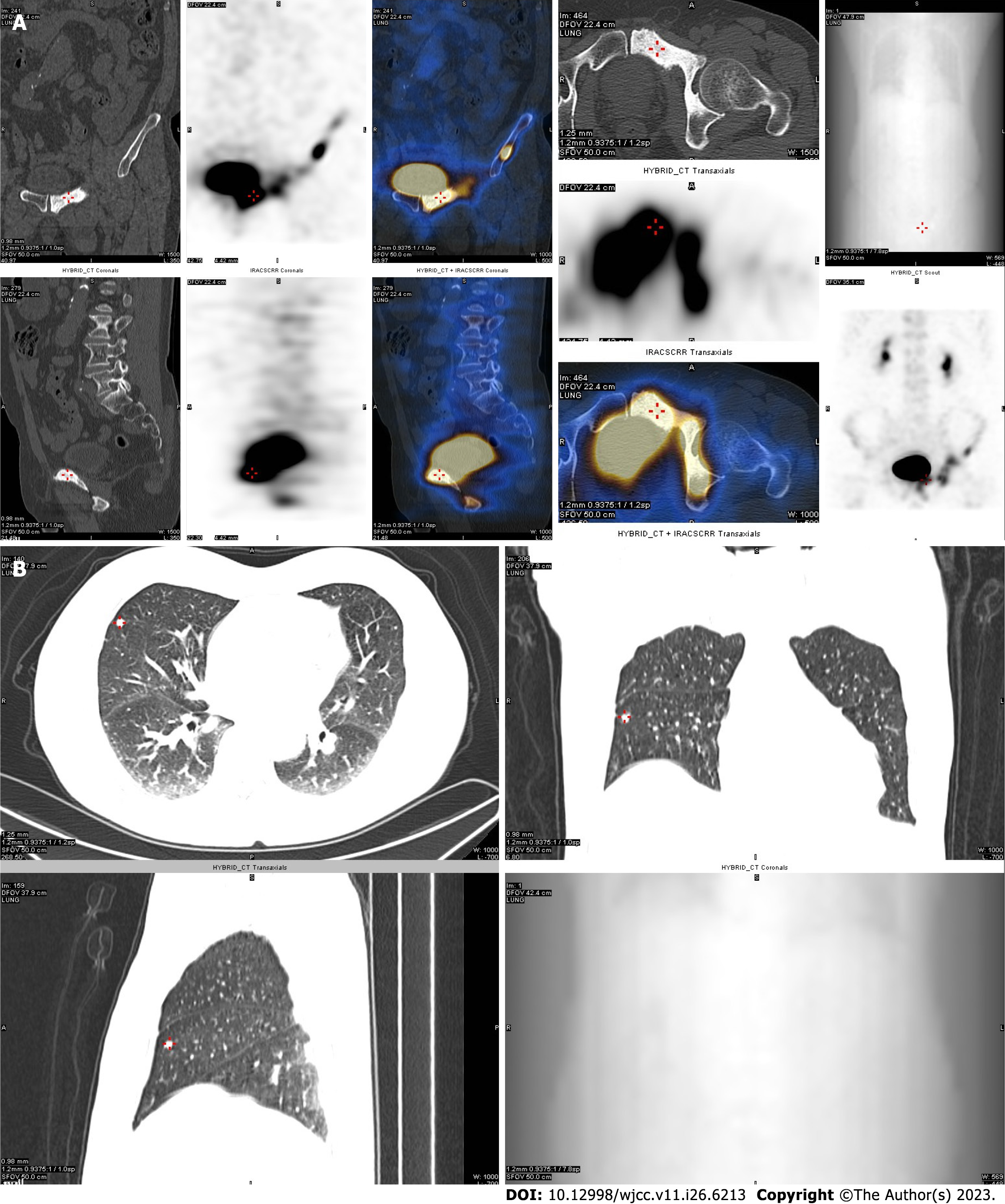
At this point, the patient began abiraterone treatment, but it was ineffective. In January 2019, the TPSA level was 4.92 ng/mL. Bone ECT showed multiple metastases in the left iliac bone, left acetabulum, left suprapubic branch, right infra-pubic branch, and both lungs (Figure 2). The patient underwent an additional five courses of novel hormone therapy (NHT) along with traditional Chinese medicine cell therapy. While there was a brief decrease in the TPSA level at first, it eventually increased rapidly. positron emission tomography-computed tomography (PET-CT) suggested enhanced metabolism in primary metastases in the lungs and focal PCA, and bone metastases were found to have areas of neo-metabolic enhancement. In December 2020, the patient underwent a second prostate puncture biopsy with the same pathological findings and Gleason score as before (Figure 3). Nine courses of docetaxel chemotherapy were started in March 2021, but they were ineffective. Pelvic MRI and bone ECT both revealed an increase in the extent of diffuse PCA restriction, with vertebral lesions involving T2 and L4. A second review at the six-month follow-up revealed an increase in the extent of diffuse cancer restriction, as well as new lesions involving T5 and L5. PET-CT analysis revealed that primary lung metastases as well as focal metabolic enhancement of PCA. Bone metastases showed areas of neo-metabolic enhancement, and neuroendocrine alterations were considered.
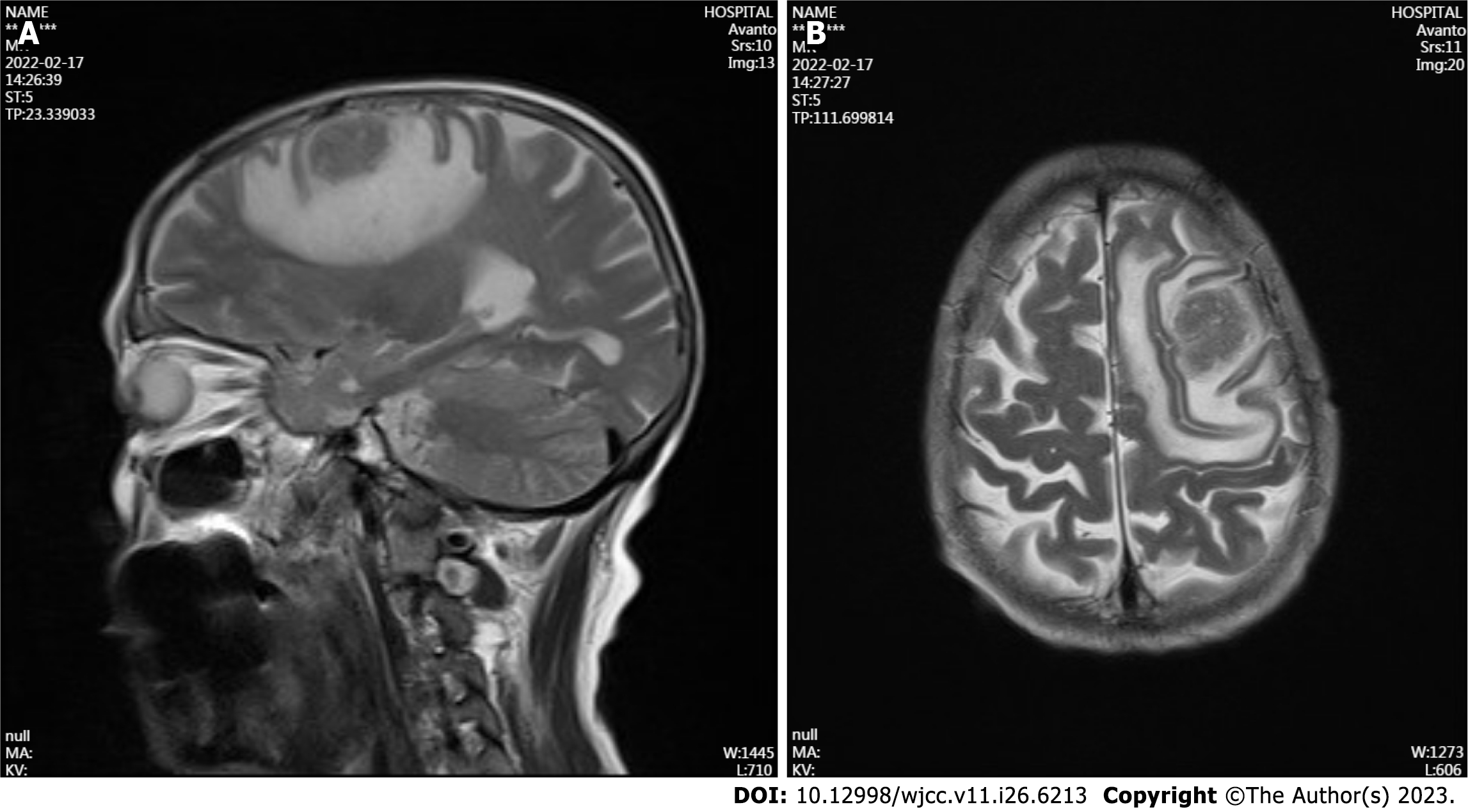
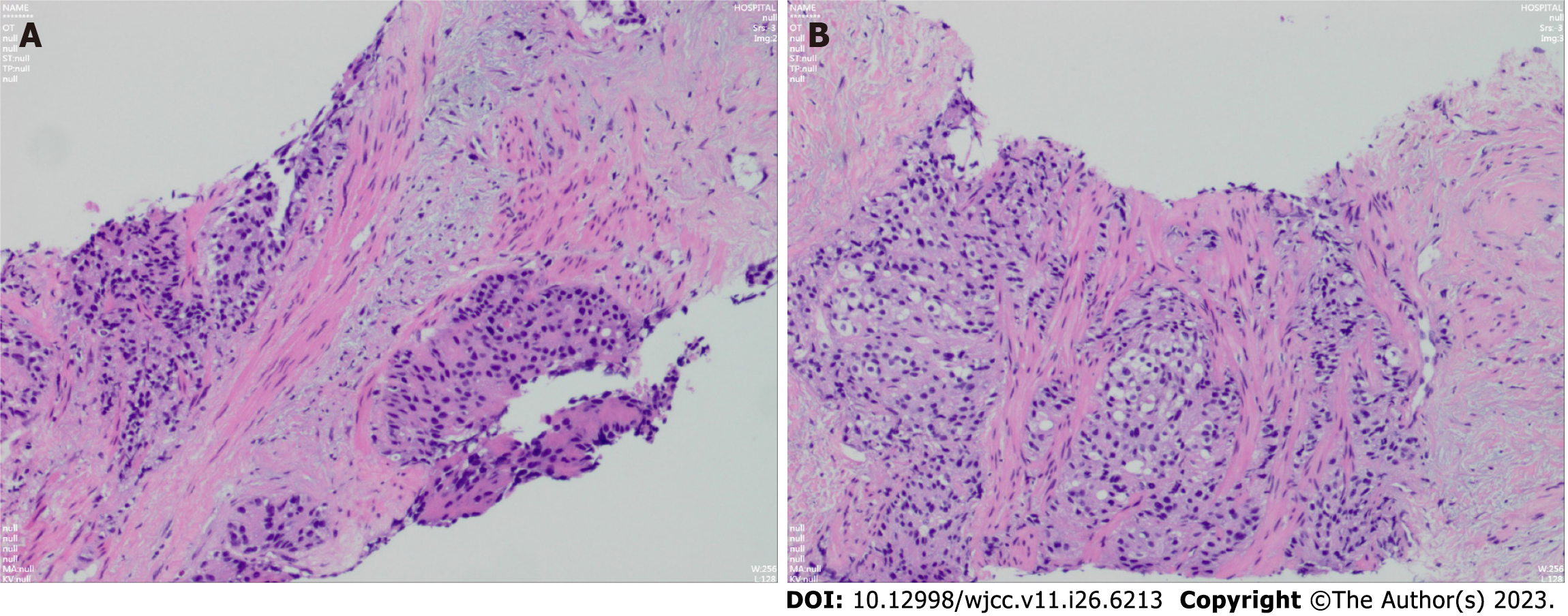
The patient was admitted in February 2022 to have his TPSA level checked, which was 3.27 ng/mL. Unexpectedly, we found that the patient's carcinoembryonic antigen (CEA) level reached 5552 ng/mL, and cranial MRI revealed left frontal lobe brain metastasis (d = 2.6 cm) (Figure 4). The patient developed coughing and shortness of breath, and chest CT revealed a new large right pleural effusion and solid right lung opacification. The left lung had a small amount of effusion, the original left lung metastases were reduced in number, and some lesions were enlarged. During hospitalization, the patient continued to receive NHT treatment combined with traditional Chinese medicine. He underwent a third prostate puncture biopsy, and the results still suggested PCA with a Gleason score of 4 + 4 = 8 (Figure 5). The genetic test results suggested variants in the AR, EXT1, MYC, and BRAF genes.
After symptomatic treatment, the patient’s symptoms improved, and he was discharged from the hospital in March 2022. The patient developed chest tightness and shortness of breath again and eventually died due to ineffective treatment.
The first-line therapy for PCA is ADT, which can effectively prolong survival and improve prognosis. However, all of them will eventually enter the CRPC stage. With the inhibition of the AR receptor signaling driving mechanism, AR receptor-independent PCA (AIPC) variants emerge. These variants, include chemotherapy sensitivity, increase expression of neuroendocrine or cluster neuromarkers, absent expression of AR receptor, and combined alterations in tumor suppressors, and they exhibit aggressive and lethal progression. The histological variants of AIPC defined by tumor morphology were classified as neuroendocrine PCA (NEPC), aggressive variant PCA (AVPC), and double negative PCA. The differences in histologic features among these three have been described in detail in the literature[6-8].
Not all androgen-independent CRPC exhibits histologic features of neuroendocrine differentiation in histological biopsies. The majority of PCA cells of the AVPC subtype have lost their typical cellular features and exhibit histologic features without NEPC but with histologically defined molecular features[9]. AVPC is used to characterize the histology of this class of "mesenchymal" tumors with non-neuroendocrine histological markers. Beltran et al[10] discovered significant genomic overlap between castration-resistant adenocarcinoma (CRPC-adeno) and neuroendocrine cancer (CRPC-NE) with AR independent clinical features by analyzing whole exome sequencing data from patients’ metastatic samples. They concluded that CRPC-adeno can evolve into CRPC-NE or other more progenitor-like cell states. Not all individuals with AR-independent CRPC demonstrate histological markers of neuroendocrine differentiation in tissue biopsies, but they all have similar clinical characteristics, such as low PSA levels, visceral or bone metastases, and insensitivity to hormone therapy. To further systematize the description of such diseases, Aparicio et al[9] termed them AVPC based on their clinical characteristics, and developed the following criteria to characterize them:
Histologic evidence of small-cell prostate carcinoma (pure or mixed).
Exclusively visceral metastases.
Radiographically predominant lytic bone metastases by plain X-ray or CT scan.
Bulky (5 cm) lymphadenopathy or bulky (5 cm) high-grade (Gleason 8) tumor mass in the prostate/pelvis.
Low PSA (≤ 10 ng/mL) at initial presentation (before ADT or at symptomatic progression in the castrate setting) plus high volume (≥ 20) bone metastases.
Presence of neuroendocrine markers in histological samples (positive staining of chromogranin A or synaptophysin) or in serum (abnormal high serum levels for chromogranin A or gastrin-releasing peptide at initial diagnosis or at progression.
Additionally, presence of any of the following in the absence of other causes: (1) Elevated serum lactate dehydrogenase (LDH) (2 IULN); (2) Malignant hypercalcemia; and (3) Elevated serum CEA (2 IULN).
Short interval (6 mo) to androgen-independent progression following the initiation of hormonal therapy with or without the presence of neuroendocrine markers.
Regardless of whether they are hormone dependent, patients with PCA with a small cell prostate cancer pattern in histological samples are considered to have AVPC.
It is estimated that approximately 30% of men with metastatic PCA meet the clinical criteria for AVPC. The lethality of AVPC is very high, and the mechanisms underlying its development are still unclear. According to one study, this subtype evolved from CRPC-adeno to CRPC-NE as a result of the organism's adaptation from AR-driven to AR-resistant conditions. It has also been proposed that this subtype results from a change in genealogical plasticity that promotes treatment resistance. Treatment-resistant stress results from the acquisition of transcription factors (SOX2, 11) and the lack of Trp53, Rb1, and phosphatase and tensor homologs (PTEN)[10-13]. Molecular analysis of AVPC revealed the combined deletion of multiple tumor suppressors, including PTEN, TP53, and Rb1, which is consistent with the understanding that genealogical plasticity in AVPC progression is assumed to be caused by genome-wide chromatin remodeling[1].
AVPC is uniquely heterogeneous in terms of its histopathological and molecular features. Its cell morphology could be that of a straightforward small cell carcinoma, the cell morphology of a typical PCA, or a mixed histological tumor consisting of adenocarcinoma together with small or large cells with neuroendocrine features[14]. All these types of cells usually exhibit low expression or absence of AR proteins, but some can express markers of neuroendocrine differentiation[2]. Loss of androgen signaling markers and trans differentiation to a neural progenitor cell phenotype are associated with antiandrogen ablation and multiorgan involvement, which characterizes AVPC. The molecular signature of AVPC exhibits combined oncogene expression defects characterized by alterations involving 2 or more of Rb1, TP53 and PTEN. Defective tumor suppressor expression is a driver of AVPC subtype variation and plays an important role in tumor cell apoptosis. The relevant effects of different combinations of alterations on clinical behavior need to be further investigated[11,15,16].
Gene detection allows for early identification of at-risk populations. Further knowledge of epigenetics can lead to a more comprehensive understanding of the possible mechanisms of AVPC subtypes. MutY DNA glycosylase (MUTYH) has been shown to be the only gene among all human DNA glycosylases whose reduced mRNA expression correlates with somatic mutational load in PCA, and its abnormal expression is a key step in the development of PCA[17]. There may be an interaction between a MUTYH mutation and AVPC subtype in this patient. The MUTYH mutation actually indicates a high tumor load, rapid disease progression and poor prognosis in this patient. TP53 variants have been found to have an elevated risk of having AVPC[18]. Thus, the addition of genetic testing of the reproductive system for PCA could help us to visualize highly aggressive subtypes and support the consideration of earlier/frequent PSA screening and MRI screening for prostate pathology in men found to have families with high-risk mutations.
Molecular imaging has been shown to have the ability to provide early surveillance of metastatic PCA. The most widely studied molecular imaging targets include prostate specific membrane antigen, and dihydrotestosterone[19]. Glumac et al[20] found that CD13 was overexpressed in specific subtypes of AVPC and independent of AR and NE. Thus, CD133 could be a promising marker for noninvasive PET imaging[20]. Mossa et al[21] noted that in AVPC patients, glycolysis is the main metabolic modality and that the tumor microenvironment enables the existence of metabolic vulnerability induced by different subtypes and sites of PCA specifically, which could be investigated in future studies to explore targeted therapeutic options. Ferrara et al[22] demonstrated ligand-directed theranostics of AVPC in vivo by establishing a preclinical model. Their findings demonstrated the viability of a ligand-directed transferase targeting the cell surface-associated glucose regulatory protein 78 KD (GPR78) in AVPCs. The differential metabolic biochemistry induced by the absence of AR signaling in CRPC can be investigated by using hyperpolarized 1- (13C) pyruvate for viable imaging biomarker science, which has research promise in PCA diagnosis and assessment of therapeutic efficacy. Hyperpolarized metabolic imaging has potential in identifying potential biological features and clinical phenotypes in patients with CRPC[23].
Exploring more sensitive and specific biomarkers for precision medicine is an urgent and topical clinical research challenge. Exploring more sensitive and specific biomarkers for precision medicine is currently an urgent and popular clinical research topic, especially for highly aggressive tumors. Liquid biopsy has the advantage of non-invasive repeat sampling and higher sensitivity and specificity than PSA, which makes it of high clinical value and a research potential for liquid biopsy. CTCs and ctDNA are more active research areas[24]. CtDNA carries transcriptional information, and tumor cells from different sources may have specific methylation profiles that help predict tumor subtypes. Sequential counting of CTCs provides early and reliable prognostic information[25]. It was further shown that single-CTC sequencing (single-CTC sequencing) could be used to assess the degree of tumor aggressiveness[26]. Liquid biopsies can also provide additional information on transcriptional plasticity. This helps us to understand the mechanisms of drug resistance, which could be a potential endpoint for clinical studies to test the efficacy of new drugs.
There is no established first-line treatment regimen for AVPC that has been defined. Platinum-based chemotherapy regimens have been shown in clinical practice to be beneficial for patients with AVPC, despite their rapid response times[27,28]. Platinum-based chemotherapy regimens, such as cisplatin/etoposide, carboplatin/etoposide, and doxorubicin/carboplatin, are advised by the 2019 National Comprehensive Cancer Network Guidelines for PCA[29]. A phase I-II study found that cabazitaxel coupled with carboplatin had superior tolerability, progression free survival, and response rates than cabazitaxel alone in mCRPC advancing to the doxorubicin stage. This study demonstrates that platinum plus paclitaxel therapy may be most beneficial for AVPC patients[28,30]. In patients with advanced CRPC for whom endocrine therapy has failed and suspicious clinical characteristics are present, we recommend rebiopsy of fast-advancing lesions for definite diagnosis and immunohistochemical analysis for routine neuroendocrine markers[31]. We also advocate for improved MSI/MMR gene deficiency testing in AVPC patients and suggest pembrolizumab as second-line therapy or as a follow-up treatment[13].
Due to the rarity of the disease and the lack of understanding of aggressive variable PCA, we did not identify this subtype in time. Consequently, we did not use platinum-based chemotherapy regimens and extended the genetic testing as early as was needed in this patient.
It has been shown that some patients who receive transplantation develop tumors after transplantation, and the prognosis is directly related to different immune environments[32]. This suggests that current detection methods are not sufficient to detect signs of early tumors. The NEPC subtype was confirmed to be the most common spreading cancer metastasis in liver transplant recipients. The location of the tumor in the transplanted organ is the most important factor affecting the prognosis of the recipient[33]. Autopsy can provide another level of evidence to help us identify early suspicious risk tumors. Assessing the presence of suspected risks by performing autopsy prior to transplantation has an important role in establishing the correct donor management pathway. In addition, it helps to better understand which cancers are still evading detection, thus refining the guideline evaluation procedures[34].
AVPC is a very rare subtype of fatal solid tumor in advanced CRPC. The clinical features of AVPC are exclusive visceral metastases, radiographically evident lytic bone metastases, bulky lymphadenopathy or bulky high-grade mass in the prostate or pelvis, elevated CEA and/or LDH, and defects in at least two of TP53, Rb1 and PTEN[35]. The primary intervention technique utilized today is platinum-based chemotherapy, but the best first-line treatment regimen is not yet known. When PSA levels are inconsistent with the disease load or when the disease progresses after treatment, tumor biopsy of metastases should be performed. Additionally, platinum-based chemotherapeutic agents can be applied early as a priority, and a more aggressive intervention and monitoring regimen must be adopted. Liquid biopsy is a highly reproducible, real-time monitoring and noninvasive detection method. Liquid biopsy is potentially valuable for early detection of cancer, clarifying disease stage, predicting disease recurrence or metastasis, monitoring therapeutic efficacy to differentiate between treatment-sensitive and treatment-insensitive patients in a timely manner, observing the characteristics of disease changes, identifying new therapeutic targets, and determining the mechanisms of drug resistance. Genetic testing is of guiding significance for predicting PCA disease progression and prognosis, making decisions on treatment options, and precision tumor therapy. In conclusion, the AIPC subtype is highly aggressive. Thus, we need to reach a consensus definition and develop tests for early recognition of this subtype to intervene as early as possible and improve prognosis. In addition, the pathologic features of lesions of the AIPC variant subtype have not been summarized. By understanding the molecular biological mechanisms involved, there have been several studies of molecularly targeted therapies for the AIPC subtype to explore genetic reprogramming and restoration of AR sensitivity, or by blocking some of this immunologically “cold” PCA to immunotherapy. These will be a promising direction for future research in advanced PCA.
The authors thank the Department of Radiology and Pathology of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine for providing the imaging and pathological data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cassell III AK, Liberia; Eccher A, Italy; Sarier M, Turkey S-Editor: Li L L-Editor: A P-Editor: Zhao S
| 1. | Sheahan AV, Ellis L. Epigenetic reprogramming: A key mechanism driving therapeutic resistance. Urol Oncol. 2018;36:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G, Huang J, True L, Gleave ME, Soule H, Logothetis C, Rubin MA. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 337] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 3. | Alanee S, Moore A, Nutt M, Holland B, Dynda D, El-Zawahry A, McVary KT. Contemporary Incidence and Mortality Rates of Neuroendocrine Prostate Cancer. Anticancer Res. 2015;35:4145-4150. [PubMed] |
| 4. | Tremont-Lukats IW, Bobustuc G, Lagos GK, Lolas K, Kyritsis AP, Puduvalli VK. Brain metastasis from prostate carcinoma: The M. D. Anderson Cancer Center experience. Cancer. 2003;98:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Masuda T, Kosaka T, Nakamura K, Hongo H, Yuge K, Nishihara H, Oya M. Multiple metastases of androgen indifferent prostate cancer in the urinary tract: two case reports and a literature review. BMC Med Genomics. 2022;15:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, Robinson BD, Troncoso P, Rubin MA. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 7. | Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Palmgren JS, Karavadia SS, Wakefield MR. Unusual and underappreciated: small cell carcinoma of the prostate. Semin Oncol. 2007;34:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, Pagliaro LC, Kim J, Millikan RE, Ryan C, Tannir NM, Zurita AJ, Mathew P, Arap W, Troncoso P, Thall PF, Logothetis CJ. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621-3630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 10. | Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV, Varambally S, Tomlins SA, Nanus DM, Tagawa ST, Van Allen EM, Elemento O, Sboner A, Garraway LA, Rubin MA, Demichelis F. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1153] [Cited by in RCA: 1272] [Article Influence: 141.3] [Reference Citation Analysis (0)] |
| 11. | Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, Goodrich MM, Labbé DP, Gomez EC, Wang J, Long HW, Xu B, Brown M, Loda M, Sawyers CL, Ellis L, Goodrich DW. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 819] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 12. | Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, Wongvipat J, Ku SY, Gao D, Cao Z, Shah N, Adams EJ, Abida W, Watson PA, Prandi D, Huang CH, de Stanchina E, Lowe SW, Ellis L, Beltran H, Rubin MA, Goodrich DW, Demichelis F, Sawyers CL. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 815] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 13. | Spetsieris N, Boukovala M, Patsakis G, Alafis I, Efstathiou E. Neuroendocrine and Aggressive-Variant Prostate Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Merkens L, Sailer V, Lessel D, Janzen E, Greimeier S, Kirfel J, Perner S, Pantel K, Werner S, von Amsberg G. Aggressive variants of prostate cancer: underlying mechanisms of neuroendocrine transdifferentiation. J Exp Clin Cancer Res. 2022;41:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 15. | He J, Kang X, Yin Y, Chao KS, Shen WH. PTEN regulates DNA replication progression and stalled fork recovery. Nat Commun. 2015;6:7620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Wang P, Guan D, Zhang XP, Liu F, Wang W. Modeling the regulation of p53 activation by HIF-1 upon hypoxia. FEBS Lett. 2019;593:2596-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Shinmura K, Kato H, Kawanishi Y, Yoshimura K, Igarashi H, Goto M, Tao H, Inoue Y, Sugiyama T, Furuse H, Ozono S, Sugimura H. Reduced expression of the DNA glycosylase gene MUTYH is associated with an increased number of somatic mutations via a reduction in the DNA repair capacity in prostate adenocarcinoma. Mol Carcinog. 2017;56:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Maxwell KN, Cheng HH, Powers J, Gulati R, Ledet EM, Morrison C, Le A, Hausler R, Stopfer J, Hyman S, Kohlmann W, Naumer A, Vagher J, Greenberg SE, Naylor L, Laurino M, Konnick EQ, Shirts BH, AlDubayan SH, Van Allen EM, Nguyen B, Vijai J, Abida W, Carlo MI, Dubard-Gault M, Lee DJ, Maese LD, Mandelker D, Montgomery B, Morris MJ, Nicolosi P, Nussbaum RL, Schwartz LE, Stadler Z, Garber JE, Offit K, Schiffman JD, Nelson PS, Sartor O, Walsh MF, Pritchard CC. Inherited TP53 Variants and Risk of Prostate Cancer. Eur Urol. 2022;81:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 19. | Boustani AM, Pucar D, Saperstein L. Molecular imaging of prostate cancer. Br J Radiol. 2018;91:20170736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Glumac PM, Gallant JP, Shapovalova M, Li Y, Murugan P, Gupta S, Coleman IM, Nelson PS, Dehm SM, LeBeau AM. Exploitation of CD133 for the Targeted Imaging of Lethal Prostate Cancer. Clin Cancer Res. 2020;26:1054-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Mossa F, Robesti D, Sumankalai R, Corey E, Titus M, Kang Y, Zhang J, Briganti A, Montorsi F, Vellano CP, Marszalek JR, Frigo DE, Logothetis CJ, Gujral TS, Dondossola E. Subtype and Site Specific-Induced Metabolic Vulnerabilities in Prostate Cancer. Mol Cancer Res. 2023;21:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Ferrara F, Staquicini DI, Driessen WHP, D'Angelo S, Dobroff AS, Barry M, Lomo LC, Staquicini FI, Cardó-Vila M, Soghomonyan S, Alauddin MM, Flores LG 2nd, Arap MA, Lauer RC, Mathew P, Efstathiou E, Aparicio AM, Troncoso P, Navone NM, Logothetis CJ, Marchiò S, Gelovani JG, Sidman RL, Pasqualini R, Arap W. Targeted molecular-genetic imaging and ligand-directed therapy in aggressive variant prostate cancer. Proc Natl Acad Sci U S A. 2016;113:12786-12791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Zacharias N, Lee J, Ramachandran S, Shanmugavelandy S, McHenry J, Dutta P, Millward S, Gammon S, Efstathiou E, Troncoso P, Frigo DE, Piwnica-Worms D, Logothetis CJ, Maity SN, Titus MA, Bhattacharya P. Androgen Receptor Signaling in Castration-Resistant Prostate Cancer Alters Hyperpolarized Pyruvate to Lactate Conversion and Lactate Levels In Vivo. Mol Imaging Biol. 2019;21:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Alix-Panabières C, Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021;11:858-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 584] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 25. | Cristofanilli M, Pierga JY, Reuben J, Rademaker A, Davis AA, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, Grisanti S, Giuliano M, Garcia-Saenz JA, Stebbing J, Caldas C, Gazzaniga P, Manso L, Zamarchi R, de Lascoiti AF, De Mattos-Arruda L, Ignatiadis M, Cabel L, van Laere SJ, Meier-Stiegen F, Sandri MT, Vidal-Martinez J, Politaki E, Consoli F, Generali D, Cappelletti MR, Diaz-Rubio E, Krell J, Dawson SJ, Raimondi C, Rutten A, Janni W, Munzone E, Carañana V, Agelaki S, Almici C, Dirix L, Solomayer EF, Zorzino L, Darrigues L, Reis-Filho JS, Gerratana L, Michiels S, Bidard FC, Pantel K. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit Rev Oncol Hematol. 2019;134:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 26. | Malihi PD, Graf RP, Rodriguez A, Ramesh N, Lee J, Sutton R, Jiles R, Ruiz Velasco C, Sei E, Kolatkar A, Logothetis C, Navin NE, Corn P, Aparicio AM, Dittamore R, Hicks J, Kuhn P, Zurita AJ. Single-Cell Circulating Tumor Cell Analysis Reveals Genomic Instability as a Distinctive Feature of Aggressive Prostate Cancer. Clin Cancer Res. 2020;26:4143-4153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Slootbeek PHJ, Duizer ML, van der Doelen MJ, Kloots ISH, Kuppen MCP, Westgeest HM, Uyl-de Groot CA, Pamidimarri Naga S, Ligtenberg MJL, van Oort IM, Gerritsen WR, Schalken JA, Kroeze LI, Bloemendal HJ, Mehra N. Impact of DNA damage repair defects and aggressive variant features on response to carboplatin-based chemotherapy in metastatic castration-resistant prostate cancer. Int J Cancer. 2021;148:385-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Ruiz de Porras V, Font A, Aytes A. Chemotherapy in metastatic castration-resistant prostate cancer: Current scenario and future perspectives. Cancer Lett. 2021;523:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Ippolito JE, Kane CJ, Kuettel MR, Lang JM, McKenney J, Netto G, Penson DF, Plimack ER, Pow-Sang JM, Pugh TJ, Richey S, Roach M, Rosenfeld S, Schaeffer E, Shabsigh A, Small EJ, Spratt DE, Srinivas S, Tward J, Shead DA, Freedman-Cass DA. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:479-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 921] [Article Influence: 184.2] [Reference Citation Analysis (0)] |
| 30. | Corn PG, Heath EI, Zurita A, Ramesh N, Xiao L, Sei E, Li-Ning-Tapia E, Tu SM, Subudhi SK, Wang J, Wang X, Efstathiou E, Thompson TC, Troncoso P, Navin N, Logothetis CJ, Aparicio AM. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomised, open-label, phase 1-2 trial. Lancet Oncol. 2019;20:1432-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 31. | Zhang Q, Han Y, Zhang Y, Liu D, Ming J, Huang B, Qiu X. Treatment-Emergent Neuroendocrine Prostate Cancer: A Clinicopathological and Immunohistochemical Analysis of 94 Cases. Front Oncol. 2020;10:571308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Eccher A, Girolami I, Marletta S, Brunelli M, Carraro A, Montin U, Boggi U, Mescoli C, Novelli L, Malvi D, Lombardini L, Cardillo M, Neil D, D'Errico A. Donor-Transmitted Cancers in Transplanted Livers: Analysis of Clinical Outcomes. Liver Transpl. 2021;27:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Eccher A, Lombardini L, Girolami I, Puoti F, Zaza G, Gambaro G, Carraro A, Valotto G, Cima L, Novelli L, Neil D, Montin U, Scarpa A, Brunelli M, Nanni Costa A, D'Errico A. How safe are organs from deceased donors with neoplasia? The results of the Italian Transplantation Network. J Nephrol. 2019;32:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Girolami I, Neil D, Segev DL, Furian L, Zaza G, Boggi U, Gambaro G, De Feo T, Casartelli-Liviero M, Cardillo M, Lombardini L, Zampicinini L, D'Errico A, Eccher A. Discovered cancers at postmortem donor examination: A starting point for quality improvement of donor assessment. Transplant Rev (Orlando). 2021;35:100608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Berchuck JE, Viscuse PV, Beltran H, Aparicio A. Clinical considerations for the management of androgen indifferent prostate cancer. Prostate Cancer Prostatic Dis. 2021;24:623-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |