Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6091
Peer-review started: June 25, 2023
First decision: July 18, 2023
Revised: August 16, 2023
Accepted: August 25, 2023
Article in press: August 25, 2023
Published online: September 16, 2023
Processing time: 74 Days and 17.8 Hours
Multinucleated giant cells (MGCs) in bladder carcinomas are poorly studied.
To describe the function, morphogenesis, and origin of mononuclear and MGCs in urothelial carcinoma (UC) of the bladder in Bulgarian and French patients.
Urothelial bladder carcinomas (n = 104) from 2016-2020 were analyzed retrospec
We confirm that MGCs in the mucosa in UC of the bladder were positive for both mesenchymal and myofibroblast markers (vimentin, smooth muscle actin, Desmin, and CD34) and the macrophage marker CD68. Furthermore, IHC studies revealed the following profile of these cells: Positive for p16; negative for epithelial (CK AE1/AE3 and GATA-3), vascular (CD31), neural (PS100 and C-KIT), cambial, blastic (CD34-blasts and C-KIT), and immune markers (IG G, immunoglobulin G4, and PD-L1); no proliferative activity, possess no specific immune function, and cannot be used to calculate the Combined Positive Score scale.
In conclusion, the giant stromal cells in non-tumor and tumor bladder can be used as a characteristic and relatively constant, although nonspecific, histological marker for chronic bladder damage, reflecting the chronic irritation or inflammation. Likewise, according to the morphological and IHC of the mono- and multinucleated giant cells in the bladder, they are most likely represent telocytes capable of adapting their morphology to the pathology of the organ.
Core Tip: Based on our results and data from the literature, we have developed and proposed an algorithm of histological, histochemical, and immunohistochemical (IHC) criteria, and also a generalized algorithm for the differential diagnosis of multinucleated giant cells (MGCs) in bladder urothelial carcinoma and benign or malignant lesions of other visceral organs. According to the data obtained by us in the morphological and IHC study, MGCS are modified telocytes. The reason for this modification is the chronic damage to the bladder mucosa (regardless of the etiological factor), which leads to the fusion of telocytes and their transformation into multinucleated cells. At the same time, they lose C-kit expression, penetrate phagocytic function, and begin to express p16, which is, as described in detail above, a sign of cellular aging.
- Citation: Gulinac M, Velikova T, Dikov D. Multinucleated giant cells of bladder mucosa are modified telocytes: Diagnostic and immunohistochemistry algorithm and relation to PD-L1 expression score. World J Clin Cases 2023; 11(26): 6091-6104
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6091.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6091
The lamina propria of the bladder consists of connective tissue, rich in different types of cells, nervus and blood vessels. In the subepithelial layer of cells, fibroblast and macrophage-like immunohistochemical and morphological structures were also identified, called by different authors by different names, such as interstitial cells, interstitial cells of Cajal, interstitial cell-like Cajal, fibroblast-like cells[1,2]. Some study from Bucharest in 2005 discovered a brand-new entity of interstitial cells in various organs, and they named them telocytes[3-5].
Since 2007, telocytes are still the subject of debate about their role. Their morphological structure resembles mostly that of Cajal interstitial cells.
In summary, we believe that it is more reasonable in the present study to determine the exact identification of these cells to use the terms: Mononuclear and multinucleated giant cells (MGCs) present in the connective tissue of the bladder wall in neoplastic and inflammatory processes.
The presence of more or less atypical mono- or MGCs resembling fibroblasts is a relatively common but poorly described finding in the vesical lamina propria[6,7]. Such cells can also be observed relatively frequently in bladder biopsies without an inflammatory context[8]. In a large number of cases, these cellular changes are nonspecific. They are observed in 1/3 (33%) of autopsy cases with chronic cystitis[9].
When MGCs are presented in large numbers, in non-tumor pathology cases, the term “giant cell cystitis” has been used in the past[8-10]. However, such cellular changes are now observed under the influence of radiation, chemotherapy[6,11-14], and around tumors[10,11]. Similarly, giant cells can occur in various benign mesenchymal tumors and the stroma of some epithelial benign gynecological lesions[8].
Urothelial carcinoma (UC) of the bladder is a multifactorial disease characterized by an aggressive course, frequent recurrences, and high mortality worldwide. The morphology of C-KIT bladder carcinoma is well known. Still, its stroma is insufficiently studied. Moreover, some of its components, such as mononuclear giant cells and MGCs, are almost unknown[15]. To date, the literature lacks detailed histoepidemiological, morphological, and immunohistochemical (IHC) studies of the immunophenotype and morphogenesis of MGCs in the mucosa and the stroma of the bladder. At the same time, in the non-tumor bladder, this question is insufficiently studied. These questions and difficulties, which pathologists, urologists, and oncologists are exposed to, motivated us to study these problems in more depth, using materials from two geographically different countries-Bulgaria and France.
Our study aimed to establish the function, morphogenesis, and origin of mononuclear giant cells and MGCs in the stroma of UC of the bladder in patients’ samples, as well as to compile a proper differential-diagnostic algorithm from histological, histochemical, and IHC criteria for the diagnosis of MGCs observed in the stroma of the UC of the bladder.
We examined 104 cases of UC (91 samples of Bulgarian patients and 13 samples of French patients) from the tissue database of University Hospital “St. George” Plovdiv, Bulgaria and Grand Hospital de l’Este Francilien, Jossigny, France, and reviewed the period from 2016 to 2020. All analyzed patients in this study have been diagnosed with invasive or non-invasive high-grade (HG) and low-grade (LG) UCs of the bladder.
The materials were divided into two main groups, where all cases of bladder UC studied in this study were primarily diagnosed: 1st group-28/104 cases with non-invasive bladder UC; 2nd group-76/104 cases with invasive LG and HG UC of the bladder.
In the quantitative examination of the MGCs in the stroma of the UC of the bladder, an autopsy of the bladder mucosa in 10 patients was used for external negative control.
Four control groups of patients with different types of cystitis without concomitant tumor pathology were also used. MGCs were detected in 7/9 cases with eosinophilic cystitis, in 5/5 cases with polypoid cystitis, in 3/5 cases with follicular cystitis, and in 3/3 cases with radiation cystitis.
Including criteria: (1) Autopsy bladder wall (mucosa) of patients who died of neurological diseases in a hospital setting. The mean age of the patients was 68.9 years (ranging from 45 to 90 years), equally distributed as men and women; and (2) Normal macroscopic bladder at autopsy.
Excluding criteria: (1) Autopsy cases in which there was no evidence of pre-death radiation therapy in the genital area, bladder, or rectum; (2) Autopsy cases in which there was no evidence of chemotherapy in the genital area, in the area of the bladder or rectum; (3) Autopsy cases with optimally preserved and fixed bladder mucosa with minimal or zero postmortem autolysis; and (4) Autopsy cases in which no significant acute or chronic inflammatory infiltrate or tumor proliferation was observed histologically.
Histological processing of the tissues was performed using automatic tissue processor “DIAPATH EN ISO 9001:2000” and 4-5 μm formalin-fixed paraffin-embedded tissue sections underwent routine staining with hematoxylin-eosin (H&E) to determine the presence of individual histological features.
The IHC examination was performed under the standard protocols of the manufacturer. The antibodies used are from Dako Denmark A/S & Dako Carpinteria, California.
From each paraffin block of 104 cases with UC of the bladder, the stroma containing MGCs, serial sections with a thickness of 4 µm were made, mounted on adhesive slides. Then, the sections were dewaxed and rehydrated to alcohols in descending concentration. Flushing was performed with BondTM Wash Solution according to the instructions for use. The IHC examination was performed according to the manufacturer’s instructions using a Novolink Polymer Detection System. Reactions have been reported positive in the presence of brownish staining of cellular structures. In addition, an IHC positive expression was observed in different cell areas, depending on the location of the desired antigen.
Counter-staining of the nuclei was performed with Mayer’s hematoxylin.
The positive external control was selected following the manufacturer’s instructions. The negative external control for each antibody was performed according to a standard IHC procedure without dripping the test antibody.
The following monoclonal antibodies, summarized in Table 1, were used to implement the IHC examination (Department of Pathology, Grand Hôpital de l’Est Francilien, Jossigny, France and Department of Clinical Pathology of St. George Hospital EAD-Plovdiv).
| Monoclonal antibodies | Suppliers | Dilutions |
| CD31 | Dako | 1:50 |
| CD34 | Dako | 1:50 |
| CD34 blasts | Dako | 1:100 |
| CD10 | Dako | 1:50 |
| CD68 | Dako | 1:100 |
| CD45 | Dako | 1:300 |
| CD1A | Dako | 1:300 |
| P-S100 | Dako | 1:200 |
| CD117 (C-KIT) | Dako | 1:100 |
| P16 | Dako | 1:25 |
| Smooth muscle actine | Dako | 1:100 |
| DESMIN | Dako | 1:100 |
| VIMENTIN | Dako | 1:100 |
| Ki-67 | Dako | 1:100 |
| CK AE1/AE3 | Dako | 1:100 |
| IG G4 | Dako | 1:100 |
| GATA3 | Ventana | 1:100 |
| PD-L1 (22C3) | Dako | 1:50 |
Statistical analysis was performed with the software package for statistical analysis (SPSS®, 92 IBM 2009, version 19) using descriptive statistics and dispersion analysis (ANOVA). We considered the results as significant if P < 0.05.
The analyzed 104 patients in our study were diagnosed with proven UC.
The quantitative study of the MGCs in the stroma of the UC and the surrounding lamina propria was performed by the methods of descriptive statistics in 104 cases (n = 104). Of the total number of patients samples (Bulgarian and French), we found the presence of mono-, bi- and multinucleated giant fibroblast-like stromal cells in the stroma of the UC and the surrounding mucosal lamina propria in 37/104 (35.6%) of the cases while in 67/104 (64.4%) of the cases such cells were not detected.
In 25/37 cases (67.6%) with giant stromal cells, patients with UC were from Bulgaria. In 12/37 cases (32.4%) with giant stromal cells, patients with UC were from France.
In the Bulgarian sample of patients, we observed giant cells in 27.5% of cases. In the French sample of patients, giant cells were found in 92.3%, while in 7.7% of them-no giant stromal cells were detected. Giant stromal multinucleated cells were not detected in any of our normal autopsy bladder control autopsies.
It is noteworthy that there was a difference in the presence of giant stromal cells in the lamina propria in UC of the bladder, according to the geographical origin of the material: giant stromal cells were more commonly found in the stroma of UC of the bladder in the French patient group (92.3% vs 27.5%, in the French materials and Bulgarian materials, respectively, P < 0.001).
The comparative analysis between the grading of the UC, according to the WHO 1973, and the presence of giant cells in the stroma of the bladder lamina propria showed the following results. Giant cells were found: (1) In well-differentiated UC (G1)-in 6/37 of cases (16.2%); (2) in moderately differentiated UC (G2)-in 17/37 of cases (45.9%); and (3) in poorly differentiated UC (G3)-in 14/37 of cases (37.8%).
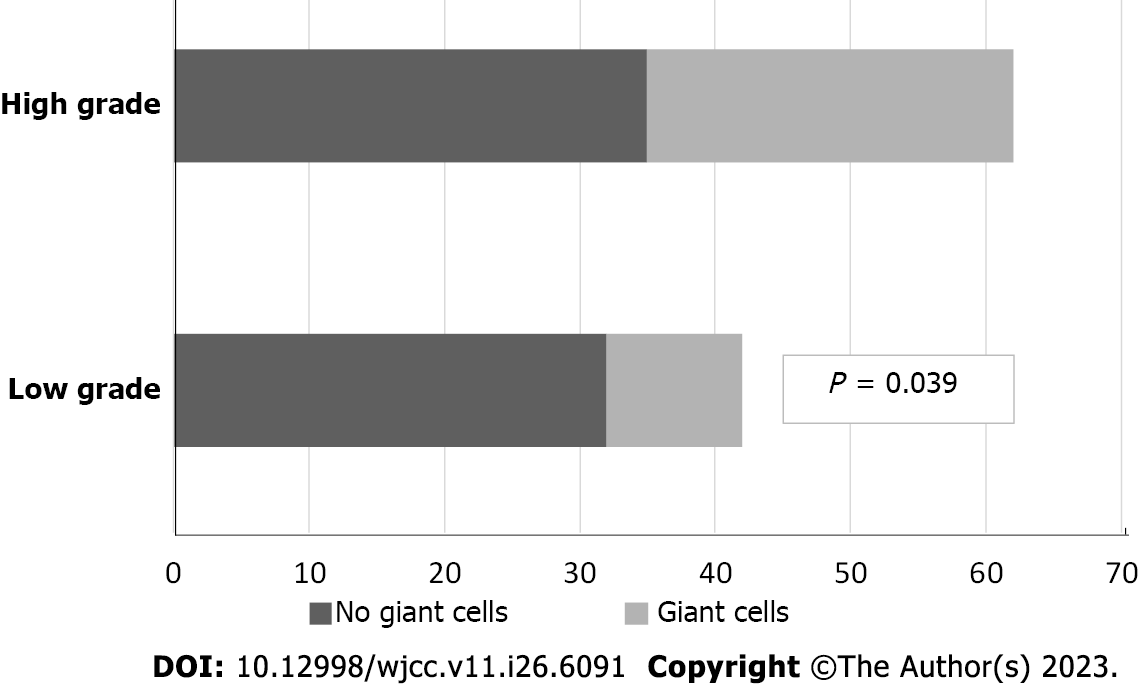
The analysis of the results mentioned above showed that giant fibroblast-like cells in the stroma of the UC of the bladder and the surrounding lamina propria, in most cases, were found in moderately and poorly differentiated UC (G2/G3). Regarding this trend, the analysis reported marginal statistical significance (P = 0.056). A comparative analysis between the gradation of UC of the bladder, according to the WHO 2016, and the presence of giant cells in the stroma of the mucosa lamina propria found that MGCs were present in 27/37 (73%) cases of HG UC and 10/37 (27%) of the cases of LG UC. The results are graphically presented in Figure 1.
The data analysis showed a statistically significant correlation between the grading of UC according to the WHO 2016 and the presence of giant stromal cells in the stroma of UC of the bladder and the surrounding lamina propria. This association was weak (coefficient of correlation, r = 0.202, P = 0.039).
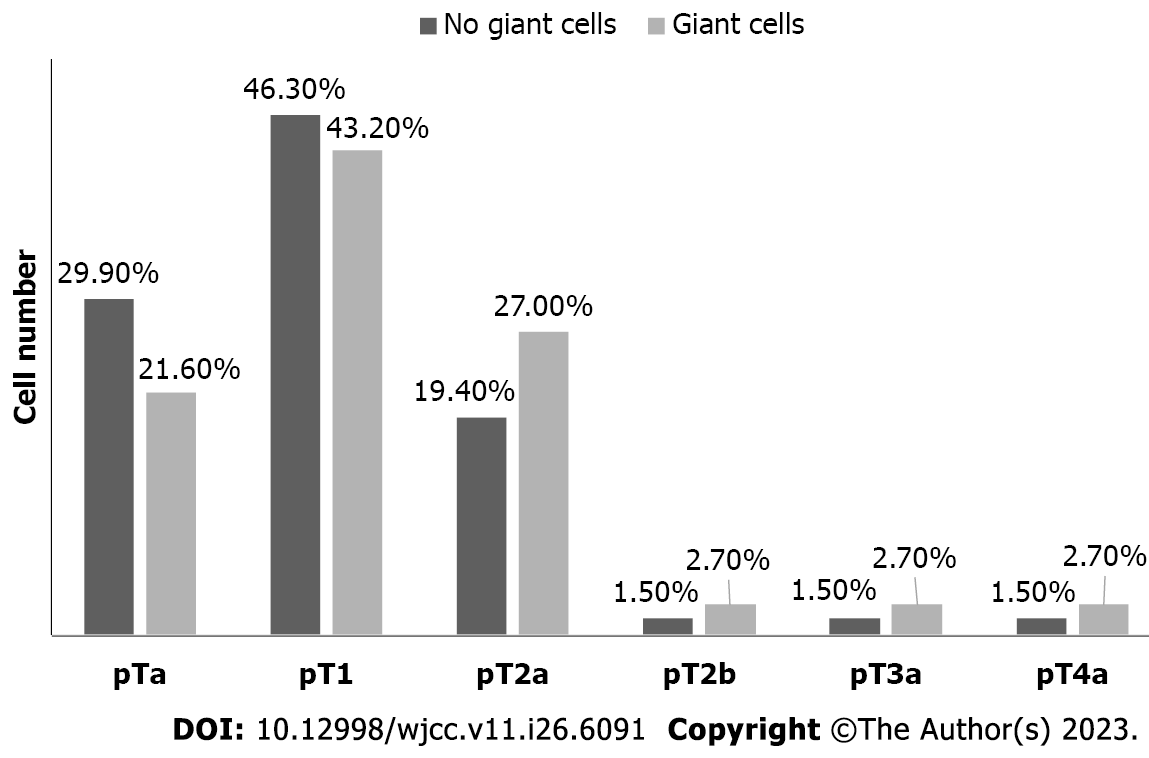
During the comparative analysis between the stage of UC of the bladder and the presence of MGCs in the stroma and the surrounding lamina propria, the following associations were established: (1) In pTa stage (non-invasive) of bladder UC, the presence of MGCs in the stroma was found in 8/37 cases (21.6%); (2) in pT1 stage of bladder UC, the presence of MGCs in the stroma was detected in 16/37 cases (43.2%); (3) in pT2a stage of bladder UC, the presence of MGCs in the stroma was detected in 10/37 cases (27.0%); (4) in pT2b stage of bladder UC, the presence of MGC in the stroma was observed only in 1/37 (2.7%) cases; (5) in stage pT3a of UC, the presence of MGCs in the stroma was detected only in 1/37 of cases (2.7%); and (6) in the pT4a stage of bladder UC, the presence of MGCs in the stroma was found in 1/37 cases (2.7%).
The descriptive analysis and chi-square (χ2) test showed no significant correlation between the stage of UC of the bladder and MGCs in its stroma (P = 0.874). However, there was a tendency for a progressive increase in the number of MGCs with an increasing degree of tumor invasion, peaking in the pT1 stage in the UC of the bladder. These considerations are graphically reflected in Figure 2.
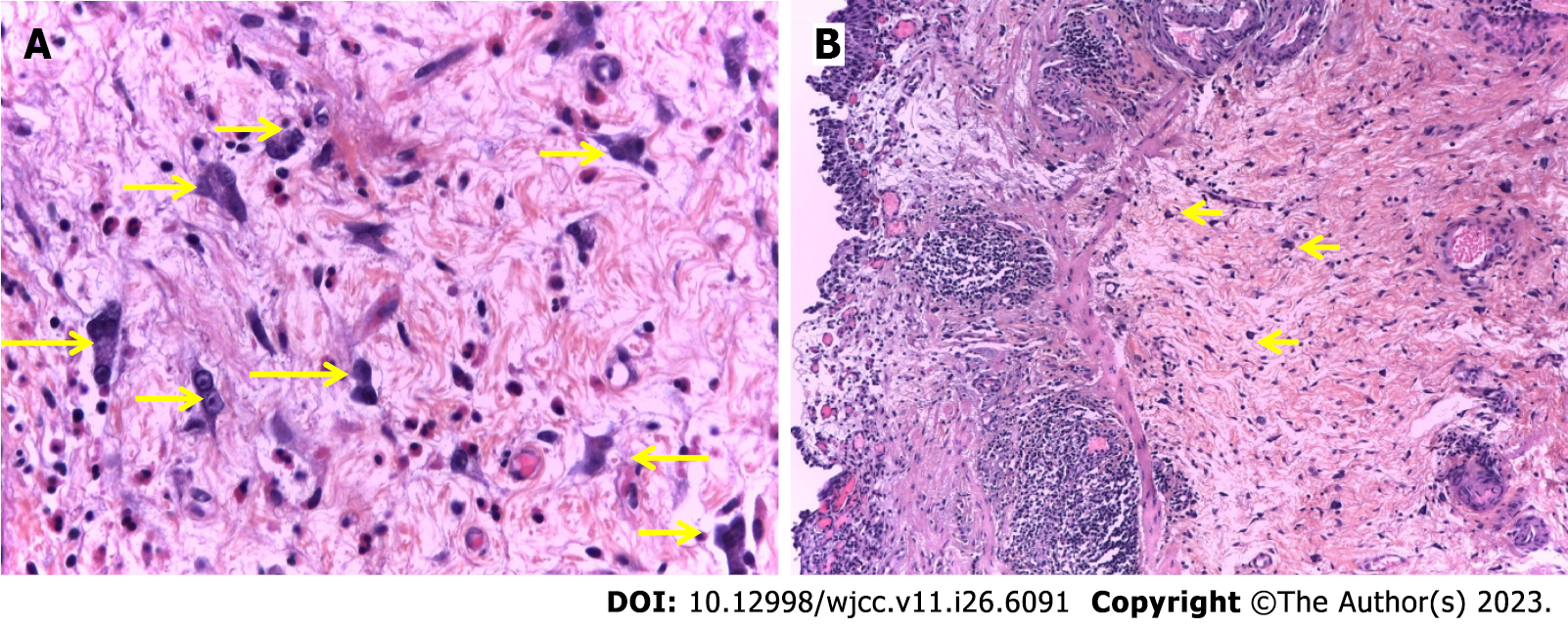
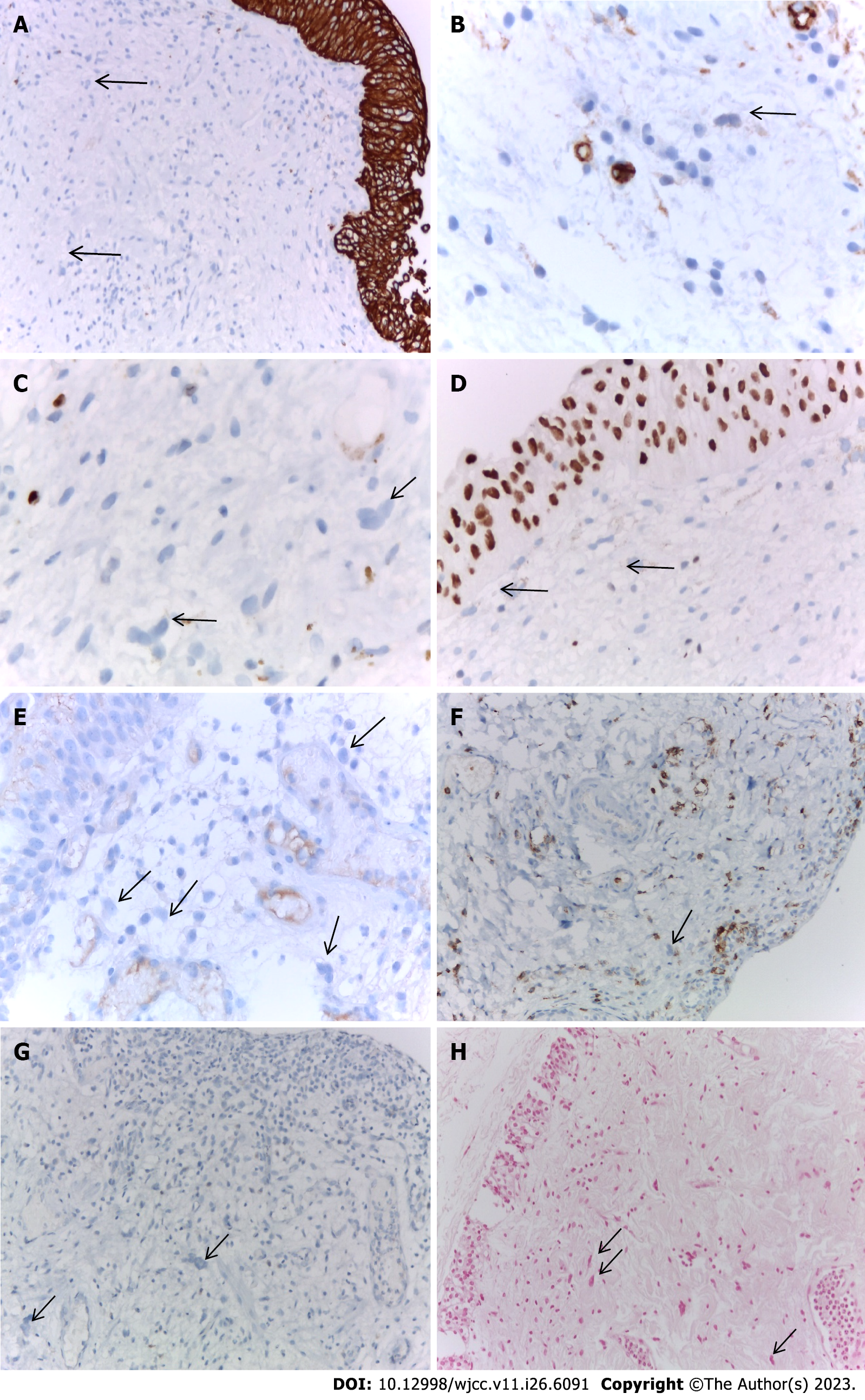
Conventional histological examination: Morphologically, on conventionally stained histological specimens, the giant cells in the stroma of the UC and the surrounding lamina propria were large-from 10 µm to 20 µm and star-shaped. Their cytoplasm was eosinophilic and sparse, with the presence of long cytoplasmic growths. The nuclei were rounded, hyperchromatic, and multilobulated, sometimes more or less atypical, but no mitotic figures were observed (Figure 3A). Thus, we have obtained the tissue context in which the MGCs were located in the stroma of the UC or the surrounding lamina propria-stromal fibrosis with congestion and edema and with more or less pronounced lymphoid inflammatory reaction-to the presence of tertiary lymphoid structures (Figure 3B).
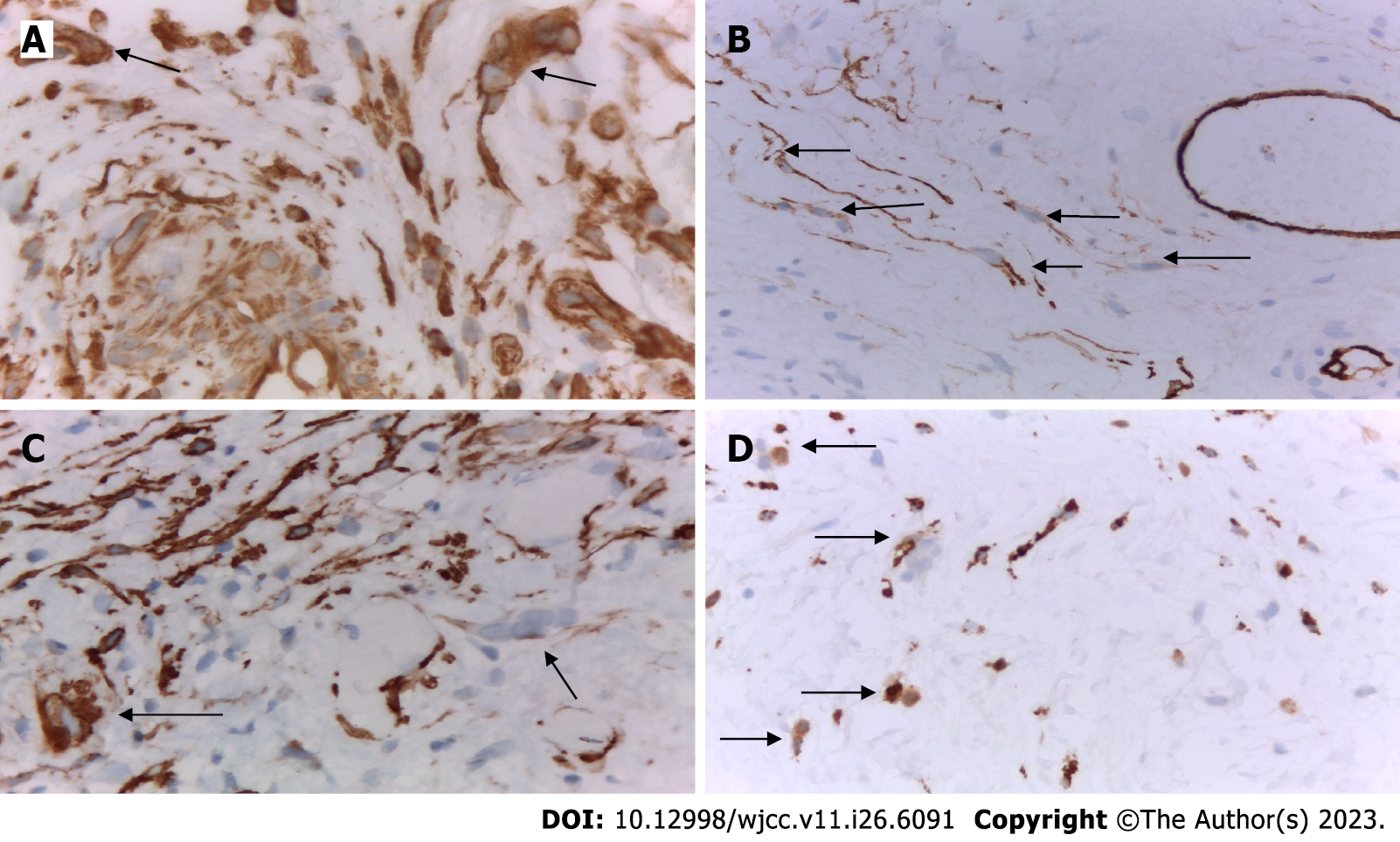
IHC examination: We performed an IHC examination of 37 cases with the presence of MGCs in the stroma of UC of the bladder. The obtained results were identical in all cases studied. The MGCs in the stroma and the surrounding mucosa in the UC of the bladder were positive for vimentin (Figure 4A). Cytoplasmic growths of MGCs in the bladder mucosa were positive for CD34 (Figure 4B). Some of the growths of MGCs in the bladder mucosa were positive for smooth muscle actin (Figure 4C). MGCs in the bladder mucosa were found positive for the macrophage marker CD68 (Figure 4D).
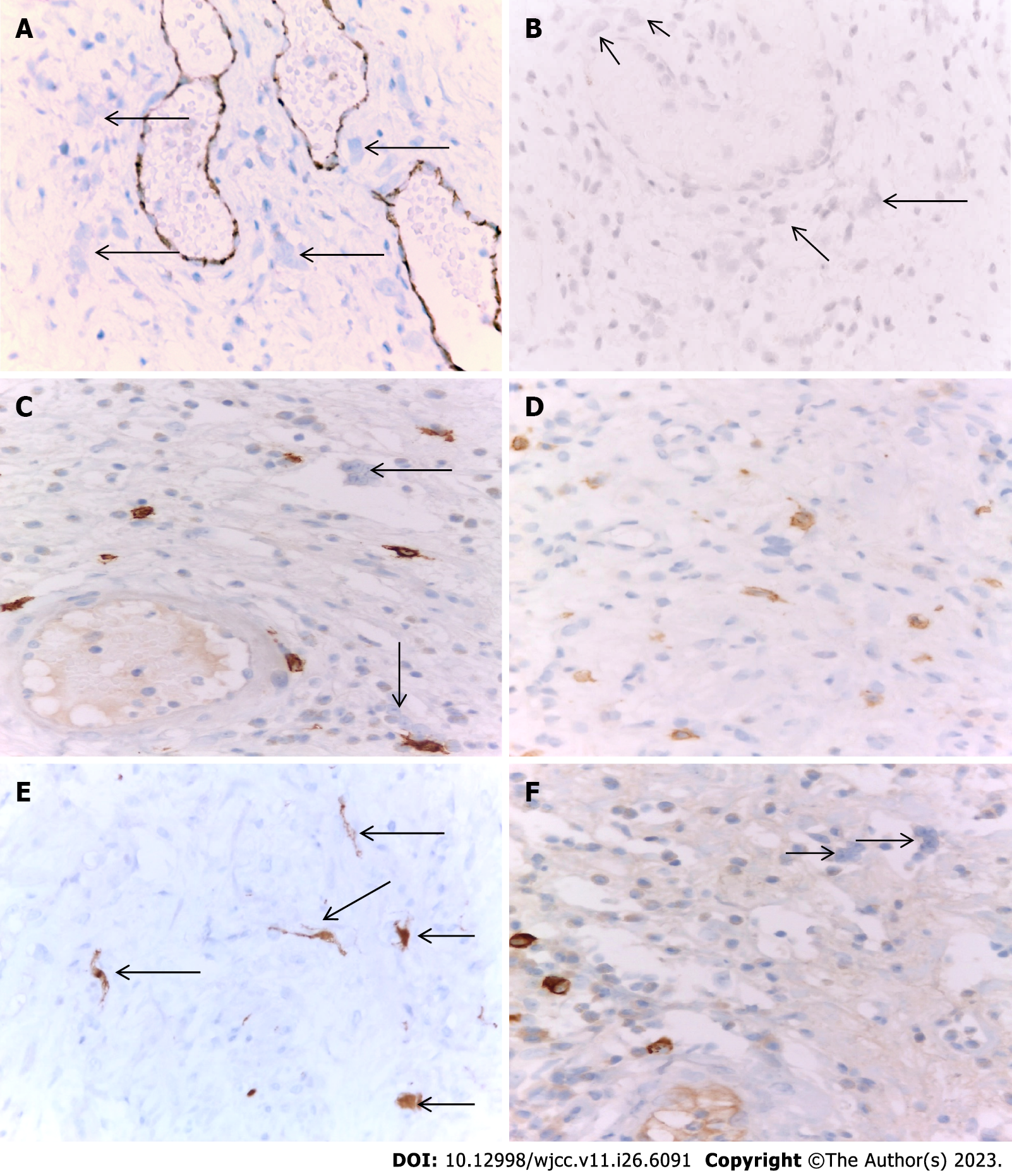
MGCs in the bladder mucosa were negative for the vascular endothelial marker CD31 (Figure 5A), the mesenchymal myofibroblast marker CD10 (Figure 5B), the mast cell, and neural marker C-KIT (Figure 5C). At the same time, it should be noted that in all the cases we have examined, the inflammatory infiltrate that accompanied giant cells was rich in mast cells and was found C-KIT-positive. This, in turn, was in favor of degranulated mast cells (Figure 5D); Giant cells were also positive for the stress marker p16 (Figure 5E). MGCs in the bladder mucosa were negative for the allergic marker immunoglobulin G4 (IG G4) (Figure 5F).
MGCs in the bladder mucosa are also negative for IHC markers: CKAE1/AE3, CD34-blast, Ki-67, GATA3, PD-L1, CD45, and CD1A, as well as for histochemical staining of Perls (Figure 6).
The results of our IHC examination are summarized in Table 2.
| IHC antibodies | Expression |
| VIMENTIN | + |
| SMA | + |
| DESMIN | + |
| CD34 | + |
| CD68 | + |
| P16 | + |
| CK AE1/AE3 | - |
| CD31 | - |
| PS100 | - |
| C-KIT (CD117) | - |
| CD34BLAST | - |
| KI-67 | - |
| IGG | - |
| PD-L1 | - |
| GATA3 | - |
| CD10 | - |
| CD45 | - |
| CD1A | - |
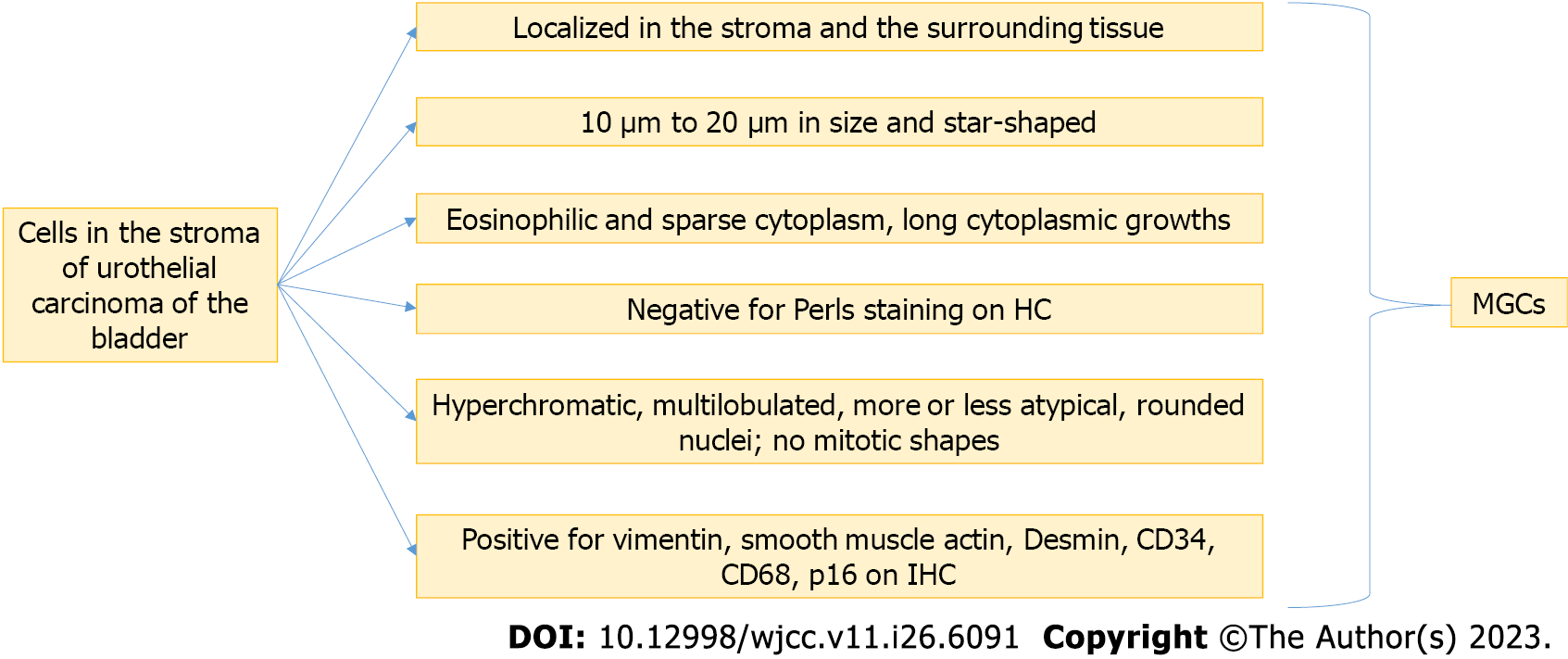
Based on the above results, we propose a generalized algorithm of histological, histochemical, and IHC-criteria for the diagnosis of MGCs observed in the stroma of UC of the bladder: MGCs, except in chronic cystitis and other bladder lesions, are localized in the stroma of the bladder carcinoma and the surrounding lamina propria. MGCs are 10 to 20 µm in size and star-shaped.
Their cytoplasm is eosinophilic and sparse, with the presence of long cytoplasmic growths.
The nuclei are rounded, hyperchromatic, and multilobulated, sometimes more or less atypical, but no mitotic figures are observed.
Histochemically, MGCs are negative for Perls staining. Immunohistochemically, MGCs are positive for mesenchymal and myofibroblast markers (vimentin, smooth muscle actin, Desmin, and CD34), for the macrophage marker CD68, and the marker of cell aging and degeneration p16.
This diagnostic algorithm is presented in Figure 7. To make a definite diagnosis, all of the criteria should be met.
Based on our results, the above diagnostic algorithm, we also propose an algorithm for the differential diagnosis of MGCs in the bladder’s UC. It should be done with MGCs in “specific” types of cystitis, e.g., in tuberculosis, in xanthogranulomatous cystitis, after bladder surgery, in the presence of surgical sutures or other foreign bodies; with MGCs in radiation cystitis; with multinucleated giant tumor cells in the stroma of the UC “in situ” or with invasive UC containing giant tumor cells; with MGCs especially those seen in mesenchymal tumors of the bladder; with MGCs observed in the stroma of some benign tumor processes of the female genital tract.
The presence of isolated giant cells is a rarely observed and poorly studied phenomenon in human pathology. Furthermore, very few articles have been published in the English-language literature related to describing these cells with different localizations, as those in the bladder are single.
To date, the literature lacks a detailed histo-epidemiological study of giant cells in the stroma of bladder UC. In our study, the quantitative results for the giant cells in the stroma of the UC and the surrounding lamina propria were obtained in 104 cases. Of the total number of patients samples (Bulgarian and French contingent), we found the presence of mono-, bi- and multinucleated giant fibroblast-like stromal cells in the stroma of the UC and the surrounding mucosal lamina propria in 37/104 (35.6%) of cases, while in 67/104 (64.4%) cases such cells were not detected. These results are similar to the results obtained by other authors and other types of material. Furthermore, it was demonstrated that stromal cell changes are nonspecific and occur in 1/3 of autopsy cases with chronic cystitis[6,10].
Using correlation analysis by the method of χ2 test in relation to the geographical origin of the studied materials, we found that in the Bulgarian samples of patients with bladder UC, the number of cases with the presence of giant stromal cells was higher than in the samples of French patients [25/37 (67.6%) vs 12/37 (32.4%)]. It is noteworthy that there was a difference in the presence of giant stromal cells in the bladder lamina propria in UC, according to the geographical origin of the material: giant stromal cells were more often presented in the stroma of UC of the bladder in the French patients-in 92.3% (12/13), compared to 27.5% (25/92) in the Bulgarian samples (P < 0.001).
To date, there is no histo-epidemiological study of giant cells in the bladder UC stroma in correlation with tumor grading in the literature. Our results showed a borderline statistically significant correlation (P = 0.056) between the presence of giant cells in the stroma of the bladder UC and tumor grading, according to the WHO, 1973. MGCs in the stroma of the bladder and the surrounding lamina propria, in the majority of cases, were found mostly in moderately and poorly differentiated UC (G2/G3, according to WHO, 1973).
Our results showed a correlation between the presence of MGCs in the stroma of the bladder UC with tumor grading, according to the WHO, 2016 (P = 0.056). MGCs in the stroma of the bladder and the surrounding lamina propria, in the majority of cases, were found in the UC with a high malignant potential of malignancy (HG) according to WHO, 2016.
There is also a strong correlation between tumor grades in both WHO 1973 and WHO 2016 classification systems (r = 0.809, P <0.001).
However, only one observational study demonstrated the presence of MGCs in the stroma of the bladder. Still, there was no quantitative statistical analysis in that study[16]. This study established that the amount of giant stromal cells in the bladder mucosa increased in intimate connection with the stromal inflammatory response and its intensity[16]. There is no histo-epidemiological study of the giant cells in the stroma of the bladder UC in correlation with the stage of the tumor in the TNM system in the literature.
Our results did not find a significant correlation between the stage of bladder UC and the presence of MGCs in its stroma (P = 0.874). However, there was a tendency for a progressive increase in the number of multinucleated giant stromal cells with an increasing degree of tumor invasion, with a peak in the pT1 stage. Ohtsuki et al[16], in the only publication on the presence of MGCs in the stroma of UC of the bladder to date, showed that the amount of giant stromal cells in the mucosa in case of UC of the bladder increased in parallel with the intensity of the stromal inflammatory response. However, the investigators did not perform a precise quantitative study and did not address the extent of tumor invasion[16].
Our results demonstrated that morphologically, on conventionally stained histological specimens, the MGCs in the stroma of the UC of the bladder and its surrounding lamina propria had large sizes-from 10 µm to 20 µm and were star-shaped. Their cytoplasm was eosinophilic and sparse, with the presence of long cytoplasmic growths. The nuclei were round, hyperchromic, and multilobular, sometimes more or less atypical, but without mitoses. According to the literature, in cases of bladder UC in which giant cells were found in the stroma, there was also a pronounced inflammatory stromal reaction[6-8,10]. In line with this, giant multinucleated stromal cells can be observed relatively frequently in bladder biopsies without an inflammatory context[8]. Still, they are most often associated with chronic inflammation. These stromal cell changes are nonspecific and occur in 1/3 of autopsy cases with chronic cystitis[10]. Similar giant stromal cell changes also occur under the influence of radiation or chemotherapy[6,11-13].
According to the literature, there is only one study with IHC examination of the MGC in the stroma of the UC[16]. From the only study so far it is known that the IHC profile of the giant stromal cells in the bladder mucosa in the urinary tract is: Vimentin/+/; MB 2/+/; Ki-67/-/; HLA-DR/-/[16]. The same authors added the following parameters, which are sometimes positive-smooth-muscle actin; CD6; and S-100 (+) (in individual cases)[16]. Ultrastructurally, giant stromal cells in the bladder mucosa in urinary UC showed superficial, focal aggregated short cytoplasmic growths[16]. The authors believe that giant stromal cells in the bladder mucosa are multipotent mesenchymal cells (CD34/+/; E-cadherin/+/) without mitotic activity (Ki-67/-/), having both fibroblast (Vimentin/+/) and histiocyte (CD68/+/) phenotype localized in the immediate vicinity of the tumor[16]. Besides, they conclude that the giant stromal cells in the bladder mucosa were most likely a result of the fusion of mononuclear stromal cells[16].
In a recently published clinical case of bladder leiomyosarcoma detected two months after histological verification as LG UC, Fiorentino et al[17] observed osteoclast-like giant multinucleated cells in the tumor stroma having the IHC phenotype of cells of non-tumor origin. The authors hypothesize that giant multinucleated cells are most likely to be regenerative mucosal muscle cells or stromal myofibroblasts. We confirm that MGCs in the mucosa in UC of the bladder were positive for both mesenchymal and myofibroblast markers (vimentin, smooth muscle actin, Desmin, and CD34) and the macrophage marker CD68. Similar results were obtained in the sole publication concerning giant cells in UC and a non-tumor context[16]. Besides, the results obtained by us complement the IHC profile of these cells. Furthermore, they were positive for the stress marker p16, which supports their degenerative nature. Giant cells were negative for epithelial (CKAE1/AE3 and GATA-3), vascular (CD31), neural (PS100 and C-KIT), cambial, blastic (CD34-blasts and C-KIT), and immune markers (IG G, IG G4 and PD-L1). They did not show proliferative activity, confirmed by the absence of mitoses and the negativity of the proliferative marker Ki-67. In addition, giant cells were PD-L1 negative, had no specific immune function, and could not be used to calculate the Combined Positive Score (CPS) scale.
Recognition and correct interpretation of MGCs are necessary and crucial for an accurate diagnosis. Misinterpretation of these abnormal cells as malignant can lead to misdiagnosis and potentially lead to inappropriate treatment. To date, there is no established and structured algorithm in the literature concerning the diagnosis and differential diagnosis of MGCs observed in the stroma of bladder UC.
Based on our results and data from the literature, we have developed and proposed an algorithm of histological, histochemical, and IHC criteria, detailed in the results section. In addition, based on our results, the above diagnostic algorithm, and the data in the literature, we also offer a generalized algorithm for the differential diagnosis of MGCs in bladder UC and benign or malignant lesions of other visceral organs.
Giant cell cystitis: MGCs in “specific” types of cystitis, e.g., in tuberculosis, in xanthogranulomatous cystitis, after bladder surgery presence of surgical sutures or other foreign bodies. In these cases, the cells have a macrophage genesis, and they are of the Langhans type, the Tuton type, or a foreign body type. They are always combined with granuloma-forming epithelioid cells with or without necrosis. For this reason, the term “giant cell cystitis”[8] is now considered an obsolete term[8].
Radiation cystitis: MGCs in radiation cystitis-the cells and their surrounding histiocytes contain very often thе cytoplasmic pigment hemosiderin, while in the stroma of the UC, the similar giant cells are negative for Perl’s staining.
Mesenchymal tumors of the urinary bladder: It is necessary to make a differential diagnosis with mesenchymal tumors of the bladder when MGCs are numerous and in the form of small groups[8]. Unlike sarcomas and other mesenchymal neoplasms, giant multinucleated cells in the bladder mucosa have no mitotic activity, probably because they reflect degenerative cellular changes[6].
Female reproductive system: MGCs may be observed in the stroma of some benign tumor processes of the female genital tract, e.g., fibroepithelial vaginal polyp[8]. Still, there, of course, the localization is different. Nevertheless, from a general pathological perspective, our results and conclusions are fully comparable with previously reported data from other research teams concerning morphologically similar cells in other visceral organs.
Breast: Rosen PP first described МGCs in breast tissue in 1979[18]. He found that the presence of MGCs did not pose a risk factor for malignant degeneration of the lesion. Similar findings were described by Heneghan et al[19] and Ryska et al[20] in breast fibroadenoma. Tissue foci containing these atypical giant cells were located in otherwise normal areas of the mammary gland. Therefore, Heneghan et al[19] conclude that these cells are most likely to arise due to a possible recovery process. Subsequently, atypical giant cells have been described in several other breast lesions, raising an exciting differential diagnosis, mainly benign processes/Lesions, and sometimes connected with neoplastic diseases. However, the pathogenesis of giant cells in breast tissue remained unclear[19,21].
Digestive tract: Sachak et al[22] make a morphological and IHC study for the first time to evaluate giant stromal cells in the gastrointestinal tract. They found that giant stromal cells result from a reactive/regenerative process, given that they are most commonly seen in reflux and chemical/reactive gastropathy. Therefore, the authors hypothesize that MGCs are most likely to be regenerative mucosal muscle cells or stromal myofibroblasts[22].
Soft tissues: The presence of MGCs in other benign tumors, including pleomorphic lipomas, leiomyomas and fibroma, schwannomas, and in some variants of dermatofibromas with atypical cells, have also been described[23].
To date, in the literature, the morphogenesis of giant cells in the bladder mucosa has been poorly studied. Epstein et al[6] believe that MGCs in the bladder mucosa represent degenerative cellular changes[22]. Ohtsuki et al[16] believe that MGCs in the vesical mucosa result from the fusion of mononuclear stromal cells. This hypothesis is also confirmed by the only ultrastructural study to date, which shows that multinucleated cells result from the fusion of mononuclear cells under conditions of chronic inflammation or irritation[16,24].
Our results support the opinions of the authors cited above. Like the other investigators who studied giant stromal cells in non-tumor and tumor bladder, we believe that MGCs in the bladder mucosa in UC are pluripotent cells with mesenchymal, myofibroblast, and histiocyte origin at the same time. Our IHC results also showed that the giant cells in the bladder UC have no hematogenous origin (CD45/-/), and no analogy should be made with morphologically similar dermal dendrocytes. We also found that the giant cells we studied in bladder UC were not similar to the Langerhans dendritic histiocytes known from the skin and lymph nodes (PS100 /-/ and CD1A /-/).
Besides, the IHC studies revealed a simultaneous expression of the marker of cellular aging and p16 degeneration, which supports theory of Epstein et al[6] for a degenerative cellular phenomenon. It is known that in addition to being an IHC marker of human papillomavirus infection, p16 is an IHC indicator of cellular stress and aging[25]. The use of p16 in clinical practice as a marker of biological aging can provide a quantitative measurement of chemotherapeutic tolerance and the “suitability” of the patient’s immune system.
According to the literature, the latter has been used as an assessment prior to organ transplantation to identify biologically “younger” donor tissues with increased potential for successful outcomes and survival[25]. LaPak and colleagues pointed out several pieces of evidence that p16 is not only a biomarker of aging but also a cause of aging in many cell types[25]. Using p16-transgenes mice, the cell-autonomous role of p16 in cellular aging was demonstrated. In the mouse hematopoietic stem cells, T cells, pancreatic β-cells, and neural progenitors of the subventricular area, p16 expression denotes a decrease in regenerative capacity.
These data also support the hypothesis that p16 expression denotes cellular aging, which leads to a decrease in regenerative potential[25]. Since the discovery of p16 more than 20 years ago, numerous scientific studies on its regulation and function have proven its complex nature. The role of p16 extends beyond cell “aging” and tumor pathology[25,26].
Induction of p16 during these highly proliferative processes is thought to be crucial for maintaining proper tissue homeostasis. However, it is not yet clear whether the same signals that trigger p16 expression under physiological conditions play a role in the process of tumorigenesis or aging[25].
For the first time, we showed the expression of p16 in giant stromal cells outside the context of granulomatous inflammation. This phenomenon’s probable explanation is the persistent mucosal irritation leading to this protein’s expression in an attempt to find an optimal thermodynamically and biochemically metabolic formula for stromal cells “exhausted” by chronic cellular stress. Similar “dynamic induction” in p16 expression is observed in other processes and other organs: involution of the glandular parenchyma of the breast, wound healing, nerve regeneration, and chronic inflammation[24,25].
From a pathogenetic point of view, we believe that giant stromal cells in non-tumor and tumor bladder represent a degenerative cellular phenomenon reflecting chronic mucosal bladder irritation (mechanical, chemical, or tumor in nature) and chronic mucosal inflammation. Moreover, we observed a significant correlation between these cells’ presence and the degree of tissue damage (tissue stress). From an oncological perspective, our results show that giant stromal cells in bladder UC are part of the stromal tumor response, although nonspecific and non-immune. This giant cell reaction is probably due to chronic mucosal irritation. It correlates with malignancy and the degree of tumor infiltration. In addition to the above considerations, the positive expression of p16 observed by us is further evidence of the histiocytic nature of the giant cells in the stroma of the bladder UC. In support of this, it is known from the literature that histiocytes and their derived tumor lesions are p16 positive.
Our results also confirm the conclusion given by Cretoiu et al[4] that telocytes, depending on their location, may exhibit different immunohistochemical properties depending on the location and the role that these cells may play, some of which may be common to the various organs and others to be specific to a given organ, such as the bladder. As for the immediate morphogenetic mechanism of this cellular phenomenon, we believe, like other authors, that it is a matter of fusion of mononuclear histiocyte, fibroblast-like cells under conditions of chronic irritation. Thus, from the perspective of general pathology, the morphogenesis of giant stromal cells in bladder UC reflects the phenomenon of cell fusion under conditions of chronic tissue stress (chronic irritation or inflammation). Also, telocytes can exhibit different immunohistochemical properties and ultrastructural features and form contact with each other or with other cell types.
Taken together, our results showed that giant cells in the bladder stroma were found in 35.6% of cases, more often in HG (G2/G3, according to WHO 1973). In addition, we observed a tendency for a progressive increase in the number of giant cells with an increase in the degree of tumor invasion in the bladder wall. Morphologically, the giant cells in the UC of the bladder’s stroma were large, star-shaped, with sparse cytoplasm and the presence of long cytoplasmic growths. They showed both mesenchymal, myofibroblast, and macrophage immunophenotype. According to the markers, MGCs were cytokeratin negative, unlike giant tumor cells in bladder cancer; p16-positive; PD-L1 negative, with no specific immune function, and could not be used to calculate the CPS scale.
To sum up, our observations give us reasons to assume that the giant stromal cells in non-tumor and tumor bladder can be used as a characteristic and relatively constant, although nonspecific, a histological marker for chronic bladder damage. In addition, our current knowledge of MGCs allows the following conclusions about the role of that cells: According to the data obtained by us in the morphological and IHC study, MGCS are modified telocytes. The reason for this modification is the chronic damage to the bladder mucosa (regardless of the etiological factor), which leads to the fusion of telocytes and their transformation into multinucleated cells. At the same time, they lose C-KIT expression, penetrate phagocytic function, and begin to express p16, which is, as described in detail above, a sign of cellular aging.
To date, the literature lacks detailed histoepidemiological, morphological, and immunohistochemical (IHC) studies of the immunophenotype and morphogenesis of multinucleated giant cells (MGCs) in the mucosa and the stroma of the bladder. At the same time, in the non-tumor bladder, this question is insufficiently studied. These questions and difficulties, which pathologists, urologists, and oncologists are exposed to, motivated us to study these problems in more depth, using materials from two geographically different countries-Bulgaria and France.
We believe that it is more reasonable in the present study to determine the exact identification of these cells to use the terms: Mononuclear and MGCs present in the connective tissue of the bladder wall in neoplastic and inflammatory processes.
To establish the function, morphogenesis, and origin of mononuclear giant cells and MGCs in the stroma of urothelial carcinoma (UC) of the bladder in patients samples, as well as to compile a proper differential-diagnostic algorithm from histological, histochemical, and IHC criteria for the diagnosis of MGCs observed in the stroma of the UC of the bladder.
We analyzed retrospectively urothelial bladder carcinomas (n = 104) from 2016-2020 using IHC and histochemical stain examination. Giant cells in the bladder stroma were found in 35.6% of cases, more often in high-grades.
From a pathogenetic point of view, we believe that giant stromal cells in non-tumor and tumor bladder represent a degenerative cellular phenomenon reflecting chronic mucosal bladder irritation (mechanical, chemical, or tumor in nature) and chronic mucosal inflammation. Moreover, we observed a significant correlation between these cells’ presence and the degree of tissue damage (tissue stress). From an oncological perspective, our results show that giant stromal cells in bladder UC are part of the stromal tumor response, although nonspecific and non-immune. This giant cell reaction is probably due to chronic mucosal irritation. It correlates with malignancy and the degree of tumor infiltration. In addition to the above considerations, the positive expression of p16 observed by us is further evidence of the histiocytic nature of the giant cells in the stroma of the bladder UC. In support of this, it is known from the literature that histiocytes and their derived tumor lesions are p16 positive.
Based on the above results, we propose a generalized algorithm of histological, histochemical, and IHC-criteria for the diagnosis of MGCs observed in the stroma of UC of the bladder: MGCs, except in chronic cystitis and other bladder lesions, are localized in the stroma of the bladder carcinoma and the surrounding lamina propria. MGCs are 10 µm to 20 µm in size and star-shaped. Their cytoplasm is eosinophilic and sparse, with the presence of long cytoplasmic growths. The nuclei are rounded, hyperchromatic, and multilobulated, sometimes more or less atypical, but no mitotic figures are observed. Histochemically, MGCs are negative for Perls staining. Immunohistochemically, MGCs are positive for mesenchymal and myofibroblast markers (vimentin, smooth muscle actin, Desmin, and CD34), for the macrophage marker CD68, and the marker of cell aging and degeneration p16.
For the first time, we showed the expression of p16 in giant stromal cells outside the context of granulomatous inflammation. This phenomenon’s probable explanation is the persistent mucosal irritation leading to this protein’s expression in an attempt to find an optimal thermodynamically and biochemically metabolic formula for stromal cells “exhausted” by chronic cellular stress. Similar “dynamic induction” in p16 expression is observed in other processes and other organs: Involution of the glandular parenchyma of the breast, wound healing, nerve regeneration, and chronic inflammation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkhatib AJ, Jordan; Eccher A, Italy; Hasan A, Egypt S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Vannucchi MG, Traini C, Guasti D, Del Popolo G, Faussone-Pellegrini MS. Telocytes subtypes in human urinary bladder. J Cell Mol Med. 2014;18:2000-2008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Wiseman OJ, Brady CM, Hussain IF, Dasgupta P, Watt H, Fowler CJ, Landon DN. The ultrastructure of bladder lamina propria nerves in healthy subjects and patients with detrusor hyperreflexia. J Urol. 2002;168:2040-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Zheng Y, Zhu T, Lin M, Wu D, Wang X. Telocytes in the urinary system. J Transl Med. 2012;10:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Cretoiu D, Giuliana Vannucchi M, Bei Y, Manetti M, Faussone-Pellegrini MS, Ibba-Manneschi L. Telocytes: New Connecting Devices in the Stromal Space of Organs [Internet]. Innovations in Cell Research and Therapy. IntechOpen, 2020. [DOI] [Full Text] |
| 5. | Popescu LM, Faussone-Pellegrini MS. TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 455] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 6. | Epstein J, Amin M, Reuter V. Biopsy interpretation of the bladder. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2010: 355. |
| 7. | Eyden B. Letter to the Editor concerning: Multinucleated Giant Cells in Submucosal Layer of Human Urinary Bladder. Path Res and Practice. 2001;197:230. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Young RH. Nonneoplastic disorders of the urinary bladder. In: Bostwick D, Cheng L. Editor. Urologic Surgical Pathology. Philadelphia: Elsevier/Saunders, 2014: 194-227. [DOI] [Full Text] |
| 9. | Wells HG. Giant cells in cystitis. Arch Pathol Lab Med. 1938;26:32-43. [DOI] [Full Text] |
| 10. | Kuipers FC. Cystitis with giant cells. Ned Tijdschr Geneeskd. 1973;117:85-88. [PubMed] |
| 11. | Johnson WW, Meadows DC. Urinary-bladder fibrosis and telangiectasia associated with long-term cyclophosphamide therapy. N Engl J Med. 1971;284:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 112] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Beyer-Boon ME, de Voogt HJ, Schaberg A. The effects of cyclophosphamide treatment on the epithelium and stroma of the urinary bladder. Eur J Cancer (1965). 1978;14:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Millard RJ. Busulfan-induced hemorrhagic cystitis. Urology. 1981;18:143-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Parekh A, Das S, Parida S, Das CK, Dutta D, Mallick SK, Wu PH, Kumar BNP, Bharti R, Dey G, Banerjee K, Rajput S, Bharadwaj D, Pal I, Dey KK, Rajesh Y, Jena BC, Biswas A, Banik P, Pradhan AK, Das SK, Das AK, Dhara S, Fisher PB, Wirtz D, Mills GB, Mandal M. Multi-nucleated cells use ROS to induce breast cancer chemo-resistance in vitro and in vivo. Oncogene. 2018;37:4546-4561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Wijesinghe HD, Malalasekera A. Giant Cell Urothelial Carcinoma of Bladder. Case Rep Urol. 2021;2021:8021947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Ohtsuki Y, Furihata M, Iwata J, Takeuchi T, Sonobe H, Chen BK, Liang SB, Kuwahara M, Ochi K, Terao N. Multinucleated giant cells in submucosal layer of human urinary bladder: an immunohistochemical and electron microscopic study. Pathol Res Pract. 2000;196:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Fiorentino V, Pierconti F, Lenci N, Calicchia M, Palermo G, Bassi P, Larocca LM, Martini M. Urinary bladder leiomyosarcoma with osteoclast-like multinucleated giant cells: a case report. BMC Cancer. 2019;19:763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Rosen PP. Multinucleated mammary stromal giant cells: a benign lesion that simulates invasive carcinoma. Cancer. 1979;44:1305-1308. [PubMed] [DOI] [Full Text] |
| 19. | Heneghan HM, Martin ST, Casey M, Tobbia I, Benani F, Barry KM. A diagnostic dilemma in breast pathology--benign fibroadenoma with multinucleated stromal giant cells. Diagn Pathol. 2008;3:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Ryska A, Reynolds C, Keeney GL. Benign tumors of the breast with multinucleated stromal giant cells. Immunohistochemical analysis of six cases and review of the literature. Virchows Arch. 2001;439:768-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Milentijević MJ, Basić M, Petrović A. Multinucleated stromal giant cells in adenoid cystic carcinoma of the breast: a case report and literature review. Vojnosanit Pregl. 2011;68:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Sachak T, Frankel WL, Arnold CA, Chen W. Multinucleated stromal giant cells in the gastroesophageal junctional and gastric mucosa: a retrospective study. Diagn Pathol. 2019;14:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Chand K, Bhardwaj RK, Rappai TJ. Study of 7 Cases of Giant Cell Tumor of Soft Tissue. Med J Armed Forces India. 2006;62:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Holt DJ, Grainger DW. Multinucleated giant cells from fibroblast cultures. Biomaterials. 2011;32:3977-3987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res. 2014;12:167-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 26. | Hasan A, Mohammed Y, Basiony M, Hanbazazh M, Samman A, Abdelaleem MF, Nasr M, Abozeid H, Mohamed HI, Faisal M, Mohamed E, Ashmawy D, Tharwat M, Morsi DF, Farag AS, Ahmed EM, Aly NM, Abdel-Hamied HE, Salama DEA, Mandour E. Clinico-Pathological Features and Immunohistochemical Comparison of p16, p53, and Ki-67 Expression in Muscle-Invasive and Non-Muscle-Invasive Conventional Urothelial Bladder Carcinoma. Clin Pract. 2023;13:806-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |