Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3158
Peer-review started: February 19, 2023
First decision: February 28, 2023
Revised: March 17, 2023
Accepted: April 6, 2023
Article in press: April 6, 2023
Published online: May 16, 2023
Processing time: 85 Days and 23.1 Hours
Brain gliomas are malignant tumors with high postoperative recurrence rates. Early prediction of prognosis using specific indicators is of great significance.
To assess changes in ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) levels in patients with glioma pre-and postoperatively.
Between June 2018 and June 2021, 91 patients with gliomas who underwent surgery at our hospital were enrolled in the glioma group. Sixty healthy vol
UCH-L1 and GFAP levels in patients with glioma decreased significantly 3 d after surgery compared to those before therapy (P < 0.05). However, UCH-L1 and GFAP levels in the glioma group were significantly higher than those in the control group before and after surgery (P < 0.05). There were no statistically significant differences in preoperative serum UCH-L1 and GFAP levels among patients with glioma according to sex, age, pathological type, tumor location, or number of lesions (P > 0.05). Serum UCH-L1 and GFAP levels were significantly lower in the patients with WHO grade I-II tumors than in those with grade III-IV tumors (P < 0.05). Serum UCH-L1 and GFAP levels were lower in the patients with tumor diameter ≤ 5 cm than in those with diameter > 5 cm, in which the differences were statistically significant (P < 0.05). Glioma recurred in 22 patients. The preoperative and 3-d postoperative serum UCH-L1 and GFAP levels were significantly higher in the recurrence group than these in the non-recurrence group (P < 0.05). Receiver operating characteristic curves were plotted. The areas under the curves of preoperative serum UCH-L1 and GFAP levels for predicting postoperative glioma recurrence were 0.785 and 0.775, respectively. However, the efficacy of serum UCH-L1 and GFAP levels 3 d after surgery in predicting postoperative glioma recurrence was slightly lower compared with their preoperative levels.
UCH-L1 and GFAP efficiently reflected the development and recurrence of gliomas and could be used as potential indicators for the recurrence and prognosis of glioma.
Core Tip: Since the recurrence rate of glioma is high, it is important to early predict its prognosis. Ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) are important markers for nervous system damages and lesions. Therefore, we evaluated the changes in UCH-L1 and GFAP levels in patients with glioma during the perioperative period and compared them with those in healthy volunteers to analyze their relationship with clinicopathological and postoperative recurrence. These results revealed that UCH-L1 and GFAP might reflect the development and recurrence of glioma and could be used as potential indicators to estimate prognosis of glioma.
- Citation: Zhu QH, Wu JK, Hou GL. Changes and significance of serum ubiquitin carboxyl-terminal hydrolase L1 and glial fibrillary acidic protein in patients with glioma. World J Clin Cases 2023; 11(14): 3158-3166
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3158.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3158
Brain glioma is an extremely common type of intracranial malignant tumor that deteriorates, grows rapidly and causes severe neurological impairment. Due to the poor sensitivity of brain gliomas to radiotherapy, they are mainly managed by surgical resection. However, some gliomas are large in size or close to important neural tissues and are difficult to completely remove intraoperatively. Therefore, the recurrence rate of brain gliomas after surgery is high[1,2]. Early prediction of postoperative prognosis in patients with gliomas is of great importance in clinical practice. Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) is a cysteine hydrolase that regulates the cell cycle, is involved in apoptosis and inflammatory responses, is present at high levels in the brain, and is regarded as a biomarker of brain injury[3]. Glial fibrillary acidic protein (GFAP) is a specific indicator of astrocytes and involved in neurological damage and lesions[4]. In this study, we evaluated changes in serum UCH-L1 and GFAP levels in patients with glioma before and after surgery. We also assessed the relationship between the two indicators and analyzed data of the patients’ clinicopathological characteristics and postoperative recurrence. We also aimed to determine the values of UCH-L1 and GFAP for predicting glioma recurrence.
A total of 91 patients with gliomas who underwent surgery in the hospital between June 2018 and June 2021 were enrolled in the glioma group. The control group included 60 healthy volunteers during the same period.
The inclusion criteria comprised patients: Who were treated surgically; with glioma that was clearly detected by postoperative pathological examination; who underwent no radiotherapy before surgery; and with complete clinical case data. Patients with: acromegaly, hepatitis, and other diseases; severe defects in vital organ function; severe complications, and postoperative death; or other malignant tumors were excluded from the study.
The age and sex ratios in the control group were similar to those in the glioma group: Both groups underwent physical examination and had no previous history of tumors, intracranial lesions, or brain injury.
All patients with gliomas were surgically treated, and 5 mL of peripheral venous blood was collected from these patients with glioma before and 3 d after surgery. In the control group, venous blood was collected during fasting in the early morning on day two after enrollment.
Blood samples were immediately centrifuged at 3000 rpm for 15 min. The liquid supernatant was separated and stored at -80°C for later use. Serum GFAP and UCH-L1 Levels were detected by ABC-ELISA, and kits were purchased from Rapid Bio, USA. The experimental procedure was performed in strict accordance with the relevant kit standards.
(1) To compare UCH-L1 and GFAP levels between the glioma and control groups; (2) To analyze data of preoperative serum UCH-L1 and GFAP levels in patients with gliomas with different clinicopathological features; and (3) The patients were followed up until February 2022 to record the preoperative recurrence of glioma and compare serum UCH-L1 and GFAP levels between the recurring and non-recurring patients. A receiver operating characteristic (ROC) curve was drawn. Furthermore, the values of preoperative and postoperative serum UCH-L1 and GFAP levels for predicting postoperative glioma recurrence were analyzed.
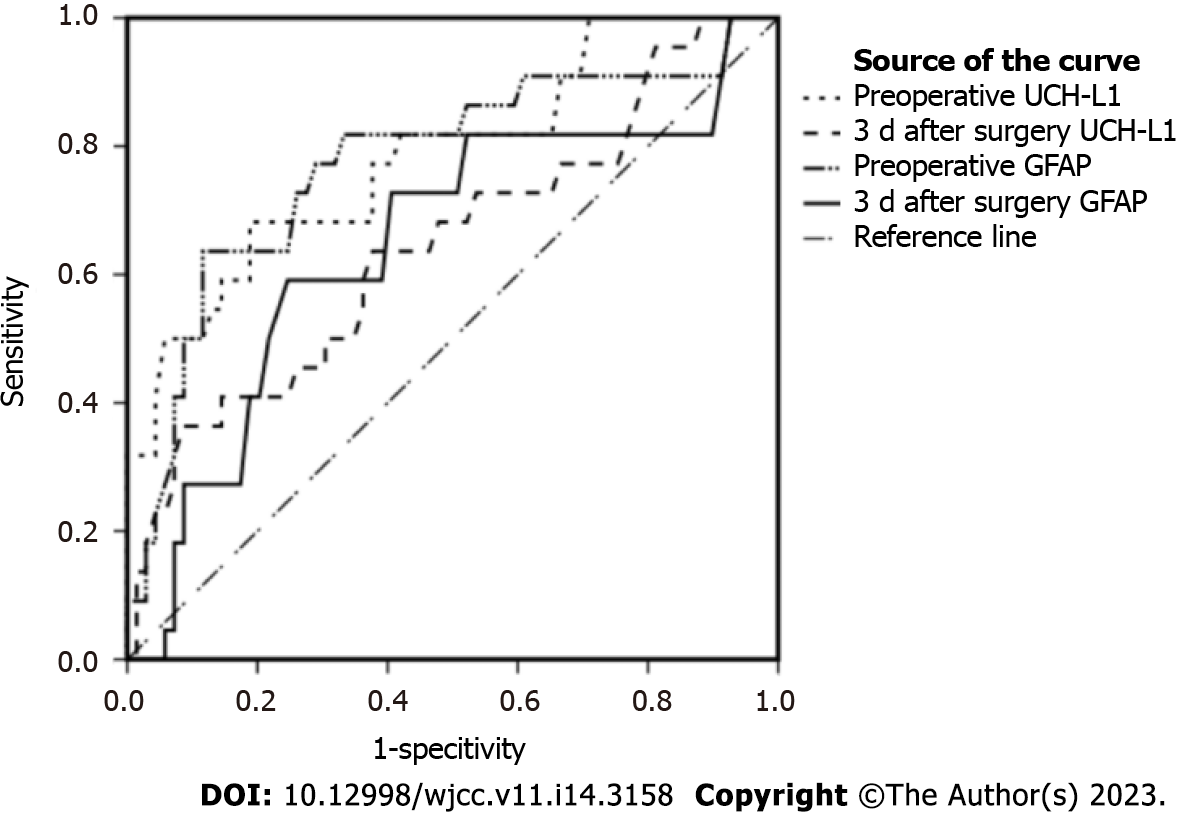
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 19.0. The measurement data was presented as mean ± SD. The independent samples t test was used for mean comparison between the two groups. The mean data before and after treatment were analyzed using the paired t test, and the count data were conveyed by case. The χ2 test was used to compare the two groups. ROC curves were drawn (Figure 1). In addition, the best critical value was calculated by the Youden index formula to evaluate the efficacy of preoperative and postoperative serum UCH-L1 and GFAP levels in predicting postoperative glioma recurrence. P value < 0.05 was considered statistically significant.
UCH-L1 and GFAP levels in the patients with glioma decreased significantly 3 d after surgery compared with their pre-therapy levels (P < 0.05). However, the UCH-L1 and GFAP levels in the glioma group were significantly higher than those in the control group before and after surgery (P < 0.05, Table 1).
There were no significant differences in the preoperative serum UCH-L1 and GFAP levels in patients with brain glioma with respect to sex, age, pathological type, tumor location, or number of lesions (P > 0.05, Table 2). The UCH-L1 and GFAP levels in the patients with WHO grade I-II tumors were lower than those in the participants with grade III-IV tumors. Additionally, the UCH-L1 and GFAP levels in the patients with tumor diameters ≤ 5 cm were lower than those in the participants with tumor diameters > 5 cm.
| Clinicopathological features | UCH-L1 (pg/mL) | GFAP (ng/L) |
| Gender | ||
| Male (n = 49) | 95.89 ± 16.79 | 16.58 ± 2.14 |
| Female (n = 42) | 98.05 ± 17.69 | 16.82 ± 2.20 |
| t value | 0.597 | 0.515 |
| P value | 0.552 | 0.608 |
| Age (yr) | ||
| < 40 (n = 44) | 99.78 ± 18.42 | 17.03 ± 2.29 |
| ≥ 40 (n = 47) | 94.19 ± 15.58 | 16.37 ± 2.01 |
| t value | 1.566 | 1.483 |
| P value | 0.121 | 0.141 |
| Pathological type | ||
| Glioblastoma (n = 76) | 98.12 ± 17.14 | 16.84 ± 2.16 |
| Medulloblastoma (n = 15) | 90.65 ± 16.35 | 15.92 ± 2.04 |
| t value | 1.554 | 1.512 |
| P value | 0.124 | 0.134 |
| Tumor location | ||
| Frontal lobe (n = 41) | 95.28 ± 16.24 | 16.50 ± 2.09 |
| Temporal lobe (n = 39) | 99.14 ± 17.59 | 16.97 ± 2.21 |
| Other locations (n = 11) | 94.93 ± 19.56 | 16.39 ± 2.29 |
| F value | 0.582 | 0.592 |
| P value | 0.561 | 0.555 |
| Tumor grade | ||
| WHO I-II grade (n = 33) | 78.89 ± 5.05 | 14.39 ± 0.84 |
| WHO III-IV grade (n = 58) | 107.13 ± 12.48 | 18.01 ± 1.47 |
| t value | 12.402 | 12.900 |
| P value | < 0.000 | < 0.001 |
| Tumor diameter | ||
| ≤ 5 cm (n = 19) | 75.23 ± 3.04 | 13.80 ± 0.59 |
| > 5 cm (n = 72) | 102.61 ± 14.55 | 17.45 ± 1.73 |
| t value | 8.123 | 9.006 |
| P value | < 0.001 | < 0.001 |
| Number of lesions | ||
| Single (n = 70) | 97.57 ± 17.70 | 16.77 ± 2.25 |
| Multiple (n = 21) | 94.63 ± 15.37 | 16.43 ± 1.84 |
| t value | 0.685 | 0.619 |
| P value | 0.495 | 0.537 |
All patients were followed up until February 2022. A total of 22 patients with gliomas experienced recurrence. The preoperative and 3 d postoperative serum UCH-L1 and GFAP levels were significantly higher in the recurrence group compared with the non-recurrence group (P < 0.05, Table 3).
| Time | UCH-L1 (pg/mL) | GFAP (ng/L) |
| Recurrence group (n = 22) | ||
| Preoperative | 120.44 ± 6.41 | 19.59 ± 0.57 |
| 3 d after surgery | 88.01 ± 2.44 | 10.00 ± 0.46 |
| t value | 37.398 | 289.806 |
| P value | < 0.001 | < 0.001 |
| Non-recurrence group (n = 69) | ||
| Preoperative | 89.38 ± 11.83 | 15.76 ± 1.58 |
| 3 d after surgery | 67.09 ± 9.60 | 6.74 ± 1.16 |
| t value | 78.571 | 172.100 |
| P value | < 0.001 | < 0.001 |
| Preoperative comparison of the two groups | ||
| t value | 11.749 | 11.118 |
| P value | < 0.001 | < 0.001 |
| Comparison of the two groups at 3 d after surgery | ||
| t value | 10.086 | 12.791 |
| P value | < 0.001 | < 0.001 |
The AUC of the preoperative serum UCH-L1 and GFAP levels for predicting postoperative glioma recurrence were 0.785 and 0.775, respectively (Table 4). The efficacy of UCH-L1 and GFAP levels 3 d after surgery in predicting postoperative glioma recurrence was slightly lower than their preoperative levels.
| Indicator | Critical value | AUC | 95%CI | P value | Sensitivity (%) | Specificity (%) |
| Preoperative UCH-L1 | 103.85 | 0.785 | 0.670-0.901 | < 0.001 | 68.2 | 81.2 |
| 3 d after surgery UCH-L1 | 85.61 | 0.646 | 0.507-0.785 | 0.040 | 63.6 | 62.3 |
| Preoperative GFAP | 18.70 | 0.775 | 0.651-0.898 | < 0.001 | 63.6 | 88.4 |
| 3 d after surgery GFAP | 8.58 | 0.648 | 0.508-0.787 | 0.038 | 59.1 | 75.4 |
Glioma is a tumor caused by glial cell lesions originating from the ectoderm of the nervous system with an incidence of approximately 5/100000. Owing to the lack of specific tumor markers related to gliomas, imaging examinations such as brain computed tomography or magnetic resonance imaging are the main methods for the clinical assessment of changes and treatment effects during the course of glioma. However, imaging examinations are particularly lagging behind clinical treatment and prognostic determination[5,6]. Therefore, finding more sensitive indicators that reflect treatment effect and prognosis in patients with glioma as soon as possible was one of the aims of the current study.
UCH-L1 is a member of the ubiquitin protease system family, which mainly consists of 223 amino acids, and is abundant in the brain. It is involved in cell proliferation, differentiation, apoptosis, and other physiological processes via the ubiquitin pathway. In addition, UCH-L1 has been shown to be relevant to brain nervous system development, brain tumors, and brain injury[7,8]. Studies have shown that[9] after acute cerebral infarction, a large amount of UCH-L1 could be released from damaged nerve cells and penetrate the blood-brain barrier into the blood circulation. Therefore, serum UCH-L1 levels were elevated in patients with cerebral infarction. Wang et al[10] found that serum UCH-L1 Levels had good clinical value for reflecting the degree of brain injury and prognosis in patients with severe craniocerebral injury. Elevated levels of UCH-L1 in the cerebrospinal fluid and peripheral blood have become effective indicators of the severity of central nervous cell damage.
This study revealed that the preoperative serum UCH-L1 levels in patients with glioma were notably higher than those in the control group. Furthermore, UCH-L1 Levels in patients with gliomas significantly decreased after surgical treatment. However, the postoperative UCH-L1 level was also higher than that in healthy controls. This may be related to the fact that under compression by glioma, part of the brain nerve tissue could have been damaged, which in turn released a large amount of UCH-L1, leading to an increase in serum UCH-L1 Levels. Subsequently, the glioma was removed to relieve the compressed brain tissues and decrease the release of UCH-L1 from damaged nerve cells.
The WHO classifies gliomas into grades I–IV, with grades I–II as low-grade and those of III–IV as high-grade gliomas. This study demonstrated that UCH-L1 Levels in the patients with WHO grade III-IVI-II tumors were higher than those in those with grade I-II tumors. Additionally, the UCH-L1 Level was greater in the patients with a tumor diameter > 5 cm than in those with diameter ≤ 5 cm. It has been suggested that serum UCH-L1 Levels reflected development of glioma.
GFAP is a cytoskeletal protein that maintains the morphological and structural stability of astrocytes and determines the degree of astrocyte response to injury[11]. Some studies have shown that after central nervous system damage, astrocytes were abnormally active, manifesting as rapid synthesis and secretion of GFAP, and the addition of GFAP-positive astrocytes could further promote astrocyte mitosis. Some studies have found[12] that positive GFAP expression in astrocytes adjacent to the cerebral cortex significantly increased after brain injury. Feng et al[13] found that an increase in GFAP levels in patients with severe craniocerebral injury after surgery was a risk factor for poor prognosis, which had a certain value in promoting postoperative survival. Wang et al[14] found that serum GFAP levels were elevated in asphyxiated preterm infants with brain injury and serum GFAP had some value in the diagnosis of brain injury and could be used as a marker for central nervous system injury and prognosis.
We found that the preoperative serum GFAP levels in the patients with glioma were higher than those in the control group. After surgery, the serum GFAP levels in these patients with gliomas decreased. However, this level was higher than that observed in healthy controls. In addition, the serum GFAP levels in the patients with WHO grade III-IVI-II tumors were dramatically higher than those in the participants with grade I-II tumors. The serum GFAP level in the patients with tumor diameter > 5 cm was higher than that in those with diameter ≤ 5 cm. It has been suggested that serum GFAP was valuable in predicting the occurrence and development of gliomas.
In the early stages of glioma, patients do not exhibit specific clinical manifestations. However, by the time the disease is diagnosed, glioma is mostly advanced, with a large tumor size involving important functional brain nerve areas[15-17]. In addition, distinguishing the boundary between the tumor and normal brain tissue becomes difficult, making complete removal of the tumor challenging and resulting in residual tumor tissue, which is considered the main reason for postoperative recurrence[18-20]. In this study, among the 91 patients with glioma, 22 experienced recurrence after surgery. In addition, the UCH-L1 and GFAP levels were higher in the recurrence group than that in the non-recurrence group before and 3 d after surgery. This indicated that serum UCH-L1 and GFAP levels had the potential to reflect postoperative glioma recurrence. By plotting ROC curves, we found that the efficacy of both preoperative UCH-L1 and GFAP levels in predicting postoperative glioma recurrence was slightly higher than that 3 d after surgery. However, limited by the study design, we did not discuss the optimal time points for serum UCH-L1 and GFAP levels to predict postoperative glioma recurrence. This study also did not consider the specific mechanisms of action of these two indicators of gliomas, which warrants further research.
The UCH-L1 and GFAP levels abnormally increased in patients with gliomas. Although the levels of these two indices decreased after the surgical treatment, they remained higher than those in the control group. Both serum UCH-L1 and GFAP levels may specifically reflect the development and postoperative recurrence of glioma. These two markers could be used as potential indicators of recurrence and prognosis in patients with postoperative glioma.
Glioma is a very common intracranial malignant tumor with a high degree of malignancy, rapid growth, and high postoperative recurrence rate, which could cause severe damage to the nervous system. Early prediction of postoperative prognosis in patients with glioma is of great clinical significance.
Ubiquitin carboxyl terminal hydrolase L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) reflect damage and lesions in the nervous system. Changes in serum UCH-L1 and GFAP levels in patients with glioma before and after surgery, and the relationship between them, have not been clarified.
This study aimed to assess the changes and correlation between pre-and postoperative serum UCH-L1 and GFAP levels in patients with glioma and predict the postoperative prognosis of patients with glioma after surgery.
Total 91 patients with glioma were included in the experimental group and 60 healthy volunteers were selected as the control group. In the experimental group, 5 mL of peripheral venous blood was collected before and 3 d after surgery to detect UCH-L1 and GFAP levels in the peripheral blood serum. In the control group, venous blood was collected on an empty stomach morning on the second day after enrollment to monitor the levels of UCH-L1 and GFAP in the peripheral blood serum. At the same time, the postoperative recurrence of glioma was recorded to determine the value of serum UCH-L1 and GFAP for predicting glioma prognosis.
UCH-L1 and GFAP levels 3 d after surgery in the patients with gliomas were significantly lower than those before surgery. Moreover, the UCH-L1 and GFAP levels in the glioma group were significantly higher than those in the control group before and after surgery. The levels of serum UCH-L1 and GFAP in 22 patients with glioma recurrence were higher compared with the non-recurrence group before and 3 d after surgery, and the difference was statistically significant.
Although serum UCH-L1 and GFAP levels in the patients with glioma were abnormally increased, these levels decreased after surgery. Serum UCH-L1 and GFAP levels may be potential indicators for predicting the postoperative recurrence and prognosis of glioma.
Future work and clinical research should be conducted to verify the accuracy of the experimental results through a more rigorous experimental design, expanded sample size, and multicenter studies and to provide favorable evidence for predicting the recurrence and prognosis of glioma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pierro A, Canada; Shah PS, Canada S-Editor: Wang JL L-Editor: A P-Editor: Yuan YY
| 1. | Ren CC, Zhang LT, Kang JS, Kang L, Wang QX, Zhao J. Expressions and Diagnostic Efficacies of Serum NSE, CA15-3, S100B and IGF-1 in Patients with Brain Glioma. Jiefangjun Yiyao Zazhi. 2022;34:25-28. [DOI] [Full Text] |
| 2. | He LJ, Ren J, Zhao YB, Gao Q, Xu JC, Wang J. Scalp electroencephalogram characteristics of ganglioglioma and its correlation with post-operative prognosis. Dianxian Yu Shenjingdianshenglixue Zazhi. 2022;31:12-21. [DOI] [Full Text] |
| 3. | Amoo M, Henry J, O'Halloran PJ, Brennan P, Husien MB, Campbell M, Caird J, Javadpour M, Curley GF. S100B, GFAP, UCH-L1 and NSE as predictors of abnormalities on CT imaging following mild traumatic brain injury: a systematic review and meta-analysis of diagnostic test accuracy. Neurosurg Rev. 2022;45:1171-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Amalia L. Glial Fibrillary Acidic Protein (GFAP): Neuroinflammation Biomarker in Acute Ischemic Stroke. J Inflamm Res. 2021;14:7501-7506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 5. | Leibetseder A, Leitner J, Mair MJ, Meckel S, Hainfellner JA, Aichholzer M, Widhalm G, Dieckmann K, Weis S, Furtner J, von Oertzen T, Preusser M, Pichler J, Berghoff AS. Prognostic factors in adult brainstem glioma: a tertiary care center analysis and review of the literature. J Neurol. 2022;269:1574-1590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Nicholson JG, Fine HA. Diffuse Glioma Heterogeneity and Its Therapeutic Implications. Cancer Discov. 2021;11:575-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 278] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 7. | Richard M, Lagares A, Bondanese V, de la Cruz J, Mejan O, Pavlov V, Payen JF; BRAINI investigators. Study protocol for investigating the performance of an automated blood test measuring GFAP and UCH-L1 in a prospective observational cohort of patients with mild traumatic brain injury: European BRAINI study. BMJ Open. 2021;11:e043635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Papa L, Ladde JG, O'Brien JF, Thundiyil JG, Tesar J, Leech S, Cassidy DD, Roa J, Hunter C, Miller S, Baker S, Parrish GA, Davison J, Van Dillen C, Ralls GA, Briscoe J, Falk JL, Weber K, Giordano PA. Evaluation of Glial and Neuronal Blood Biomarkers Compared With Clinical Decision Rules in Assessing the Need for Computed Tomography in Patients With Mild Traumatic Brain Injury. JAMA Netw Open. 2022;5:e221302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Shan HL, Jiao GM, Cheng X, Ma Z, Gao YJ, Yang N, Dou ZJ. Changes and significance of serum UCH-L1 and Fibulin-5 levels in patients with acute cerebral infarction. Shandong Yiyao. 2021;61:32-36. [DOI] [Full Text] |
| 10. | Wang J, Zhang HY, Du P, Wan J. The Predictive Value of the Serum Ubiquitin Carboxyl-terminal Hydrolase L1 and Neutrophil Gelatinase-associated Lipocalin to the Ill Condition and Prognosis in Patients with Severe Brain Injury. Biaojimianyifenxi Yu Linchuang. 2020;27:195-199, 205. |
| 11. | Yuan W, Lu L, Rao M, Huang Y, Liu CE, Liu S, Zhao Y, Liu H, Zhu J, Chao T, Wu C, Ren J, Lv L, Li W, Qi S, Liang Y, Yue S, Gao J, Zhang Z, Kong E. GFAP hyperpalmitoylation exacerbates astrogliosis and neurodegenerative pathology in PPT1-deficient mice. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Hausmann R, Riess R, Fieguth A, Betz P. Immunohistochemical investigations on the course of astroglial GFAP expression following human brain injury. Int J Legal Med. 2000;113:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Feng AP, Wang W, Du C. The relationship between the postoperative levels of serum copeptin and GFAP and the prognosis of patients with severe traumatic brain injury. Shiyong Yiyuan Linchuang Zazhi. 2022;19:132-135. [DOI] [Full Text] |
| 14. | Wang T, Li YF, Wang XS, Liu ZHJ. Diagnostic value of serum HMGB1, GFAP, and UCH-L1 for brain injury in asphyxia premature infants. Guoji Jianyan Yixue Zazhi. 2021;42:1549-1553. [DOI] [Full Text] |
| 15. | Yang Y. The factors related to postoperative recurrence in frontal low-grade gliomas after neurosurgeon determined gross-total resection. Litidingxiang He Gongnengxing Shenjingwaike Zazhi. 2020;33:280-284. [DOI] [Full Text] |
| 16. | Zhang QH, Duan WC, Liu XZ, Zhang ZHY. Clinical characteristics and postoperative survival of asymptomatic patients with WHO grade Ⅱ gliomas. Zhonghua Shenjingwaike Zazhi. 2020;36:405-409. [DOI] [Full Text] |
| 17. | Ng S, Lemaitre AL, Moritz-Gasser S, Herbet G, Duffau H. Recurrent Low-Grade Gliomas: Does Reoperation Affect Neurocognitive Functioning? Neurosurgery. 2022;90:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Rubin MC, Sagberg LM, Jakola AS, Solheim O. Primary versus recurrent surgery for glioblastoma-a prospective cohort study. Acta Neurochir (Wien). 2022;164:429-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Teyateeti A, Geno CS, Stafford SS, Mahajan A, Yan ES, Merrell KW, Laack NN, Parney IF, Brown PD, Jethwa KR. Does the dural resection bed need to be irradiated? Neurooncol Pract. 2021;8:190-198. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Strand PS, Berntsen EM, Fyllingen EH, Sagberg LM, Reinertsen I, Gulati S, Bouget D, Solheim O. Brain infarctions after glioma surgery: prevalence, radiological characteristics and risk factors. Acta Neurochir (Wien). 2021;163:3097-3108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |