Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5252
Peer-review started: April 20, 2023
First decision: June 7, 2023
Revised: June 10, 2023
Accepted: July 3, 2023
Article in press: July 3, 2023
Published online: August 6, 2023
Processing time: 104 Days and 12.6 Hours
It is common for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection to occur in the gastrointestinal tract, which can present itself as an initial symptom. The severity of coronavirus disease 2019 (COVID-19) is often reflected in the prevalence of gastrointestinal symptoms. COVID-19 can damage the nerve supply to the digestive system, leading to gastrointestinal autonomic dysfunction. There is still much to learn about how COVID-19 affects the autonomic nervous system and the gastrointestinal tract.
To thoroughly explore the epidemiology and clinical aspects of COVID-19-induced gastrointestinal autonomic dysfunction, including its manifestations, potential mechanisms, diagnosis, differential diagnosis, impact on quality of life, prognosis, and management and prevention strategies.
We conducted a thorough systematic search across various databases and performed an extensive literature review. Our review encompassed 113 studies published in English from January 2000 to April 18, 2023.
According to most of the literature, gastrointestinal autonomic dysfunction can seriously affect a patient's quality of life and ultimate prognosis. Numerous factors can influence gastrointestinal autonomic nervous functions. Studies have shown that SARS-CoV-2 has a well-documented affinity for both neural and gastrointestinal tissues, and the virus can produce various gastrointestinal symptoms by reaching neural tissues through different pathways. These symptoms include anorexia, dysgeusia, heartburn, belching, chest pain, regurgitation, vomiting, epigastric burn, diarrhea, abdominal pain, bloating, irregular bowel movements, and constipation. Diarrhea is the most prevalent symptom, followed by anorexia, nausea, vomiting, and abdominal pain. Although COVID-19 vaccination may rarely induce autonomic dysfunction and gastrointestinal symptoms, COVID-19-induced autonomic effects significantly impact the patient's condition, general health, prognosis, and quality of life. Early diagnosis and proper recognition are crucial for improving outcomes. It is important to consider the differential diagnosis, as these symptoms may be induced by diseases other than COVID-19-induced autonomic dysfunction. Treating this dysfunction can be a challenging task.
To ensure the best possible outcomes for COVID-19 patients, it is essential to take a multidisciplinary approach involving providing supportive care, treating the underlying infection, managing dysfunction, monitoring for complications, and offering nutritional support. Close monitoring of the patient's condition is crucial, and prompt intervention should be taken if necessary. Furthermore, conducting thorough research on the gastrointestinal autonomic dysfunction caused by COVID-19 is vital to manage it effectively.
Core Tip: As a systemic disease, coronavirus disease 2019 (COVID-19) can impact various organs in the human body, including the autonomic nervous system and gastrointestinal tract. Our team conducted a systematic review to better understand the clinical range of COVID-19's impact on gastrointestinal autonomic dysfunction. We examined the clinical manifestations, potential mechanisms, diagnosis, differential diagnosis, effects on quality of life, prognosis, management, and prevention of COVID-19-induced gastrointestinal autonomic dysfunction. Most of the literature suggests that gastrointestinal autonomic dysfunction can be severe and negatively impact a patient's quality of life and prognosis. As a result, a multidisciplinary approach is needed to manage this dysfunction. However, further research is necessary to study COVID-19-induced gastrointestinal autonomic dysfunction effectively.
- Citation: Elbeltagi R, Al-Beltagi M, Saeed NK, Bediwy AS. COVID-19-induced gastrointestinal autonomic dysfunction: A systematic review. World J Clin Cases 2023; 11(22): 5252-5272
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5252.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5252
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first officially described in Wuhan, China. This virus has quickly spread worldwide and caused a pandemic known as coronavirus disease 2019 (COVID-19). The pandemic has presented humanity with numerous challenges, including the rapid transmission rate of the virus, variations in clinical presentations, fast mutation rates, differing laboratory diagnosis guidelines, and various disease management methods[1]. Although the SARS-CoV-2 primarily affects the respiratory system, it can impact any organ or system in the human body[2]. Since its emergence at the end of 2019, COVID-19 has presented in unusual and atypical ways worldwide, with a surge in unfamiliar symptoms and manifestations. This suggests that the pandemic may reshape how diseases present themselves, either by generating new types of illnesses or altering the clinical severity and manifestations of existing ones. These atypical presentations of COVID-19 further complicate the burden imposed by the pandemic, making diagnosis and treatment difficult and increasing the risk of disease transmission[3].
It is common for SARS-CoV-2 to infect the gastrointestinal tract, which can lead to initial symptoms, particularly in children[1]. This virus can cause inflammation and damage to the lining mucosa of the intestine by infecting gastrointestinal tract cells. Studies have found that up to 50% of patients experience gastrointestinal symptoms, such as lack of appetite, nausea, vomiting, abdominal pain, and other enteric symptoms[4,5]. Additionally, the severity of the disease often correlates with the frequency of gastrointestinal symptoms[1]. Current research indicates that the gastrointestinal tract can impact the progression of COVID-19, as demonstrated by gastrointestinal symptoms before respiratory symptoms and the persistence of viral shedding from the gut in severe cases with an aggressive clinical course[6,7].
Autonomic dysfunction is a disruption of the autonomic nervous system that can cause gastrointestinal issues due to damage to the nerve supply in the digestive system. This can be caused by various conditions, including viral infections, impacting appetite, digestion, bowel movements, and sexual function. The dysfunction may occur directly due to the underlying disorder's impact on both the autonomic nerve and the gut or indirectly as a delayed consequence of autonomic dysfunction in the gut[8,9]. COVID-19 is a systemic disease that can affect the autonomic nervous system, which supplies the gut, leading to gastrointestinal symptoms[10]. While there are many hypotheses about the mechanism behind this dysfunction, we still need to understand how COVID-19 alters the autonomic nervous system and gastrointestinal tract during long COVID-19.
This systematic review aims to gather and analyze the current evidence regarding the impact of COVID-19 on the gastrointestinal autonomic system. This newly emerged condition has become a concern during the pandemic, and we will examine its epidemiology, clinical manifestations, potential mechanisms, diagnosis, differential diagnosis, effects on quality of life and prognosis, as well as management and prevention.
To understand our objective thoroughly, we systematically reviewed the relevant literature using the "Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)" guidelines. We searched multiple electronic databases, including PubMed, PubMed Central, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, Embase, Library and Information Science Abstracts, Google Scholar, Scopus, and the National Library of Medicine catalog up until April 18, 2023. Our search keywords included COVID-19, SARS-CoV-2, Gastrointestinal Autonomic Dysfunction, Long COVID, Post-COVID, and Autonomic Nervous System. We limited our search to studies published in English or with an abstract in English. Our search strategy included both "free-full text terms" and "Medical Subject Headings (MeSH) terms", tailored for each database. We considered studies that discussed patients with COVID-19 and gastrointestinal dysfunction with or without autonomic dysfunction, as well as studies on COVID-19 vaccination and its potential side effects on the autonomic nervous and gastrointestinal systems. We included individuals of all ages, sexes, and ethnicities.
We identified 1453 articles, 137 of which were excluded due to duplication. Upon screening the titles and abstracts, we excluded 971 articles. Out of the remaining 345 full-text articles, only 113 met the eligibility criteria. Our study included 33 full-text research articles, two clinical guidelines and consensus, one meta-analysis, three systematic reviews, 59 reviews, five letters to the editor, and 11 case reports. Figure 1 shows the study flow chart. We also conducted citation searches and reviewed articles available as abstracts only. However, articles with a commercial background were excluded. We extracted data from the qualified studies using a standard form and assessed bias using the "Cochrane Risk of Bias tool." Publication bias was also evaluated using "funnel plots and Egger's test."
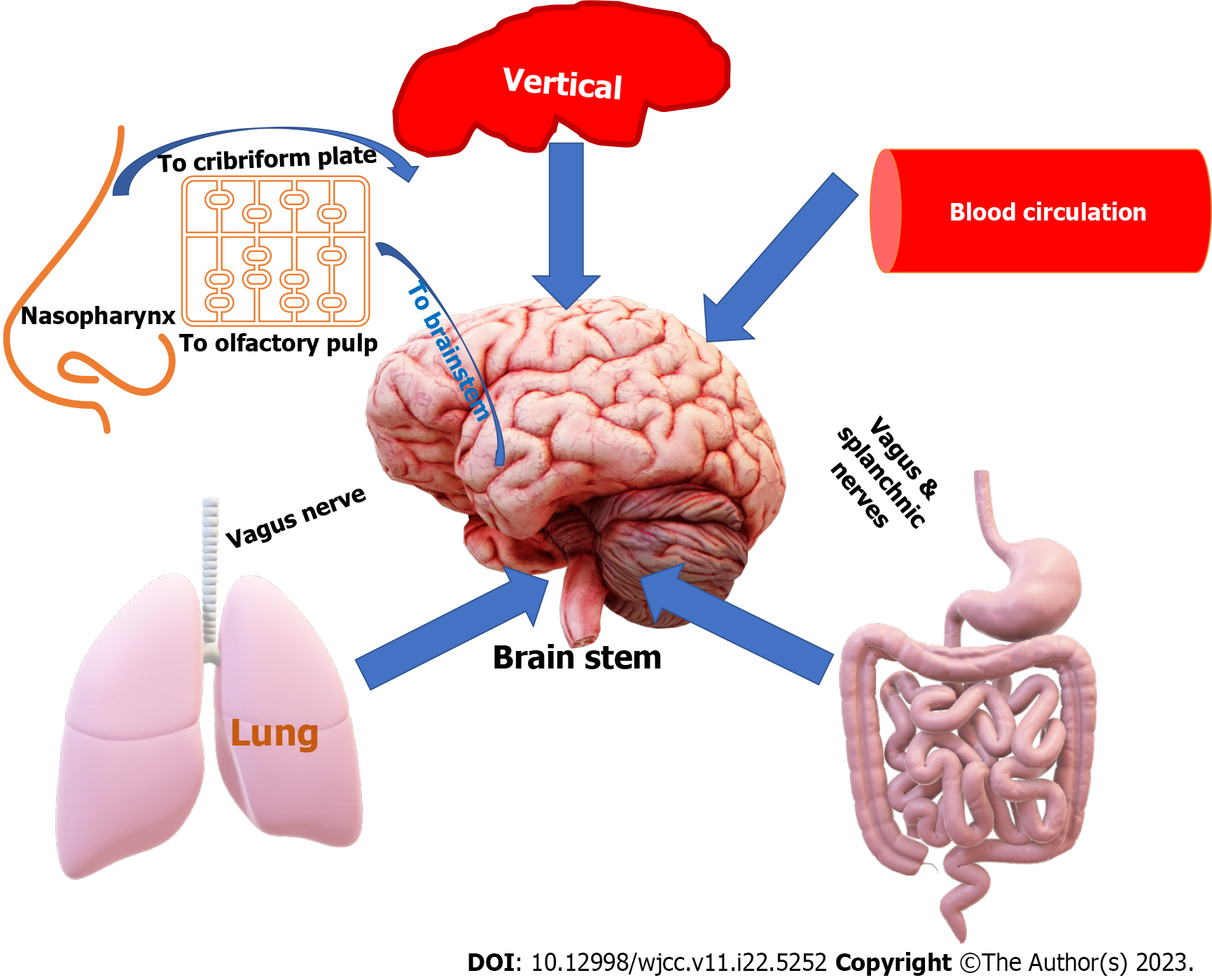
Based on our analysis of the studies provided, we discovered that although gastrointestinal autonomic dysfunction is a rare complication of COVID-19, it can significantly impact the patient's quality of life and prognosis. Table 1 outlines various factors that could affect gastrointestinal autonomic nervous functions. Most studies agree that SARS-CoV-2 has a documented affinity for neural and gastrointestinal tissues and can reach the neural tissues through different pathways, as shown in Figure 2. Patients with COVID-19 may experience different gastrointestinal symptoms, as summarized in Table 2. We have included four studies on the prevalence of gastrointestinal autonomic dysfunction, detailed in Table 3, along with the risks of developing these autonomic manifestations in COVID-19, as shown in Table 4. Table 5 and Figure 3 provide information on the prevalence and underlying mechanisms of common gastrointestinal autonomic dysfunction in patients with COVID-19. It is important to note that vaccination against COVID-19 may induce autonomic dysfunction, including gastrointestinal symptoms, in rare but significant situations, as evidenced by the nine studies outlined in Table 6. COVID-19-induced autonomic effects can significantly impact the patient's condition, general health, prognosis, and quality of life, making early recognition and diagnosis crucial for a positive outcome. Differential diagnosis should also be considered, as detailed in Table 7, as other diseases could induce these manifestations. Treating autonomic dysfunction can be a challenge, as highlighted in Table 8, which provides a simplified description of various treatment modalities.
| 1 Psychological factors: Vigilance, stress, anxiety, and depression |
| 2 Diet: A high-fat or protein diet slows digestion, while a high-fiber diet stimulates peristalsis |
| 3 Exercise training: Moderate exercise can increase the motility and blood flow of the gastrointestinal tract. In contrast, prolonged or intense exercise can reduce blood flow to the gastrointestinal tract and shift blood flow to the muscles |
| 4 Hormones: Cholecystokinin, gastrin, and secretin |
| 5 Toxins: Such as toxic heavy metals (e.g., mercury or lead), alcohol, and industrial chemicals |
| 6 Aging: Age-related neuronal loss in both intestinal myenteric and submucosal plexuses |
| 7 Neurological disorders: Parkinson's disease, multiple sclerosis, amyloidosis, autonomic neuropathy, multiple system atrophy, amyotrophic lateral sclerosis, and autonomic dysreflexia such as in spinal cord injuries |
| 8 Chronic metabolic disorders: Such as diabetes mellitus, hypothyroidism (hypothyroid gastroparesis and constipation), Addison's Disease (constipation, bloating, and abdominal discomfort), chronic kidney disease, chronic liver disease, Celiac disease, and inflammatory bowel disease |
| 9 Infections: Viral gastroenteritis, bacterial infections (diphtheritic polyneuropathy, tetanus, and botulism), COVID-19, acute tick-borne encephalitis virus infections, Lyme disease, Chagas disease, human rabies, Guillain-Barre syndrome, and HIV/AIDS |
| Part of the digestive system | Symptoms |
| General symptoms | Anorexia (22.3%), early satiety |
| Oral cavity | Dysgeusia (early symptom 71% to 88.8%) |
| Esophagus | Heartburn, belching, chest pain, regurgitation, vomiting |
| Gastroduodenal tract | Epigastric burn, nausea, vomiting, peptic ulcer, bleeding |
| Pancreas | Elevated lipase, acute pancreatitis |
| Liver & biliary tract | Elevated liver enzymes, jaundice, acute cholecystitis |
| Lower gastrointestinal tract | Diarrhea (most common, 3% to 96%), abdominal pain (6.2%), bloating, irregular bowel movements, bleeding, ischemic colitis, constipation, intussusception |
| Ref. | Type of Study | Number of patients | GIT manifestation(s) | Number & percentage | |
| Patients | Controls | ||||
| Erdal et al[57], 2022 | Cross-sectional | 112 | 106 | Diarrhoea | 24 (21.8) |
| Varma-Doyle et al[58], 2023 | Retrospective | 6 (4 with previous dysautonomia and 2 with new onsets) | - | Nausea, vomiting, early satiety, bloating, irregular bowel movements, constipation | 5 (83) |
| Scala et al[59], 2022 | Observational, cross-sectional | 38 | 38 | Diarrhoea | 10 (26.3) |
| Tan et al[60], 2020 | Retrospective study | 69 (without ACEI/ARB) | 31 (ACEI/ARB) | Diarrhea (6.5% vs 14.5%), nausea and vomiting (9.7% vs 11.6%), abdominal pain (2.9% vs 6.5%) | 40 (58) |
| 1 Severe disease |
| 2 Presence of hyperinflammation and hypoxia |
| 3 Longer duration of disease |
| 4 Old age |
| 5 Down-regulation of the IKBKAP gene |
| 6 Up-regulation of N-type calcium channels |
| 7 Presence of smell loss or dyspnoea |
| 8 Presence of comorbidities characterized by high sympathetic activity, such as diabetes mellitus and hypertension |
| Symptom(s) | Incidence | Underlying mechanism |
| Nausea and/or vomiting | Nausea: 1.0%-12.5%, vomiting 1.0%-27.5% | Release of neuroactive agents from enteroendocrine cells and inflammatory mediations → abdominal vagal nerve stimulation of dorsal medulla → projection of information to higher brain regions → nausea and vomiting |
| Diarrhea | 2% and 50% | Targeting intestinal ACE2 by the virus → cytokine storms, increased intestinal barrier permeability, and gut dysbiosis → diarrhoea. Hepatic and pancreatic injuries may also cause diarrhea. Antibiotic-induced iatrogenic diarrhea caused by activating Clostridium spp. should also be considered |
| Anorexia | Up to 40% | Social pressure. Neuromodulation → miscommunication between brain-gut-adipose tissues → changes in brain serotonin and tryptophan concentrations → anorexia, anosmia, and related odor perception impairment → development and aggravation of anorexia |
| Abdominal pain | 6.0% (vary depending on the population) | Inflammation-induced release of many cytokines and chemokines → activating pain-sensing neurons. ↑ Eosinophils → ↑eosinophil-derived neurotoxins → abdominal pain. Intestinal inflammatory infiltration → ↑ intestinal mucosal permeability and the direct effect of viruses can aggravate dysbiosis and cause changes in. Tryptophan metabolism → initiating peristaltic and secretory reflexes in the viscera and exacerbating inflammatory bowel disease symptoms, including abdominal pain. COVID-19 infection → ↓Na, K, Ca, and Mg → abdominal pain |
| Acid reflux | 1.1% | Increased serotonin levels. Esophageal mucosal barrier damage caused by cytokine storms. Relaxation of the lower esophageal sphincter. Gastric and duodenal dysfunction leading to obstruction of gastric emptying |
| Gastrointestinal bleeding | 1%-4.5% | Peptic and rectal ulcers. Impaired gastrointestinal mucosa integrity. Treatment-related (secondary bleeding) |
| Intestinal ischemia injury | Not well established | Venous thrombosis, pulmonary embolism, and mesenteric ischemia |
| Ref. | Type of Study | Country | Type of vaccine | Time-interval | Number of patients | Age | Sex | Main presentation | GIT manifestations |
| Hermel et al[79], 2022 | Case report | United States | Pfizer-BioNTech | 2 wk | 1 | 46 | F | POTS | Elevated AST |
| Reddy et al[80], 2021 | Case report | United States | Pfizer-BioNTech | 6 d | 1 | 42 | M | POTS | His symptoms increased after heavy meals |
| Lanman et al[81], 2022 | Case report | United States | Pfizer/BioNTech mRNA-BNT162b2 | 3 d (1st dose) | 1 | 58 | F | GBS | Constipation |
| Eldokla et al[82], 2022 | Case report | United States | Moderna (1) Pfizer/BioNTech (4) | 1-3 wk | 5 | 17- 46 | F | POTS | Dry mouth (1), constipation (1), nausea (2), bloating (1) |
| Tahir et al[83], 2021 | Case report | United States | Johnson & Johnson/Janssen | 10 d | 1 | 44 | F | Transverse myelitis & Bell's palsy | Nausea |
| Hilts et al[84], 2022 | Case report | United States | Moderna | 3 d | 1 | 58 | M | GBS | Diarrhoea (stopped before symptoms) |
| Fitzsimmons et al[85],2021 | Case report | United States | Moderna | 1 d (2nd dose) | 1 | 63 | M | Transverse myelitis | Constipation |
| Akaishi et al[86], 2022 | Retrospective cohort study | Japan | Moderna | 131544 | ≥ 16 | M/F 1.2:1 | Variable | Nausea (0.27%); abdominal pain (0.006%) | |
| Antonelli et al[87], 2022 | Prospective community-based case-control | United Kingdom | BNT162b2, ChAdOx1 nCoV-19, mRNA-1273 | 1-2 wk | 9462 | > 18 Mean age: 52.9 years | Variable | Abdominal pain, nausea, diarrhoea |
| Condition | Comments | |
| Gastrointestinal disorders | Gastrointestinal dysmotility | Gastrointestinal dysmotility refers to abnormal movement in the digestive system that can cause symptoms such as nausea, vomiting, abdominal pain, and bloating. COVID-19-induced gastrointestinal autonomic dysfunction can cause similar symptoms, making it essential to distinguish between the two |
| Gastroesophageal Reflux disease | Gastroesophageal reflux disease is a common condition that occurs when stomach acid flows back into the esophagus, causing heartburn and other symptoms. It can be diagnosed by an upper endoscopy or an esophageal pH test | |
| Gastroparesis | Gastroparesis is a disorder in which the stomach takes too long to empty its contents, causing symptoms such as nausea, vomiting, and bloating | |
| Gastritis | This condition is inflammation of the gastric mucosa. It can cause abdominal pain, nausea, and vomiting | |
| Peptic ulcer disease | This condition is characterized by sores in the lining of the stomach or duodenum and can cause abdominal pain and vomiting | |
| Gastrointestinal infections | Viral, bacterial, or parasitic infections can cause similar symptoms to COVID-19-induced gastrointestinal autonomic disorders and may require specific testing and treatment. For example: Other viral infections, such as norovirus, adenovirus, and rotavirus, can also cause gastrointestinal symptoms like COVID-19-induced gastrointestinal autonomic disorders. Considering these infections in the differential diagnosis is important, especially if the patient has a recent travel history or exposure to infected individuals. Some bacterial infections, such as Clostridium difficile infection, can cause gastrointestinal symptoms like those of COVID-19. Clostridium difficile infection can also cause autonomic dysfunction, leading to symptoms such as dizziness and fainting. Other bacteria like Salmonella, Shigella, Campylobacter, and Escherichia coli can produce gastrointestinal symptoms. Parasitic infections such as infection with Giardia lamblia, Entamoeba histolytica, and Cryptosporidium can cause diarrhea and other gastrointestinal symptoms | |
| Acute pancreatitis | This condition can cause abdominal pain, vomiting, and diarrhea, which may be like COVID-19-induced gastrointestinal autonomic dysfunction. Blood tests and imaging studies can help diagnose pancreatitis | |
| Gallstones | Gallstones can cause abdominal pain, nausea, and vomiting | |
| Cholecystitis | It is gallbladder inflammation, which can cause symptoms such as abdominal pain, nausea, vomiting, and fever | |
| Inflammatory bowel disease | Crohn's disease and ulcerative colitis can cause diarrhea, abdominal pain, and vomiting, which may be like COVID-19-induced gastrointestinal autonomic dysfunction. Endoscopy and stool tests can help to distinguish inflammatory bowel disease from other causes | |
| Irritable bowel syndrome | Irritable bowel syndrome is a chronic condition that causes abdominal pain, bloating, and changes in bowel habits. These symptoms may be similar to COVID-19-induced gastrointestinal autonomic dysfunction, but irritable bowel syndrome is a non-inflammatory condition | |
| Post-infectious irritable bowel syndrome | Post-infectious irritable bowel syndrome is a subtype of irritable bowel syndrome that occurs following an infection. This condition can cause similar symptoms to COVID-19-induced gastrointestinal autonomic dysfunction | |
| Celiac disease | Celiac disease is an autoimmune disorder that affects the small intestine, causing symptoms such as diarrhea, abdominal pain, and bloating | |
| Small intestinal bacterial overgrowth | Small intestinal bacterial overgrowth is a condition in which there is an overgrowth of bacteria in the small intestine, leading to symptoms such as diarrhea, bloating, and abdominal pain. It can be diagnosed through a breath test and a physical exam | |
| Gastrointestinal malignancies | Gastrointestinal malignancies such as colon cancer, gastric cancer, or pancreatic cancer, can cause abdominal pain, bloating, nausea, vomiting, and changes in bowel habits | |
| Food-related disorders | Food poisoning | Food poisoning is a bacterial or viral infection caused by consuming contaminated food, causing symptoms such as diarrhea, nausea, vomiting, and abdominal pain |
| Food intolerance | Lactose intolerance or gluten intolerance can cause gastrointestinal symptoms | |
| Endocrine disorders | Hyperthyroidism, diabetes, adrenal insufficiency | Certain hormonal imbalances, such as hyperthyroidism, diabetes, or adrenal insufficiency, can cause gastrointestinal symptoms and autonomic dysfunction. For example, in diabetic neuropathy, diabetes can damage the nerves that control the gastrointestinal system, leading to symptoms such as delayed gastric emptying and gastroparesis. Diabetic gastroparesis symptoms include nausea, vomiting, bloating, and early satiety |
| Psychiatric disorders | Stress, anxiety, somatization disorders, depression | Conditions such as stress, anxiety, somatization disorders, and depression can cause gastrointestinal symptoms such as nausea and vomiting and symptoms of autonomic dysfunction, such as rapid heartbeat and sweating. It is essential to assess the patient's mental health and history of psychiatric disorders to determine if psychological factors may contribute to the patient's presentation |
| Neurological disorders | Parkinson's disease, multiple sclerosis, multiple system atrophy, pure autonomic failure | Certain neurological disorders can cause autonomic dysfunction, including Parkinson's disease, multiple sclerosis, multiple system atrophy, and pure autonomic failure. For example, Parkinson's disease can cause gastrointestinal symptoms such as constipation and difficulty swallowing |
| Autonomic nervous system dysfunction | Postural orthostatic tachycardia syndrome | Postural orthostatic tachycardia syndrome is a condition that causes an abnormal increase in heart rate when changing position from lying down to standing up. Symptoms can include gastrointestinal symptoms such as nausea, vomiting, and abdominal pain |
| Autonomic neuropathy | Autonomic neuropathy is a condition that affects the nerves that control the body’s automatic functions, including the gastrointestinal system. It can cause symptoms such as delayed gastric emptying, constipation, and gastroparesis | |
| Metabolic disorders | Metabolic disorders such as liver disease, kidney disease, and electrolyte imbalances can cause gastrointestinal and autonomic symptoms | |
| Medications | Certain medications include opioids, anticholinergics, antibiotics, antihypertensives (e.g., calcium channel and beta blockers), nonsteroidal anti-inflammatory drugs, corticosteroids, chemotherapy drugs, and proton pump inhibitors, which can cause gastrointestinal symptoms | |
| Treatment | Comments |
| Infection control measures | To prevent the spread of COVID-19, following the public health guidelines, such as social distancing, wearing a face mask, and practicing good hand hygiene |
| Treatment of COVID-19 | Treatment of COVID-19 should be made on a case-by-case basis, based on the severity of COVID-19 and the patient's overall health status |
| Antiviral therapy such as Remdesivir and Molnupiravir, could be used in patients with COVID-19 to reduce the severity and duration of the illness. However, there is limited evidence for the effectiveness of antiviral therapy for COVID-19-induced gastrointestinal autonomic dysfunction | |
| Immunomodulatory therapy in severe cases of COVID-19-induced gastrointestinal autonomic dysfunction, such as corticosteroids or monoclonal antibodies such as Tocilizumab, may be needed to reduce inflammation and prevent complications | |
| Oxygen therapy may be needed | |
| Manage any other COVID-19-related complications that may arise | |
| Supportive management | ORT, intravenous fluids, and electrolyte replacement therapy |
| Getting enough rest is crucial to enhance recovery | |
| Symptomatic treatment | Management for abdominal pain with antispasmodics and analgesics such as acetaminophen or nonsteroidal anti-inflammatory drugs. Avoid using anticholinergics or opioids, as they can exacerbate gastrointestinal autonomic dysfunction |
| For nausea and vomiting: Antiemetic and prokinetics such as ondansetron or metoclopramide | |
| For diarrhoea: Antidiarrheals such as loperamide or bismuth subsalicylate | |
| Constipation: Laxatives | |
| Caution should be taken as symptomatic treatment may mask underlying symptoms of COVID-19 | |
| Managing autonomic dysfunction | Using medications such as alpha-adrenergic agonists, beta-blockers, or anticholinergic medications to regulate the autonomic nervous system, depending on the specific symptoms and severity of autonomic dysfunction. However, these medications must be used cautiously, as they can induce side effects and interact with other medications. Specific treatment for orthostatic hypotension, tachycardia, and syncope should be according to the guidelines |
| Treatment of complications | Treatment of dehydration and electrolyte imbalances, especially in children and the elderly |
| Treatment of gastroparesis: Prokinetic agents, such as erythromycin or metoclopramide, may be needed to promote gastric emptying, improve gastrointestinal motility, relieve gastroparesis symptoms, and relieve symptoms of constipation | |
| Treatment of malnutrition | |
| Treatment of bowel obstruction or perforation: Surgical management | |
| Treatment of bacterial superinfections and/or sepsis | |
| Severe disease may may require ICU admission, mechanical ventilation, or ECMO support | |
| Treating underlying conditions | IBD: Anti-inflammatory drugs, immunosuppressants such as corticosteroids, immunomodulators, biologic therapies, and antibiotics with adjustments to their regular treatment regimen or referral to a specialist. Diet modifications by avoiding trigger foods that exacerbate symptoms may help manage IBD symptoms. A low FODMAP diet may be beneficial for some people with IBD. Stress management and regular exercise could help. Surgery may be indicated in severe cases |
| IBS: Diet modifications medications such as antispasmodics, laxatives, and antidepressants to help alleviate symptoms, probiotics, stress management, and regular exercise | |
| Malignancy therapy should be personalized according to the type of malignancy by surgery, chemotherapy, radiotherapy, palliative therapy, immunomodulators, and supportive care | |
| Nutritional support | Maintain adequate caloric intake, prevent malnutrition, follow a bland diet, and avoid spicy, fatty, or acidic foods that may worsen symptoms |
| Nutritional support may be necessary for patients with decreased appetite or weight loss | |
| Nutritional supplements or enteral or parenteral nutrition according to the situation | |
| Probiotics positively affect the gut microbiota, improving gut dysbiosis that is common in gastrointestinal disorders and helping improve gastrointestinal symptoms such as bloating, constipation, and diarrhea. Prebiotics promote the growth of beneficial gut bacteria, which can improve gut health and potentially alleviate the associated symptoms. The optimal dosage, duration, and strains of probiotics/prebiotics could differ depending on the patient’s characteristics and the underlying pathophysiology of gastrointestinal autonomic dysfunction | |
| Psychological support | Treatment of psychological distress induced by the symptoms of gastrointestinal autonomic dysfunction |
| Psychological support, such as counseling or psychotherapy | |
| Follow-up and Rehabilitation care | Regular check-ups with a healthcare professional may be necessary depending on the severity of the condition |
| Aiming to monitor the resolution of symptoms, prevent long-term complications, and improve the patient's quality of life and functional status | |
| Rehabilitation may include occupational therapy, physical therapy, speech therapy, and psychological support to manage potential complications and improve the overall quality of life | |
| Physical therapy can help manage autonomic dysfunction symptoms, including gastrointestinal symptoms. Physical activities such as postural changes and deep breathing exercises can facilitate improved blood flow and reduce the symptoms |
The enteric nervous system is also known as the second brain due to its complex network of neurons lining the gut, with over 100 million neurons and plenty of essential neurotransmitters. It consists of intrinsic enteric neurons, extrinsic sympathetic and parasympathetic projections, and visceral afferent neurons. The intrinsic neurons comprise the outer myenteric and inner submucosal plexuses. The myenteric plexus (Auerbach) is found between the muscle layers of the gastrointestinal tract, while the submucosal plexus (Meissner) lies between the mucosa and the smooth muscle layers. The myenteric plexus is crucial in initiating and controlling smooth muscle motor activities, including peristalsis[11].
The submucosal plexus coordinates gastrointestinal reflexes, such as enzymatic secretion, nutrient absorption, and smooth muscle activities. The intrinsic enteric system operates independently of the autonomic nervous system, providing substantial autonomy over gastrointestinal functions. However, it does receive central input from the parasympathetic and sympathetic nervous systems[11,12]. In addition to neurons, non-neuronal related cells are present, such as enteric glial cells and interstitial cells of Cajal. Enteric glial cells create a diffusion barrier surrounding the capillaries around the ganglia, similar to the blood-brain barrier. Interstitial cells of Cajal are electrically coupled to the muscle and act as pacemaker cells, generating spontaneous electrical slow waves and facilitating inputs from motor neurons[13].
The gastrointestinal tract receives its sympathetic neural supply from the abdominal prevertebral ganglia located in the thoracic and lumbar segments of the spinal cord, such as the superior mesenteric, coeliac, and inferior mesenteric ganglia. These ganglia bridge the central and peripheral enteric nervous systems to regulate various intestinal functions, including blood flow, motility, secretion, epithelial transport, and endocrine cells. The sympathetic stimulation mainly inhibits gastrointestinal motor activity and secretion. It also constantly inhibits mucosal secretion and enhances the contraction of gastrointestinal sphincters and blood vessels via neural-mediated vasoconstriction[14,15].
The parasympathetic nervous system plays a vital role in regulating gastrointestinal functions, helping to maintain normal secretion, absorption, and movement in the gastrointestinal tract. It is primarily supplied by the vagus nerve from the medulla oblongata and the sacral spinal nerves from sacral segments 2 to 4. The vagus nerve supplies the upper part of the tract from the esophagus to the small intestine, while the sacral nerves supply the lower part from the colon to the anus. When stimulated, the parasympathetic system causes smooth muscle contraction, promotes peristalsis, stimulates glandular secretion, enhances intestinal secretions, increases blood flow, and relaxes the internal anal sphincter, all essential for proper gastrointestinal function[16,17].
Various factors can affect the autonomic nerve supply of the gastrointestinal tract, such as psychological and emotional stress, diet, medications, toxins, hormones, and aging (Table 1). Anxiety and stress stimulate the sympathetic supply while inhibiting the parasympathetic supply to the gastrointestinal tract. On the other hand, moderate exercise can enhance motility and blood flow in the gastrointestinal tract. Still, prolonged or intense exercise can decrease blood flow to the gastrointestinal tract and shift it to the muscles[18]. Certain foods and drugs can also inhibit or stimulate the parasympathetic nervous system. Hormones like cholecystokinin, gastrin, and secretin can enhance the parasympathetic nervous system and stimulate digestive activity[19]. Toxic heavy metals, such as mercury or lead, and alcohol can damage the autonomic nerves of the gastrointestinal tract. Aging also causes neuronal loss in intestinal myenteric and submucosal plexuses, particularly in cholinergic neurons, and corresponding losses of enteric glia[20]. Chronic diseases like diabetes mellitus negatively impact the gastrointestinal autonomic nervous system. Moreover, many neurological disorders, such as Parkinson's disease and multiple sclerosis, can impair digestion and excretion by affecting the gastrointestinal autonomic nerve system[9]. Finally, various infections can induce autonomic dysfunction of the gastrointestinal tract, such as viral gastroenteritis, bacterial infections like diphtheritic polyneuropathy, tetanus, and botulism, COVID-19, acute tick-borne encephalitis virus infections, Lyme disease, Chagas disease, human rabies, Guillain-Barre syndrome, and human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS)[21].
COVID-19 is a highly infectious disease caused by the SARS-CoV-2 that can affect any body part. While most people experience mild to moderate symptoms and recover without complications, some may require hospitalization and even face fatal consequences, with more than 6 million deaths worldwide[22]. Unfortunately, some patients, particularly those with severe COVID-19, may experience prolonged effects after recovering from their initial infection, known as long COVID or post-COVID conditions. This condition is identified by persistent symptoms and other COVID-19-related conditions that continue or develop for more than 4 wk after the initial stage of infection. It can be multisystemic and may even worsen over time. Additionally, there is a possibility of severe and life-threatening events that can occur even months or years after the primary infection. Long COVID is not a single condition but a collection of potentially over
Long COVID-19 can cause various symptoms, from mild to severe, affecting different body parts. These symptoms may include fatigue, joint pain, cough, chest pain, shortness of breath, headache, sleep problems, brain fog, dizziness, numbness, smell or taste disorders, depression, anxiety, and different patterns of cognitive problems[24]. The root causes of long COVID-19 are not yet fully understood, but it is believed to be related to the body's immune response against the virus or possible organ damage during the acute phase of the viral illness. It is also possible that some viral particles may remain in the body even after the acute phase ends, triggering ongoing symptoms[25]. Many of the symptoms of long COVID-19 are due to autonomic dysfunction, which can lead to cardiac rhythm disturbances, palpitations, postural hypotension, tachycardia, dyspnoea, chest pain, and various gastrointestinal and digestive symptoms like nausea, vomiting, and diarrhea[10].
Research has shown that although SARS-CoV-2 primarily affects the respiratory system, it can also cause dysfunction in multiple organs. Liu and colleagues discovered that the virus could target cells in different organs, including the small intestine, pancreas, liver, and other tissues. They also found a potential link between the virus's ability to infect multiple organs and the distribution of angiotensin-converting enzyme 2 (ACE2) receptors in these tissues[26]. ACE2 receptors are most highly expressed in the microvilli of the small intestine, particularly in the brush border of intestinal enterocytes in the ileum[27]. They are also found in the colon, oesophageal epithelium, and gastric and duodenal glandular cells. These findings suggest that the gastrointestinal tract could be a potential transmission route and amplification factory for the virus[28,29]. This high viral tropism to the gastrointestinal tract could explain why gastrointestinal symptoms may appear before respiratory symptoms and why the virus can continue in stool even after the infection has resolved[30].
Studies have shown that SARS-CoV-2 can infect gastrointestinal and neuronal cells within 2 d of exposure and robustly replicate in human cortical astrocytes, converting them to reactive cells with increased growth factor signaling and activated cellular stress[31]. This can result in damage to and impairments of neurological tissues. The virus can enter the cerebrospinal fluid through the blood-brain barrier and reach the brain via the damaged motor or sensory nerve endings[32,33]. Additionally, it is suggested that the enteric nervous system may serve as a portal of entry for the virus into the central nervous system due to the abundance of ACE-2 receptors and TMPRSS2 expression in enteric nerves and glial cells[34]. With the rich neural network supplying the gastrointestinal tract, the enteric nerves play a significant role in SARS-CoV-2 entry into the central nervous system[35].
SARS-CoV-2 can invade the central nervous system by attacking the nerves that supply the respiratory system, including the olfactory, trigeminal, facial, glossopharyngeal, and vagus nerves[36]. In addition, it can enter the central nervous system through the blood or via the vagus nerve from the lungs. In two cases, newborns whose mothers had COVID-19 during the second trimester of pregnancy experienced seizures shortly after birth, indicating that the virus can be transmitted to the brain through the placenta[37]. The virus can replicate inside neural cells, a characteristic known as neurotropism, which is increased by the spike protein produced by furin site insertion, resulting in neuronal infections. COVID-19 can also cause a range of neurological complications during both acute and post-acute stages due to severe respiratory disease, cytokine storms, and immune reactions that lead to hypoxia and impaired brain function[38].
Although COVID-19 primarily affects the respiratory system, it also significantly impacts the gastrointestinal tract, particularly children. The exact mechanism behind SARS-CoV-2-induced gastrointestinal symptoms remains unclear, but it is suggested that fecal-oral transmission may play a role due to the presence of SARS-CoV-2 RNA particles in fecal matter[39]. The virus can strongly infect cells in the gastrointestinal tract, particularly in the upper esophagus, absorptive enterocytes, hepatocytes, and cholangiocytes, which have increased expression of ACE2 receptors. These receptors allow the virus to enter host cells and interact with the spike protein of SARS-CoV-2, leading to an inflammatory response that can cause gut dysbiosis, leaky gut syndrome, and invasion of host cells by the virus. The severity of these changes is directly linked to the expression of ACE2 receptors and can worsen or improve gut dysbiosis and the severity of gastrointestinal leakage[40]. SARS-CoV-2 infection also causes gut mucosal infiltration by plasma cells and lymphocytes, which can lead to interstitial edema and deterioration of the intestinal-blood barrier, allowing the spread of endotoxins, bacteria, viruses, and other microbial metabolites into the systemic circulation. This can further impair the host's response to SARS-CoV-2 infection and lead to multisystem dysfunction, potentially resulting in septic shock[41].
Shedding the virus in the stool can negatively impact the gut, creating an environment that supports the virus's replication and release. This shedding may be a factor in the poor prognosis associated with the virus. Additionally, respiratory disorders caused by SARS-CoV-2 can harm the respiratory tract microbiota, negatively affecting the gut microbiota and digestive tract due to immune dysregulation[42]. This disruption can lead to a long-term reduction in beneficial bacteria, such as Lactobacillus, Bifidobacterium, and Faecalibacterium prausnitzii, and an increase in harmful bacteria, such as Clostridium ramosum, Clostridium hathewayi, Coprobacillus, Aspergillus, and Candida. Medical comorbidities and the use of certain medications, such as antibiotics, antifungals, antivirals, and steroids, can worsen this dysbiosis[43]. Maintaining a diverse gut microbiota with a predominance of beneficial bacteria can help combat SARS-CoV-2 infection.
Gastrointestinal symptoms can manifest during any stage of COVID-19, either at the beginning or as the disease progresses[44]. Table 2 provides a summary of the gastrointestinal symptoms associated with COVID-19. Diarrhea and dysgeusia are the most prevalent, followed by anorexia, nausea and vomiting, and abdominal pain[45].
In some cases, patients may experience gastrointestinal symptoms before respiratory symptoms, which could indicate the gut's influence on the progression of COVID-19. Fecal viral shedding in severe cases may also suggest the gut's impact on prognosis[7]. Studies show that patients with gastrointestinal symptoms tend to have a longer time between symptom onset and hospital admission than those without[46]. Additionally, severe COVID-19 patients are more likely to have gastrointestinal symptoms, with up to 92% of cases and 80% of those in intensive care experiencing them[47].
The results of endoscopic biopsies have revealed that SARS-CoV-2 can enter gastrointestinal cells directly by binding with ACE2 receptors in various locations of the gastrointestinal tract[48]. Additionally, individuals with severe COVID-19 and multisystem inflammatory syndrome may experience hypercoagulable states due to systemic inflammation, hyperinflammation from cytokine storms, and diffuse endotheliitis. This could result in mesenteric vascular thrombosis and small vessel thrombosis of submucosal arterioles, which could cause bowel necrosis[49,50].
Patients can experience ongoing gastrointestinal symptoms even after recovering from a disease. According to Tian and Rong, a woman suffered from diarrhea for 2 mo and later developed abdominal distension and constipation for about 3 mo after fully recovering from the initial illness[51]. They hypothesized that these symptoms could be attributed to the development of irritable bowel syndrome, which may have occurred due to damage to the enteric nervous system or the central nervous center controlling the vagus nerve in the brainstem. This area regulates various autonomic functions, such as cardiac and respiratory functions, alongside appetite, nausea, and vomiting[52].
Although the pandemic has been relatively short-lived, an increasing number of studies show the involvement of the autonomic system in COVID-19. These studies suggest that autonomic dysfunction is not uncommon and could be a critical factor in increasing mortality rates related to the virus[53]. In particular, patients with severe COVID-19 have been shown to experience gastrointestinal autonomic dysfunction, which can cause symptoms such as anorexia, nausea, vomiting, abdominal pain, bloating, diarrhea, or constipation. Other autonomic dysfunctions have also been observed, including abnormal heart rate variability and blood pressure regulation. These symptoms are more prevalent in patients with older age, longer illness duration, and more severe or critical disease. Nevertheless, further research is needed to determine these dysfunctions' prevalence and clinical significance in COVID-19 patients, especially those affecting the gastrointestinal tract[54-56].
Table 3 displays some studies that examine how frequently COVID-19 patients experience gastrointestinal symptoms. One such study by Erdal et al[57] found that 21.8% of their 112 COVID-19 patients experienced diarrhea. Another study by Varma-Doyle et al[58] noted that five of six patients with dysautonomia experienced gastrointestinal symptoms such as bloating, nausea, vomiting, constipation, irregular bowel movements, and early satiety. Scala et al's research showed that 26.3% of COVID-19 patients developed diarrhea, compared to only 7.9% of the control group[59]. Moreover, Tan et al[60] found that patients who did not receive angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB) had higher rates of gastrointestinal symptoms compared to those who did, such as diarrhea (14.5% vs 6.5%), nausea and vomiting (11.6% vs 9.7%), and abdominal pain (6.5% vs 2.9%). Finally, a meta-analysis by Mao et al[45] found that 15% of COVID-19 patients in 35 studies experienced digestive symptoms, with anorexia, nausea, vomiting, and diarrhea being the most common. Patients who presented with gastrointestinal symptoms may also have a more severe disease course. Further research is needed to understand better the epidemiological characteristics and clinical significance of COVID-19-induced gastrointestinal dysfunction. Table 4 Lists the risk factors that increase the incidence of gastrointestinal autonomic dysfunction.
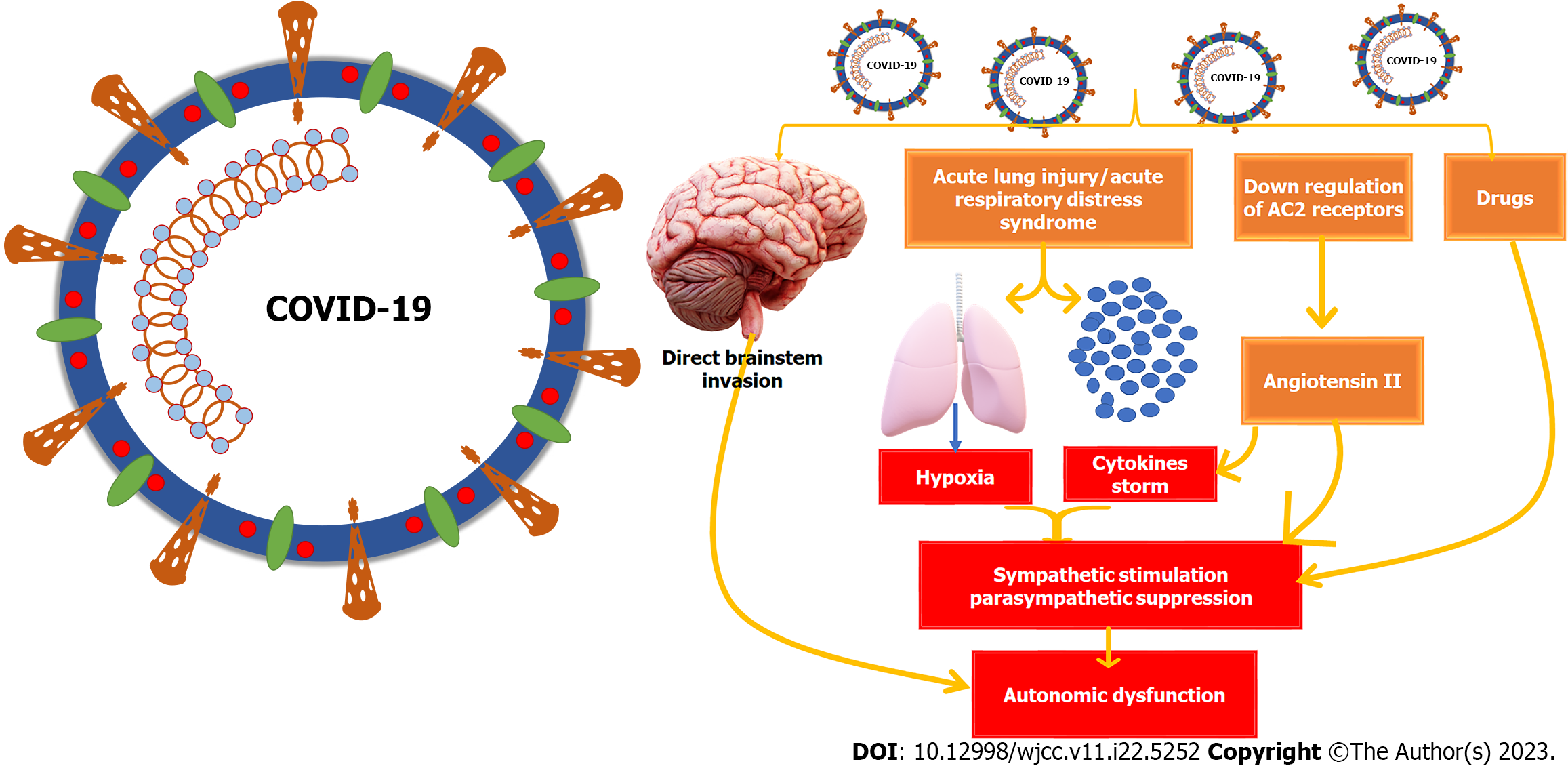
The exact cause of autonomic dysfunction in COVID-19 and long COVID-19 is still not fully understood. It could be due to the direct effects of the virus on the nervous system, gut dysbiosis, an overactive robust immune response, the impact of hypoxia on neurological tissues, or medication used in the patient's treatment. The virus may invade the autonomic pathway directly or induce injury through the immune response, which can stimulate the sympathetic nervous system and activate pro-inflammatory cytokines, causing further stimulation of the sympathetic system. This can result in the development of a sympathetic storm[10]. Direct SARS-CoV-2 invasion of the medulla or hypothalamus can trigger autonomic dysfunction through retrograde neuronal or systematic hematogenous routes. At the hypothalamus, targeting GABAergic or glutamatergic interneurons can directly or indirectly impact the paraventricular nucleus (PVN) plasticity, leading to neuroinflammation and autonomic nervous system disruption. Similarly, SARS-CoV-2 invasion of astrocytes and GABAergic interneurons in the nucleus tractus solitaries in the medulla can modulate the normal autonomic function and/or initiate cell death[54].
Studies have shown that COVID-19 patients with excessive sympathetic activation are more likely to experience severe pulmonary capillary leakage, alveolar damage, and an increased risk of developing acute respiratory distress syndrome. This over-activation of the sympathetic nervous system is also linked to parasympathetic inhibition and renin-angiotensin system activation[61,62]. On the other hand, stimulating the vagus nerve can have anti-inflammatory effects by inhibiting the release of pro-inflammatory cytokines and modulating sympatho excitation. This could be a therapeutic approach for the autonomic nervous system[63]. Pulmonary chemoreceptors and mechanoreceptors may play a role in spreading SARS-CoV-2 from the lungs to the respiratory center in the medulla oblongata, leading to sudden central respiratory failure[64]. To combat this, beta-receptor antagonists can reduce sympathetic stimulation and limit the interaction between SARS-CoV-2 and ACE2 receptors[65].
Studies have shown that autoimmune antibodies exist in many autonomic nervous system disorders, including postural orthostatic tachycardia syndrome, a subtype of orthostatic intolerance. It is believed that COVID-19 may trigger an autoimmune mechanism that causes dysfunction in the autonomic nerves. According to Al-Beltagi et al[66], the effects of SARS-CoV-2 on the immune system are related to the sizeable viral RNA molecules, molecular mimicry, and interaction with the host immune system, as well as the overlap between some viral and human peptides. The virus can also induce tissue damage and create a strong binding between the SARS-CoV-2 S protein and soluble ACE-2. A growing body of evidence suggests that autonomic nervous system dysfunction observed in COVID-19 may be due to an autoimmune response. In a study by Chadda et al[67], COVID-19 patients experienced various autonomic nervous system dysfunction symptoms, including tachycardia, orthostatic intolerance, and gastrointestinal dysfunction. The researchers suggested that these symptoms may be due to an autoimmune response against the autonomic nervous system. Lavi et al[68] found that COVID-19 patients had significantly higher levels of autoantibodies against the ANS than healthy controls, which may contribute to the autonomic nervous system dysfunction observed in COVID-19 patients. Additionally, Paterson et al[69] found that COVID-19 may trigger autoimmune responses against the nervous system, including the autonomic nervous system, which may explain the diverse neurological symptoms observed in COVID-19 patients.
Severe COVID-19 often leads to hypoxia, which can cause damage to different parts of the brain, including the dorsal vagal complex located in the medulla oblongata, the lower part of the brainstem. This complex is responsible for various autonomic functions like heart rate, respiration, and food intake. Injuries to the dorsal vagal complex can lead to nausea, vomiting, and other autonomic dysfunction symptoms[70]. Disturbance of the nucleus of tractus solitaries, part of the dorsal vagal complex beside the hypothalamus, causes impaired food intake. Impairment of the gelatinous nucleus, subnucleus part of the nucleus of tractus solitaries, causes respiratory failure in severe cases of COVID-19. It may also be responsible for sudden infant death syndrome due to the alteration of the catecholaminergic neurochemical system[71,72]. COVID-19 can also cause gut dysbiosis, which increases pro-inflammatory cytokines and ultimately leads to neuroinflammation. The cytokines storm associated with severe COVID-19 causes hyperinflammation with increased pro-inflammatory cytokines resulting in gut dysbiosis and compromising intestinal barrier integrity. The increased permeability and leaky gut increase levels of circulating lipopolysaccharides, which ultimately may initiate microglial cell activation and neuroinflammation[73]. Additionally, COVID-19 can induce systemic vascular changes all over the body, including in the gastrointestinal tract, which may result in gastrointestinal autonomic dysfunction[74].
It is imperative to understand that COVID-19 has a severe negative impact on the autonomic nervous system. The downregulation of the IKBKAP gene and the up-regulation of N-type calcium channels at the molecular level can lead to fatal gastrointestinal dysfunction and complications, including mortality[75,76]. Autonomic dysregulation can manifest in various ways, such as at the level of autonomic centers like the medulla and hypothalamus, in the sympathetic and parasympathetic supply to the gut, or the target organs of the digestive system. The prevalence and mechanism of common gastrointestinal symptoms due to autonomic nervous system dysfunction in COVID-19 patients are detailed in Table 5. Figure 3 comprehensively summarizes the mechanisms that can induce autonomic dysfunction in COVID-19. It is essential to take these findings seriously and take necessary precautions to avoid any complications.
The COVID-19 vaccine has undoubtedly been a great benefit in ending the COVID-19 pandemic. However, some individuals who have taken it have experienced certain effects, such as autonomic dysfunction that affects various body organs and systems, including the gastrointestinal tract. This side effect is uncommon and has been reported infrequently, particularly after receiving certain types of COVID-19 vaccines that rely on DNA and RNA technology, like the Moderna, Pfizer-BioNTech, and Johnson & Johnson vaccines. These vaccines use immunologic liposomes to achieve powerful antigenic stimulation of target cells[77].
Autonomic dysfunction can cause various symptoms, from mild dizziness and light-headedness to more severe symptoms like fainting and arrhythmia. These symptoms may occur shortly after vaccination and last for varying leng
The exact cause of autonomic dysfunction associated with COVID-19 vaccination is not fully understood. The vaccine's robust immune response may trigger inflammation in the autonomic nervous system, leading to symptoms of autonomic dysfunction[89]. Some patients have experienced reflex syncope or presyncope due to delayed vasovagal reaction, possibly related to stress caused by vaccination[90]. It is important to note that the risk of developing autonomic dysfunction after COVID-19 vaccination is very low. These rare complications should not deter us from reaping the significant protective benefits of these vaccines. The benefits of vaccination in preventing severe COVID-19, hospitalization, and death far outweigh the risks.
Gastrointestinal manifestations may occur during the early phase of COVID-119 and could be considered an early indicator of infection, particularly in asymptomatic or mildly symptomatic patients. However, these symptoms are more common in older patients, those with underlying medical conditions, or severe COVID-19. Such patients are at an increased risk of delayed diagnosis, higher levels of inflammatory markers, prolonged illness, severe morbidity, hospitalization, post-COVID-19 complications, and mortality[42,91]. Consequently, gastrointestinal autonomic dysfunction can significantly affect patients' overall health and well-being. Patients with gastrointestinal symptoms may experience a variety of symptoms that can impair their quality of life, such as dysphagia, anorexia, esophageal dysmotility, gastroparesis, bloating, nausea, abdominal pain, small intestinal bacterial overgrowth, diarrhea, or constipation[9]. COVID-19 patients may also experience autonomic nervous system dysregulation, leading to post-infectious irritable bowel syndrome. This condition is characterized by chronic abdominal pain, bloating, and altered or abnormal bowel habits that can persist for at least 3 mo after the initial SARS-CoV-2 infection. It is believed to occur due to persistent neuroinflammation, immune dysregulation, and gut dysbiosis[92].
Additionally, COVID-19 can cause autonomic dysfunction, which may lead to reduced blood flow to the gut and worsen gastrointestinal symptoms. It also increases the risk of thrombotic complications, such as deep vein thrombosis and possibly pulmonary embolism, due to activation of the coagulation cascade and developing endothelial dysfunction[93]. Furthermore, autonomic dysfunction may affect cardiac functions, resulting in changes in heart rate, labile blood pressure, and impaired cardiac output. COVID-19-induced gastrointestinal dysfunction may also be associated with other symptoms of autonomic dysfunction, such as dizziness and fainting[94].
COVID-19 can cause various symptoms that can hinder a patient's recovery. These symptoms may make eating difficult and lead to dehydration, malnutrition, and weight loss, further impacting the patient's health. They can also make a person more susceptible to complications such as gastric ulcers, bleeding, small bowel obstruction, or even perforation[95]. Gastrointestinal symptoms can also significantly impact a patient's mental health, leading to anxiety, depression, and social isolation, which can further worsen their symptoms and impair their ability to function in their daily life[96]. The impact depends on various factors, such as the severity of the illness, the extent of gastrointestinal involvement, general patient condition, and any existing medical conditions. While most cases resolve themselves within a few weeks, some individuals may experience prolonged symptoms[97]. Therefore, it is crucial to identify and treat these symptoms and provide adequate supportive care to enhance patient outcomes. Long-term follow-up is also essential to evaluate potential sequelae in patients with COVID-19-induced gastrointestinal manifestations[98].
Diagnosing gastrointestinal autonomic dysfunction in COVID-19 patients depends on their symptoms and clinical examination. However, diagnosing it can be challenging as the symptoms may be similar or overlap with other conditions, and no specific test exists for it. Therefore, a comprehensive evaluation is necessary based on the patient's medical history, symptoms, laboratory tests, imaging studies, and specific tests to assess autonomic nervous system function. A high level of clinical suspicion is also required[99].
To diagnose COVID-19-induced gastrointestinal autonomic dysfunction, a thorough clinical evaluation is necessary. This includes a detailed medical history and physical examination, emphasizing the duration and onset of autonomic dysfunction symptoms such as nausea, vomiting, abdominal pain, early satiety, diarrhea, weight loss, dizziness, fainting, and blood pressure fluctuations. COVID-19 symptoms like high fever, cough, or shortness of breath should also be evaluated. Medical history should include pre-existing gastrointestinal conditions, systemic premorbid conditions, recent medications, recent travel, and a past or family history of autonomic dysfunction or disorders. Such a history increases the risk of developing COVID-19-induced gastrointestinal autonomic dysfunction[55,100].
During a physical examination, it is important to evaluate vital signs such as blood pressure, heart rate, respiratory rate, hydration status, and sweating. An abdominal examination should also be conducted to check for signs of distension, tenderness, masses, or abnormal bowel sounds[101]. The SCOPA-AUT scale is useful for assessing autonomic function, particularly in individuals with neurological diseases like Parkinson's disease. This questionnaire assesses multiple aspects of autonomic dysfunction by covering 25 items related to various body regions, including the gastrointestinal tract, urinary system, cardiovascular system, thermoregulatory mechanism, pupillomotor function, and sexual dysfunction (different in males than females). Each item is scored from 0 (never) to 3 (often happening), with higher scores indicating more severe dysfunction[102,103]. Researchers have used this scale to evaluate autonomic dysfunction in patients with COVID-19 and found that many patients might have persistent symptoms of autonomic nervous system dysfunction following the acute phase of COVID-19 that might deteriorate and impair clinical recovery[57].
To determine the underlying cause of a patient's symptoms, laboratory tests can help rule out other diseases. It is important to confirm a diagnosis of COVID-19 to establish the presence of SARS-CoV-2. Blood tests can provide valuable information, such as a complete blood count, inflammatory markers like C-reactive protein, blood glucose levels, comprehensive metabolic panel, serum electrolyte levels, serum amylase and lipase levels, liver function, and kidney function. These tests can help evaluate for anaemia, dehydration, electrolyte imbalances, kidney function, pancreatic function, liver function, and other underlying disorders contributing to the patient's symptoms. Additionally, stool analysis can identify signs of inflammation or infection and detect any pathogens, such as viruses or bacteria, that could be causing gastrointestinal symptoms or any other abnormalities[104,105].
To assess autonomic dysfunction in specific areas of the gastrointestinal tract, specialized tests may be necessary. For instance, COVID-19 can cause gastrointestinal dysfunction, which may manifest as esophageal motility issues. Evaluating esophageal motility can help determine the functionality of the muscles responsible for moving food into the stomach. Delayed gastric emptying is a symptom of gastroparesis, which can be assessed through electrogastrography or gastric emptying studies to determine the speed at which food moves through the stomach[9]. A colonic transit study can also evaluate food movement through the colon in cases of suspected constipation or delayed colonic transit. Anorectal manometry may be used to assess the function of the anal or rectal areas in cases of suspected anorectal dysfunction[106]. Tests can also be conducted to evaluate the autonomic nervous system, such as heart rate variability measurements, blood pressure positional changes, and sweat testing. Electrocardiograms may be utilized to evaluate heart rate and rhythm, as arrhythmia and abnormal heart rate variability are common symptoms of autonomic dysfunction[107,108]. Sweat testing using quantitative sudomotor axon reflex may also be used to diagnose autonomic dysfunction by evaluating the function of sweat glands, as abnormal sweating is a possible symptom. In addition, the tilt table test can also be utilized to investigate unexplained fainting, which may be associated with gastrointestinal autonomic dysfunction[109]. Endoscopy may be performed to evaluate the structure and function of the gut or to confirm the presence of inflammation, hemorrhage, perforation, damage, or infection in the gastrointestinal tract[110]. Imaging, such as abdominal X-rays, ultrasonography, computed tomography, or magnetic resonance imaging, may also be necessary to evaluate for bowel obstruction, perforation, motility disorders, structural abnormalities, inflammation, or other underlying conditions that may be causing gastrointestinal symptoms[111,112].
Diagnosing gastrointestinal autonomic dysfunction can be a difficult task, as its symptoms can mimic those of other gastrointestinal disorders. Conducting a thorough differential diagnosis is important to rule out other conditions and confirm the diagnosis of COVID-19-induced gastrointestinal autonomic dysfunction. This can be achieved through detailed physical examinations, stool and blood tests, imaging studies, and other diagnostic methods. Table 7 outlines some conditions that should be considered during the differential diagnosis process.
Managing COVID-19-induced gastrointestinal autonomic dysfunction is an evolving and growing field with a multi-faceted approach. The optimal management approach depends on the individual patient's presentation and underlying conditions. The treatment needs a multidisciplinary approach with symptomatic treatment, specific treatment, pharmacological and non-pharmacological interventions, dietary modifications and management, supportive care, management of complications, and follow-up care[55]. The collaboration of a gastroenterologist, neurologist, intensivist, nutritionist, and infectious disease specialist helps to achieve proper management. Other specialties may be needed according to the patient’s condition[97]. Early identification and well-timed management of these disorders can improve patient outcomes. In severe conditions, timely management of complications is essential to prevent adverse outcomes. Patients should be instructed to obtain medical attention if symptoms persist or worsen. Follow-up is mandatory, and the resolution of the infection should be confirmed with repeated testing when necessary[113]. A summary of the proposed treatment is shown in Table 8.
Managing COVID-19-induced gastrointestinal autonomic dysfunction is a constantly evolving field that requires a multifaceted approach. The best approach for managing this condition depends on the individual patient's symptoms and any underlying medical conditions that they may have. Treatment involves a multidisciplinary team that provides symptomatic and specific treatment, pharmacological and non-pharmacological interventions, dietary modifications, supportive care, management of complications, and follow-up care[55]. A collaboration between a gastroenterologist, neurologist, intensivist, nutritionist, and infectious disease specialist is necessary to ensure proper management. Depending on the patient's condition, other specialties may also need to be consulted[97]. Early identification and timely management of gastrointestinal autonomic dysfunction can improve patient outcomes. In severe cases, prompt management of complications is crucial to prevent adverse outcomes. Patients should seek medical attention if their symptoms persist or worsen. Follow-up care is essential, and the resolution of the infection should be confirmed with repeated testing when necessary[113]. Please refer to Table 8 for a summary of the proposed treatments.
It is important to note that the study has certain limitations which may impact its findings. Due to the inclusion of a vast number of studies, there is some variation in the study design, quality, population, and outcomes measured. This variation can make drawing comparisons and synthesizing the results difficult. Furthermore, it is worth noting that not all relevant studies were reviewed, particularly those published in languages other than English or those not accessible through major databases.
The gastrointestinal tract is a common target of SARS-CoV-2 infection and could induce the initial presenting manifestation. COVID-19-induced gastrointestinal autonomic dysfunction can be a significant complication affecting patients' well-being and prognosis. Due to the unclear mechanism behind these manifestations, further research is necessary. Additionally, some COVID-19 vaccines may trigger autonomic dysfunction. Several factors can increase the risk of developing gastrointestinal autonomic nervous functions and exacerbate their impact on patients. Diagnosing COVID-19-induced gastrointestinal autonomic dysfunction can be challenging, requiring careful clinical evaluation, specific laboratory tests, and imaging studies. Early detection and management of these disorders can improve patient outcomes and prevent long-term complications.
It is typical for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection to affect the gastrointestinal tract, which may manifest as an early warning sign. The frequency of gastrointestinal symptoms often indicates the seriousness of coronavirus disease 2019 (COVID-19). COVID-19 may impair the nerve supply to the digestive system, resulting in autonomic dysfunction of the gastrointestinal tract. There is still much to discover about the impact of COVID-19 on the autonomic nervous system and the gastrointestinal tract.
Due to the importance of gastrointestinal tract autonomic dysfunction in patients with COVID-19, we are motivated to conduct this systematic review concerning this newly emerged condition that has become a concern during the pandemic.
Our objective is to thoroughly investigate the epidemiology, clinical manifestations, potential mechanisms, diagnosis, differential diagnosis, and impact of gastrointestinal autonomic dysfunctions on the quality of life and prognosis of individuals who have contracted SARS-CoV-2. Additionally, we will explore management and prevention strategies.
We conducted a comprehensive and systematic exploration of multiple databases, followed by an extensive analysis of the relevant literature. Our review included 113 studies published in English between January 2000 and April 18, 2023.
According to most of the literature, gastrointestinal autonomic dysfunction can greatly impact a patient's quality of life and prognosis. Many factors can affect the functioning of the gastrointestinal autonomic nervous system. Studies have shown that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has a documented affinity for both neural and gastrointestinal tissues, which can lead to various gastrointestinal symptoms. These symptoms include anorexia, dysgeusia, heartburn, belching, chest pain, regurgitation, vomiting, epigastric burn, diarrhea, abdominal pain, bloating, irregular bowel movements, and constipation. Diarrhea is the most common symptom, followed by anorexia, nausea, vomiting, and abdominal pain. While COVID-19 vaccination may rarely cause autonomic dysfunction and gastro
To achieve optimal results for individuals with COVID-19, it is crucial to adopt a multidisciplinary approach, which includes providing supportive care, treating the underlying infection, managing any dysfunction, monitoring for complications, and offering nutritional assistance. It is imperative to closely monitor the patient's condition and take swift action if necessary. Additionally, conducting comprehensive research on the gastrointestinal autonomic dysfunction caused by COVID-19 is critical for effective management.
A multi-faceted approach is essential to ensure the best outcome for those affected by coronavirus disease 2019 (COVID-19). This includes providing supportive care, addressing the root infection, managing dysfunctions, monitoring for complications, and providing nutritional aid. It is crucial to closely monitor the patient's condition and take prompt action if needed. Furthermore, in-depth research on COVID-19-induced gastrointestinal autonomic dysfunction is vital for proper management.
We thank the anonymous referees for their valuable suggestions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Mukhopadhyay A, India; Taghizadeh-Hesary F, Iran S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Al-Beltagi M, Saeed NK, Bediwy AS, El-Sawaf Y. Paediatric gastrointestinal disorders in SARS-CoV-2 infection: Epidemiological and clinical implications. World J Gastroenterol. 2021;27:1716-1727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Jain U. Effect of COVID-19 on the Organs. Cureus. 2020;12:e9540. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Yang Z, Chen X, Huang R, Li S, Lin D, Yang Z, Sun H, Liu G, Qiu J, Tang Y, Xiao J, Liao Y, Wu X, Wu R, Dai Z. Atypical presentations of coronavirus disease 2019 (COVID-19) from onset to readmission. BMC Infect Dis. 2021;21:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1205] [Article Influence: 241.0] [Reference Citation Analysis (0)] |
| 5. | Groff A, Kavanaugh M, Ramgobin D, McClafferty B, Aggarwal CS, Golamari R, Jain R. Gastrointestinal Manifestations of COVID-19: A Review of What We Know. Ochsner J. 2021;21:177-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Ojetti V, Saviano A, Covino M, Acampora N, Troiani E, Franceschi F; GEMELLI AGAINST COVID‐19 group. COVID-19 and intestinal inflammation: Role of fecal calprotectin. Dig Liver Dis. 2020;52:1231-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Cheng PK, Wong DA, Tong LK, Ip SM, Lo AC, Lau CS, Yeung EY, Lim WW. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699-1700. [PubMed] [DOI] [Full Text] |
| 8. | Chelimsky G, Chelimsky TC. Evaluation and treatment of autonomic disorders of the gastrointestinal tract. Semin Neurol. 2003;23:453-458. [PubMed] [DOI] [Full Text] |
| 9. | Kornum DS, Terkelsen AJ, Bertoli D, Klinge MW, Høyer KL, Kufaishi HHA, Borghammer P, Drewes AM, Brock C, Krogh K. Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 10. | Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, Lim PB. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med (Lond). 2021;21:e63-e67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 419] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 11. | Fleming MA 2nd, Ehsan L, Moore SR, Levin DE. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol Res Pract. 2020;2020:8024171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1068] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 13. | Nezami BG, Srinivasan S. Enteric nervous system in the small intestine: pathophysiology and clinical implications. Curr Gastroenterol Rep. 2010;12:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4:1339-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 368] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 15. | Vermeulen W, De Man JG, Pelckmans PA, De Winter BY. Neuroanatomy of lower gastrointestinal pain disorders. World J Gastroenterol. 2014;20:1005-1020. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Altaf MA, Sood MR. The nervous system and gastrointestinal function. Dev Disabil Res Rev. 2008;14:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Ali MK, Saha S, Milkova N, Liu L, Sharma K, Huizinga JD, Chen JH. Modulation of the autonomic nervous system by one session of spinal low-level laser therapy in patients with chronic colonic motility dysfunction. Front Neurosci. 2022;16:882602. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Daniela M, Catalina L, Ilie O, Paula M, Daniel-Andrei I, Ioana B. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 19. | Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Carod-Artal FJ. Infectious diseases causing autonomic dysfunction. Clin Auton Res. 2018;28:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2083] [Cited by in RCA: 3172] [Article Influence: 793.0] [Reference Citation Analysis (0)] |
| 23. | Centers for Disease Control and Prevention: Long COVID or Post-COVID Conditions. Available at: Long COVID or Post-COVID Conditions | CDC last accessed on 7th of April 2023. |
| 24. | Sundar Shrestha D, Love R. Long COVID Patient Symptoms and its Evaluation and Management. JNMA J Nepal Med Assoc. 2021;59:823-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity and Progression. Physiology (Bethesda). 2020;35:288-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 26. | Liu J, Li Y, Liu Q, Yao Q, Wang X, Zhang H, Chen R, Ren L, Min J, Deng F, Yan B, Liu L, Hu Z, Wang M, Zhou Y. SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov. 2021;7:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 27. | Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 742] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 28. | Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Zhixiu, Li Zifu, Cui X, Xiao J, Zhan J, Meng T, Zhou W, Liu J, Xu H. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010-1018. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 380] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 29. | Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 631] [Cited by in RCA: 710] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 30. | Saeed NK, Al-Biltagi M, Bediwy AS. Molecular Testing for COVID-19: What the Clinician Should Know. Dr. SulaimanAl Habib Medical Journal 2021; 53-59 [DOI: 10.2991/dsahmj.k.210427.002] . |
| 31. | Bauer L, Laksono BM, de Vrij FMS, Kushner SA, Harschnitz O, van Riel D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022;45:358-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 32. | De Felice FG, Tovar-Moll F, Moll J, Munoz DP, Ferreira ST. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the Central Nervous System. Trends Neurosci. 2020;43:355-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 33. | Swanson PA 2nd, McGavern DB. Viral diseases of the central nervous system. Curr Opin Virol. 2015;11:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 34. | Esposito G, Pesce M, Seguella L, Sanseverino W, Lu J, Sarnelli G. Can the enteric nervous system be an alternative entrance door in SARS-CoV2 neuroinvasion? Brain Behav Immun. 2020;87:93-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Deffner F, Scharr M, Klingenstein S, Klingenstein M, Milazzo A, Scherer S, Wagner A, Hirt B, Mack AF, Neckel PH. Histological Evidence for the Enteric Nervous System and the Choroid Plexus as Alternative Routes of Neuroinvasion by SARS-CoV2. Front Neuroanat. 2020;14:596439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Andrews MG, Mukhtar T, Eze UC, Simoneau CR, Ross J, Parikshak N, Wang S, Zhou L, Koontz M, Velmeshev D, Siebert CV, Gemenes KM, Tabata T, Perez Y, Wang L, Mostajo-Radji MA, de Majo M, Donohue KC, Shin D, Salma J, Pollen AA, Nowakowski TJ, Ullian E, Kumar GR, Winkler EA, Crouch EE, Ott M, Kriegstein AR. Tropism of SARS-CoV-2 for human cortical astrocytes. Proc Natl Acad Sci U S A. 2022;119:e2122236119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (3)] |
| 37. | Benny M, Bandstra ES, Saad AG, Lopez-Alberola R, Saigal G, Paidas MJ, Jayakumar AR, Duara S. Maternal SARS-CoV-2, Placental Changes and Brain Injury in 2 Neonates. Pediatrics. 2023;151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Veleri S. Neurotropism of SARS-CoV-2 and neurological diseases of the central nervous system in COVID-19 patients. Exp Brain Res. 2022;240:9-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Troisi J, Venutolo G, Pujolassos Tanyà M, Delli Carri M, Landolfi A, Fasano A. COVID-19 and the gastrointestinal tract: Source of infection or merely a target of the inflammatory process following SARS-CoV-2 infection? World J Gastroenterol. 2021;27:1406-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 40. | Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 990] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 41. | Kim S, Rigatto K, Gazzana MB, Knorst MM, Richards EM, Pepine CJ, Raizada MK. Altered Gut Microbiome Profile in Patients With Pulmonary Arterial Hypertension. Hypertension. 2020;75:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 42. | Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 43. | Saeed NK, Al-Beltagi M, Bediwy AS, El-Sawaf Y, Toema O. Gut microbiota in various childhood disorders: Implication and indications. World J Gastroenterol. 2022;28:1875-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (5)] |
| 44. | Marasco G, Lenti MV, Cremon C, Barbaro MR, Stanghellini V, Di Sabatino A, Barbara G. Implications of SARS-CoV-2 infection for neurogastroenterology. Neurogastroenterol Motil. 2021;33:e14104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 45. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 754] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 46. | Chigr F, Merzouki M, Najimi M. Autonomic Brain Centers and Pathophysiology of COVID-19. ACS Chem Neurosci. 2020;11:1520-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 47. | Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1856] [Article Influence: 371.2] [Reference Citation Analysis (0)] |
| 48. | Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 49. | Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA, Catalano O, Gee MS, Hahn PF, Harisinghani M, Kilcoyne A, Lee SI, Mojtahed A, Pandharipande PV, Pierce TT, Rosman DA, Saini S, Samir AE, Simeone JF, Gervais DA, Velmahos G, Misdraji J, Kambadakone A. Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology. 2020;297:E207-E215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 227] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 50. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4583] [Article Influence: 916.6] [Reference Citation Analysis (0)] |
| 51. | Tian Y, Rong L. Letter: gastrointestinal symptoms pre-admission are associated with greater severity of COVID-19-authors' reply. Aliment Pharmacol Ther. 2020;52:1231-1232. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Chen X, Laurent S, Onur OA, Kleineberg NN, Fink GR, Schweitzer F, Warnke C. A systematic review of neurological symptoms and complications of COVID-19. J Neurol. 2021;268:392-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 53. | Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, González E, Redondo-Peñas I, Perona-Moratalla AB, Del Valle-Pérez JA, Gracia-Gil J, Rojas-Bartolomé L, Feria-Vilar I, Monteagudo M, Palao M, Palazón-García E, Alcahut-Rodríguez C, Sopelana-Garay D, Moreno Y, Ahmad J, Segura T. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95:e1060-e1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 545] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 54. | Jammoul M, Naddour J, Madi A, Reslan MA, Hatoum F, Zeineddine J, Abou-Kheir W, Lawand N. Investigating the possible mechanisms of autonomic dysfunction post-COVID-19. Auton Neurosci. 2023;245:103071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 55. | Carmona-Torre F, Mínguez-Olaondo A, López-Bravo A, Tijero B, Grozeva V, Walcker M, Azkune-Galparsoro H, López de Munain A, Alcaide AB, Quiroga J, Del Pozo JL, Gómez-Esteban JC. Dysautonomia in COVID-19 Patients: A Narrative Review on Clinical Course, Diagnostic and Therapeutic Strategies. Front Neurol. 2022;13:886609. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 56. | Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3262] [Cited by in RCA: 3000] [Article Influence: 750.0] [Reference Citation Analysis (0)] |
| 57. | Erdal Y, Atalar AC, Gunes T, Okluoglu T, Yavuz N, Emre U. Autonomic dysfunction in patients with COVID-19. Acta Neurol Belg. 2022;122:885-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 58. | Varma-Doyle A, Villemarette-Pittman NR, Lelorier P, England J. Demonstrating new-onset or worsened sudomotor function post-COVID-19 on comparative analysis of autonomic function pre-and post-SARS-CoV-2 infection. eNeurologicalSci. 2023;30:100445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 59. | Scala I, Bellavia S, Luigetti M, Brunetti V, Broccolini A, Gabrielli M, Zileri Dal Verme L, Calabresi P, Della Marca G, Frisullo G. Autonomic dysfunction in non-critically ill COVID-19 patients during the acute phase of disease: an observational, cross-sectional study. Neurol Sci. 2022;43:4635-4643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Tan ND, Qiu Y, Xing XB, Ghosh S, Chen MH, Mao R. Associations Between Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blocker Use, Gastrointestinal Symptoms, and Mortality Among Patients With COVID-19. Gastroenterology. 2020;159:1170-1172.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 61. | Hyoju SK, Zaborina O, van Goor H. SARS-CoV-2 and the sympathetic immune response: Dampening inflammation with antihypertensive drugs (Clonidine and Propranolol). Med Hypotheses. 2020;144:110039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Porzionato A, Emmi A, Barbon S, Boscolo-Berto R, Stecco C, Stocco E, Macchi V, De Caro R. Sympathetic activation: a potential link between comorbidities and COVID-19. FEBS J. 2020;287:3681-3688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 63. | Seitz T, Szeles JC, Kitzberger R, Holbik J, Grieb A, Wolf H, Akyaman H, Lucny F, Tychera A, Neuhold S, Zoufaly A, Wenisch C, Kaniusas E. Percutaneous Auricular Vagus Nerve Stimulation Reduces Inflammation in Critical Covid-19 Patients. Front Physiol. 2022;13:897257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Gyebi GA, Batiha GE. Covid-19-Induced Dysautonomia: A Menace of Sympathetic Storm. ASN Neuro. 2021;13:17590914211057635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 65. | Vasanthakumar N. Beta-Adrenergic Blockers as a Potential Treatment for COVID-19 Patients. Bioessays. 2020;42:e2000094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 66. | Al-Beltagi M, Saeed NK, Bediwy AS. COVID-19 disease and autoimmune disorders: A mutual pathway. World J Methodol. 2022;12:200-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (5)] |
| 67. | Chadda KR, Blakey EE, Huang CL, Jeevaratnam K. Long COVID-19 and Postural Orthostatic Tachycardia Syndrome- Is Dysautonomia to Be Blamed? Front Cardiovasc Med. 2022;9:860198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 68. | Lavi Y, Vojdani A, Halpert G, Sharif K, Ostrinski Y, Zyskind I, Lattin MT, Zimmerman J, Silverberg JI, Rosenberg AZ, Shoenfeld Y, Amital H. Dysregulated Levels of Circulating Autoantibodies against Neuronal and Nervous System Autoantigens in COVID-19 Patients. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 69. | Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, Jayaseelan DL, Kumar G, Raftopoulos RE, Zambreanu L, Vivekanandam V, Khoo A, Geraldes R, Chinthapalli K, Boyd E, Tuzlali H, Price G, Christofi G, Morrow J, McNamara P, McLoughlin B, Lim ST, Mehta PR, Levee V, Keddie S, Yong W, Trip SA, Foulkes AJM, Hotton G, Miller TD, Everitt AD, Carswell C, Davies NWS, Yoong M, Attwell D, Sreedharan J, Silber E, Schott JM, Chandratheva A, Perry RJ, Simister R, Checkley A, Longley N, Farmer SF, Carletti F, Houlihan C, Thom M, Lunn MP, Spillane J, Howard R, Vincent A, Werring DJ, Hoskote C, Jäger HR, Manji H, Zandi MS. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104-3120. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 798] [Cited by in RCA: 795] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 70. | Cholankeril G, Podboy A, Aivaliotis VI, Tarlow B, Pham EA, Spencer SP, Kim D, Hsing A, Ahmed A. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology. 2020;159:775-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 71. | Chigr F, Merzouki M, Najimi M. Comment on "The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients". J Med Virol. 2020;92:703-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 72. | Zafra MA, Agüera AD, Molina F, Puerto A. Relevance of the nucleus of the solitary tract, gelatinous part, in learned preferences induced by intragastric nutrient administration. Appetite. 2017;118:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 73. | Follmer C. Gut Microbiome Imbalance and Neuroinflammation: Impact of COVID-19 on Parkinson's Disease. Mov Disord. 2020;35:1495-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Ghazanfar H, Kandhi S, Shin D, Muthumanickam A, Gurjar H, Qureshi ZA, Shaban M, Farag M, Haider A, Budhathoki P, Bhatt T, Ghazanfar A, Jyala A, Patel H. Impact of COVID-19 on the Gastrointestinal Tract: A Clinical Review. Cureus. 2022;14:e23333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Dai Y, Qiang W, Gui Y, Tan X, Pei T, Lin K, Cai S, Sun L, Ning G, Wang J, Guo H, Sun Y, Cheng J, Xie L, Lan X, Wang D. A large-scale transcriptional study reveals inhibition of COVID-19 related cytokine storm by traditional Chinese medicines. Sci Bull (Beijing). 2021;66:884-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 76. | Mahgoub S, Kotb El-Sayed MI, El-Shehry MF, Mohamed Awad S, Mansour YE, Fatahala SS. Synthesis of novel calcium channel blockers with ACE2 inhibition and dual antihypertensive/anti-inflammatory effects: A possible therapeutic tool for COVID-19. Bioorg Chem. 2021;116:105272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Hofman K, Shenoy GN, Chak V, Balu-Iyer SV. Pharmaceutical Aspects and Clinical Evaluation of COVID-19 Vaccines. Immunol Invest. 2021;50:743-779. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43:3-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 152] [Article Influence: 50.7] [Reference Citation Analysis (1)] |
| 79. | Hermel M, Sweeney M, Abud E, Luskin K, Criado JP, Bonakdar R, Gray J, Ahern T. COVID-19 Vaccination Might Induce Postural Orthostatic Tachycardia Syndrome: A Case Report. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Reddy S, Reddy S, Arora M. A Case of Postural Orthostatic Tachycardia Syndrome Secondary to the Messenger RNA COVID-19 Vaccine. Cureus. 2021;13:e14837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 81. | Lanman TA, Wu C, Cheung H, Goyal N, Greene M. Guillain-Barré Syndrome with Rapid Onset and Autonomic Dysfunction Following First Dose of Pfizer-BioNTech COVID-19 Vaccine: A Case Report. Neurohospitalist. 2022;12:388-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Eldokla AM, Numan MT. Postural orthostatic tachycardia syndrome after mRNA COVID-19 vaccine. Clin Auton Res. 2022;32:307-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | 83- Tahir N, Koorapati G, Prasad S, Jeelani HM, Sherchan R, Shrestha J, Shayuk M. SARS-CoV-2 Vaccination-Induced Transverse Myelitis. Cureus. 2021 Jul 25;13(7):e16624.. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Hilts A, Schreiber A, Singh A. A Clinical Case of COVID-19 Vaccine-Associated Guillain-Barré Syndrome. Am J Case Rep. 2022;23:e936896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 85. | Fitzsimmons W, Nance CS. Sudden Onset of Myelitis after COVID-19 Vaccination: An Under-Recognized Severe Rare Adverse Event. Cited May 2021. Available from: https://europepmc.org/article/PPR/PPR342408. |
| 86. | Akaishi T, Onodera T, Takahashi T, Harigae H, Ishii T. Reports of acute adverse events in mRNA COVID-19 vaccine recipients after the first and second doses in Japan. Sci Rep. 2022;12:15510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 87. | Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, Canas LS, Graham MS, Klaser K, Modat M, Murray B, Kerfoot E, Chen L, Deng J, Österdahl MF, Cheetham NJ, Drew DA, Nguyen LH, Pujol JC, Hu C, Selvachandran S, Polidori L, May A, Wolf J, Chan AT, Hammers A, Duncan EL, Spector TD, Ourselin S, Steves CJ. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 537] [Cited by in RCA: 539] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 88. | Freise NF, Kivel M, Grebe O, Meyer C, Wafaisade B, Peiper M, Zeus T, Schmidt J, Neuwahl J, Jazmati D, Luedde T, Bölke E, Feldt T, Jensen BEO, Bode J, Keitel V, Haussmann J, Tamaskovics B, Budach W, Fischer JC, Knoefel WT, Schneider M, Gerber PA, Pedoto A, Häussinger D, van Griensven M, Rezazadeh A, Flaig Y, Kirchner J, Antoch G, Schelzig H, Matuschek C. Acute cardiac side effects after COVID-19 mRNA vaccination: a case series. Eur J Med Res. 2022;27:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 89. | Naeem FN, Hasan SFS, Ram MD, Waseem S, Ahmed SH, Shaikh TG. The association between SARS-CoV-2 vaccines and transverse myelitis: A review. Ann Med Surg (Lond). 2022;79:103870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Takase B, Hayashi K, Takei S, Hisada T, Masaki N, Nagata M. Delayed Vasovagal Reaction with Reflex Syncope Following COVID-19 Vaccination. Intern Med. 2022;61:2167-2170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1132] [Article Influence: 226.4] [Reference Citation Analysis (1)] |
| 92. | Settanni CR, Ianiro G, Ponziani FR, Bibbò S, Segal JP, Cammarota G, Gasbarrini A. COVID-19 as a trigger of irritable bowel syndrome: A review of potential mechanisms. World J Gastroenterol. 2021;27:7433-7445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 93. | Rodríguez C, Luque N, Blanco I, Sebastian L, Barberà JA, Peinado VI, Tura-Ceide O. Pulmonary Endothelial Dysfunction and Thrombotic Complications in Patients with COVID-19. Am J Respir Cell Mol Biol. 2021;64:407-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 94. | Hassani M, Fathi Jouzdani A, Motarjem S, Ranjbar A, Khansari N. How COVID-19 can cause autonomic dysfunctions and postural orthostatic syndrome? A Review of mechanisms and evidence. Neurol Clin Neurosci. 2021;9:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 95. | Cipriano M, Ruberti E, Giacalone A. Gastrointestinal Infection Could Be New Focus for Coronavirus Diagnosis. Cureus. 2020;12:e7422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 96. | Ferreira N, Mikocka-Walus A, van Tilburg MAL, Graff LA, Apputhurai P, Barreiro-de Acosta M, Bennebroek Evertsz F, Burisch J, Lo B, Petrik M, Trindade IA, Jedel S, Moser G, Mokrowiecka A, Bernstein CN, Dumitrascu D, Ford AC, Stengel A, Gearry R, Knowles SR. The impact of the coronavirus (COVID-19) pandemic on individuals with gastrointestinal disorders: A protocol of an international collaborative study. J Psychosom Res. 2021;148:110561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Hunt RH, East JE, Lanas A, Malfertheiner P, Satsangi J, Scarpignato C, Webb GJ. COVID-19 and Gastrointestinal Disease: Implications for the Gastroenterologist. Dig Dis. 2021;39:119-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (2)] |
| 98. | Bogariu AM, Dumitrascu DL. Digestive involvement in the Long-COVID syndrome. Med Pharm Rep. 2022;95:5-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 99. | Blitshteyn S, Whiteson JH, Abramoff B, Azola A, Bartels MN, Bhavaraju-Sanka R, Chung T, Fleming TK, Henning E, Miglis MG, Sampsel S, Silver JK, Tosto J, Verduzco-Gutierrez M, Putrino D. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R. 2022;14:1270-1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 100. | Shouman K, Vanichkachorn G, Cheshire WP, Suarez MD, Shelly S, Lamotte GJ, Sandroni P, Benarroch EE, Berini SE, Cutsforth-Gregory JK, Coon EA, Mauermann ML, Low PA, Singer W. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31:385-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 189] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 101. | Cheshire WP Jr, Goldstein DS. The physical examination as a window into autonomic disorders. Clin Auton Res. 2018;28:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Westergren A, Wictorin K, Hansson O, Hagell P. Novel insights regarding the measurement properties of the SCOPA-AUT. BMC Neurol. 2022;22:478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 103. | Sellami L, Kacem I, Nasri A, Djebara MB, Sidhom Y, Gargouri A, Gouider R. Evaluation of an Arabic version of the non-motor symptoms scale in Parkinson's disease. Neurol Sci. 2016;37:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 104. | Goudouris ES. Laboratory diagnosis of COVID-19. J Pediatr (Rio J). 2021;97:7-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 105. | Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta. 2020;510:475-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 106. | Gutiérrez C, Marco A, Nogales A, Tebar R. Total and segmental colonic transit time and anorectal manometry in children with chronic idiopathic constipation. J Pediatr Gastroenterol Nutr. 2002;35:31-38. [PubMed] [DOI] [Full Text] |
| 107. | Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch Med Sci. 2010;6:11-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 108. | Illigens BM, Gibbons CH. Sweat testing to evaluate autonomic function. Clin Auton Res. 2009;19:79-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 109. | Chrysant SG. The tilt table test is useful for the diagnosis of vasovagal syncope and should not be abolished. J Clin Hypertens (Greenwich). 2020;22:686-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Peixoto A, Silva M, Pereira P, Macedo G. Biopsies in Gastrointestinal Endoscopy: When and How. GE Port J Gastroenterol. 2016;23:19-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Nelms DW, Kann BR. Imaging Modalities for Evaluation of Intestinal Obstruction. Clin Colon Rectal Surg. 2021;34:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 112. | Caraiani C, Yi D, Petresc B, Dietrich C. Indications for abdominal imaging: When and what to choose? J Ultrason. 2020;20:e43-e54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 113. | Jin S, Lu X, Xu C. COVID-19 induces gastrointestinal symptoms and affects patients' prognosis. J Int Med Res. 2022;50:3000605221129543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |