Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.177
Peer-review started: September 13, 2022
First decision: October 14, 2022
Revised: October 21, 2022
Accepted: December 19, 2022
Article in press: December 19, 2022
Published online: January 6, 2023
Processing time: 113 Days and 23.5 Hours
A 70-year-old man with hepatitis C virus-related recurrent hepatocellular carcinoma was admitted for further diagnosis of a 1 cm iso-hyperechoic nodule in segment (S) 5.
Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) revealed the nodule in S5 with a defect at the hepatobiliary phase, hyperintensity on diffusion weighted imaging (DWI) and hypointensity on apparent diffusion coefficient (ADC) map. Contrast-enhanced computed tomography revealed hypervascularity at the early phase, and delayed contrast-enhancement was observed at the late phase. Contrast-enhanced ultrasound (US) revealed incomplete defect at the late vascular phase. Inflammatory liver tumor, lymphoproliferative disease, intrahepatic cholangiocarcinoma (small duct type) and bile duct adenoma were suspected through the imaging studies. US guided biopsy, however, showed a noncaseating hepatic sarcoid-like epithelioid granuloma (HSEG), and histopathological analysis disclosed spindle shaped epithelioid cells harboring Langhans-type multinucleated giant cells. One month after admission, EOB-MRI signaled the disappearance of the defect at the hepatobiliary phase, of hyperintensity on DWI, of hypointensity on ADC map, and no stain at the early phase.
That the patient had received BNT162b2 messenger RNA (mRNA) coronavirus disease 2019 vaccination 3 mo before the occurrence of HSEG, and that its disappearance was confirmed 4 mo after mRNA vaccination suggested that the drug-induced sarcoidosis-like reaction (DISR) might be induced by the mRNA vaccination. Fortunately, rechallenge of drug-induced DISR with the third mRNA vaccination was not confirmed.
Core Tip: We describe a case of drug-induced sarcoidosis-like reaction (DISR) a noncaseating hepatic sarcoid-like epithelioid granuloma (HSEG). Histopathological analysis disclosed, characteristic spindle-shaped epithelioid cells harboring Langhans-type multinucleated giant cells. Two months and 5 mo after the third BNT162b2 messenger RNA (mRNA) coronavirus disease 2019 vaccination, the occurrence of HSEG was not confirmed before rechallenging the drug-induced DISR by the third mRNA vaccination.
- Citation: Kim SR, Kim SK, Fujii T, Kobayashi H, Okuda T, Hayakumo T, Nakai A, Fujii Y, Suzuki R, Sasase N, Otani A, Koma YI, Sasaki M, Kumabe T, Nakashima O. Drug-induced sarcoidosis-like reaction three months after BNT162b2 mRNA COVID-19 vaccination: A case report and review of literature. World J Clin Cases 2023; 11(1): 177-186
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/177.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.177
A drug-induced sarcoidosis-like reaction (DISR) displaying a systemic granulomatous tissue reaction is indistinguishable from sarcoidosis and occurs in a temporal manner initiated by an antagonistic drug[1]. To date, there is no clinical distinction between DISR and sarcoidosis; both have been associated with bilateral hilar adenopathy, cutaneous lesions, uveitis, granulomatous infiltration of scars, hypercalcemia, elevated serum angiotensin-converting enzyme levels, and 18F-fluorodeoxyglucose uptake, all of which appear on positron emission tomography (PET) scans[1].
A 1 cm hepatic sarcoid-like epithelioid granuloma (HSEG) was diagnosed through histopathological examination in a 70-year-old man 3 mo after his receiving two BNT162b2 messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccinations. The disappearance of the HSEG was confirmed through imaging studies 4 mo after the mRNA vaccination.
A 70-year-old man with hypertension and diabetes mellitus was in October 2021 admitted to Kobe Asahi Hospital for evaluation of a 1 cm iso-hyperechoic nodule in segment (S) 5.
The patient had overcome hepatitis C virus (HCV) infection 16 years earlier with Pegylated interferon (PEG-IFN) α2b + Ribavirin for 24 wk, and a 1 cm hepatocellular carcinoma (HCC) in S2 was completely resected in 2017.
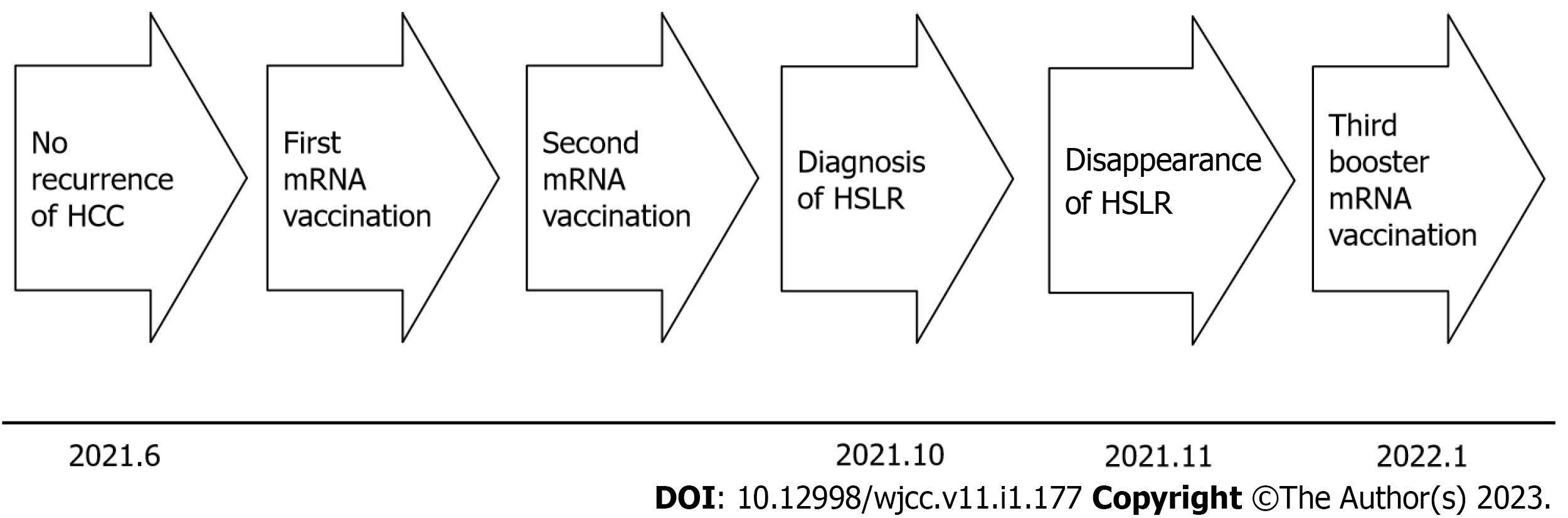
A 4 cm HCC between S7 and S8 was removed by microwave ablation in April 2020; however, due to local recurrence, the HCC was re-ablated in February 2021, and subsequent imaging studies including gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) signaled disappearance of the HCC. Follow-up imaging studies with EOB-MRI in June 2021 also signaled disappearance of any recurrent tumor. Thereafter, the first, and after a three-week interval, the second mRNA vaccination were administered to the patient in June, without any particular side effects such as anaphylaxis, fever, fatigue, general malaise or muscle pain (Figure 1). From 2020 to 2021, except for the mRNA vaccine, no other particular drugs, injections, immune checkpoint inhibitors (ICIs), highly active antiretroviral therapy (HAART), IFNs, tumor necrosis factor (TNF)-α antagonists, BRAF inhibitors, methotrexate or Bacille de Calmette et Guérin (BCG) were administered.
He suffered a cerebral hemorrhage in 2008, myocardial infarction in 2010, and from prostatic cancer, bladder cancer and lower right ureter cancer in 2019. At surgery for bladder cancer, BCG was not administered.
Nothing particular.
On admission, the patient weighed 59.0 kg, was 157.5 cm tall and had a BMI of 23.8; a physical examination showed no remarkable abnormalities.
Laboratory values and tumor markers at admission are shown in Table 1.
| AST | 34 U/L (7-38 IU/L) | HbA1c | 6.3% (4.6%-6.2%) |
| ALT | 15 U/L (4-44 IU/L) | HCV Ab | (+) |
| ALP | 118 U/L (36-126 IU/mL) | HCV RNA | (-) |
| LDH | 206 U/L (120-240 U/L) | HBs Ag | (-) |
| γ-GTP | 104 U/L (< 40 IU/L) | HBs Ab | (-) |
| T-Bil | 1.0 mg/dL (0.2-1.2 mg/dL) | HBc Ab | (-) |
| TP | 9.0 g/dL (6.5-8.2 g/dL) | sIL-2R | 937 U/mL (122-496 U/mL) |
| Alb | 4.6 g/dL (3.9-4.9 g/dL) | Lysozyme | 16.7 µg/mL (5.0-10.0 µg/mL) |
| AMY | 126 U/L (38-136 U/L) | ACE | 12.5 U/L (7.7-29.4 IU/L) |
| CRP | 0.52 mg/dL (< 0.30 mg/dL) | IgG | 2464 mg/dL (800-1750 mg/dL) |
| BUN | 21.2 mg/dL (< 40 IU/L) | IgA | 547 mg/dL (100-450 mg/dL) |
| Cre | 1.28 mg/dL (0.61-1.04 mg/dL) | IgM | 50 mg/dL (45-300 mg/dL) |
| WBC | 5200/µL (3600-9000/µL) | ANA | (-) |
| RBC | 325 × 104/µL [(410-530) × 104/µL] | AMA | (-) |
| Hb | 10.6 g/dL (13-18 g/dL) | AFP | 3.3 ng/mL (< 10.0 ng/mL) |
| Plt | 17.2 × 104/µL [(12-30) × 104/µL] | PIVKA-II | 26 mAU/mL (< 40 mAU/mL) |
| FBG | 110 mg/dL (60-110 mg/dL) | CA19-9 | 26.8 U/mL (< 37.0 U/mL) |
| CEA | 1.4 ng/mL (< 5.0 ng/mL) |
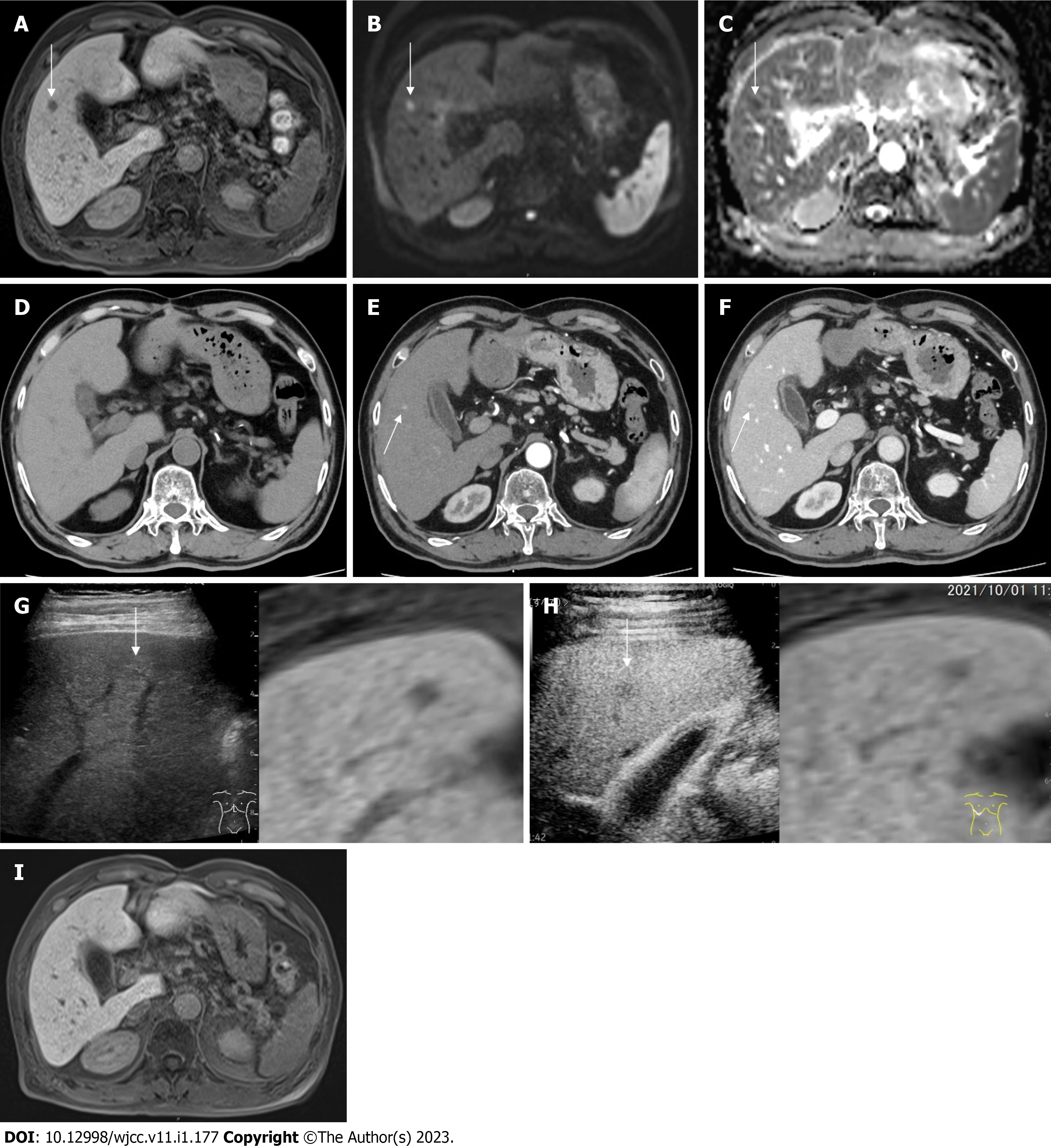
MRI findings: Follow-up imaging studies with EOB-MRI in October, 2021 revealed a 1 cm defect in S5 at the hepatobiliary phase (Figure 2A).
EOB-MRI revealed hyperintensity on diffusion weighted image (DWI) (b value = 800 s/mm3) (Figure 2B) and hypointensity on apparent diffusion coefficient (ADC) map, respectively (Figure 2C); however, imaging at the early phase was unattainable because of artifacts attributed to the patient’s restlessness.
Computed tomography findings: Plain computed tomography (CT) revealed no nodule in S5 (Figure 2D). Contrast-enhanced CT (CECT) revealed a hypervascular nodule at the early phase (Figure 2E) and delayed contrast enhancement at the late phase in S5 (Figure 2F) in October, 2021.
Ultrasound findings: Plain ultrasound (US) revealed a 1cm iso-hyperechoic nodule in S5 (Figure 2G). Contrast-enhanced US (CEUS) revealed incomplete defect at the late vascular phase in S5 (Figure 2H) in October, 2021.
From all the above imaging findings inflammatory liver tumor, lymphoproliferative disease, intrahepatic cholangiocarcinoma (iCCA, small duct type) and bile duct adenoma (BDA) were suspected.
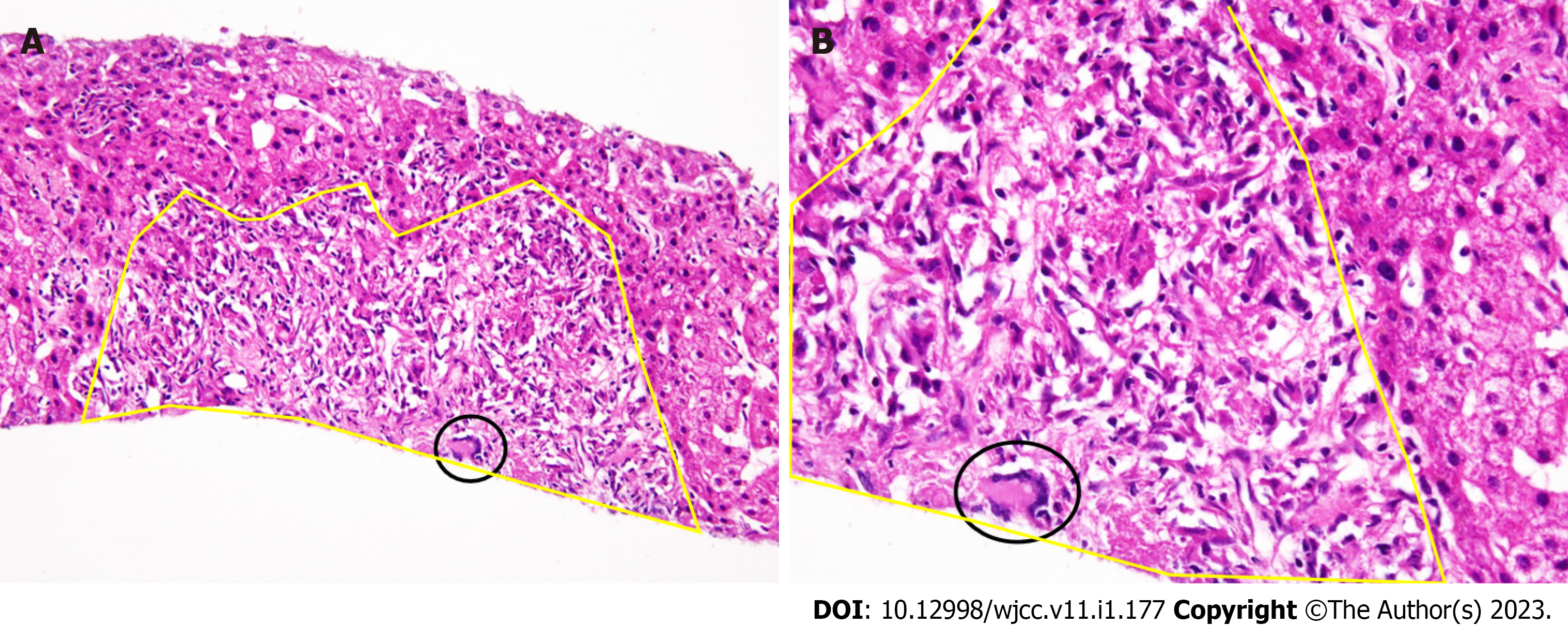
US guided biopsy revealed a noncaseating HSEG with spindle shaped epithelioid cells harboring Langhans-type multinucleated giant cells, as determined by histopathological studies in October, 2021 (Figure 1, Figure 3A and B).
Based on the above findings, the present case was diagnosed as drug-induced hepatic sarcoidosis-like reaction (HSLR).
Dermatological and ophthalmological examinations signified no suspicion of sarcoidosis. Also, lung CECT, US cardioechography and fluorine-18 fluorodeoxyglucose-PET (F-18 FDG-PET) pointed to no suspicion of sarcoidosis. The patient’s clinical course was monitored without any treatment. EOB-MRI, one month after the diagnosis of HSEG and four months after the mRNA vaccination, signified the disappearance of the defect at the hepatobiliary phase (Figure 2I), the hyperintensity on DWI, the hypointensity on ADC map and no stain at the early phase, in November 2021 (Figure 1).
According to his will, the third booster mRNA vaccination was administered to the patient in January 2022 seven months after the second mRNA (Figure 1). Follow up-imaging studies with EOB revealed no stain at the early phase, no defect at the hepatobiliary phase, no hyperintensity on DMI and no hypointensity on ADC map in March and in July 2022, 2 mo and 5 mo after the third mRNA vaccination.
Four common categories of drugs that have been associated with the development of DISR are ICIs[2-4], HAART[5-7], IFNs[8-10], and TNF-α antagonists[11-13]. Also, several drugs such as BRAF[14-16] inhibitors, methotrexate and BCG have been associated with the development of syndromes indistinguishable from sarcoidosis, and are described as DISR. Like sarcoidosis, DISR does not necessarily require treatment because it causes no significant symptoms, quality of life impairment, or organ dysfunction. Standard anti-sarcoidosis regimens seem to be effective in treating DISR; alternatively, discontinuing any antagonistic drug tends to ameliorate or resolve DISR, thus constituting another effective treatment. Unlike sarcoidosis, DISR often resolves after discontinuation of the antagonistic agent, but may recur with rechallenge[1].
In the present case, one month after the diagnosis of HSLR, and four months after the mRNA vaccinations, EOB-MRI signified the disappearance of the defect at the hepatobiliary phase, the hyperintensity on DWI, the hypointensity on ADC map and the stain at the early phase, in November 2021, without the administration of any treatment, all of which is compatible with the clinical course of DISR.
Because DISR can be confused with other clinical conditions, including infections, other drug reactions, and malignancies, it is important to recognize this disease entity because misdiagnosing it may lead to unnecessary or inappropriate testing and treatment[1].
During follow-up of the present case, frequent imaging studies for HCV-related recurrent HCC were conducted to survey any multicentric occurrence and intrahepatic metastasis.
Imaging studies disclosed a 1cm defect in S5 at the hepatobiliary phase, diffusion restriction with hyperintensity on DWI and hypointensity on ADC-map through EOB-MRI, hypervascularity in the early phase and delayed contrast enhancement at the late phase through CECT, and incomplete defect in the late vascular phase through CEUS.
From above imaging studies, inflammatory liver tumor[17], lymphoproliferative disease[18], iCCA (small duct type)[19] and BDA[20] were suspected.
Nonetheless, histopathological examination with the use of US guided biopsy revealed a noncaseating HSEG with spindle shaped epithelioid cells harboring Langhans-type multinucleated giant cells.
IFN-α has been widely used for the treatment of chronic hepatitis B virus, hepatitis C infection, and various cancers such as chronic leukemia, malignant melanoma, and renal cell carcinoma. In many cases, IFN-α-induced DISR has been detected between 6 and 104 wk after the start of therapy[1].
In the present study, however, the relation between IFN-α and DISR was ruled out, especially that 16 years earlier the patient had undergone PEG-INF-α2b + Ribavirin treatment that resulted in SVR.
The exact immunopathogenesis of DISR is unknown; however, several hypotheses have been proposed according to the kinds of drugs and injections administered[21-23].
Ipilimumab, an ICI, enhances patient capability to mount an antitumor immune response; the resulting T-cell proliferation and increased expression of T-helper (Th) 1-associated markers can potentially induce DISR because these abundant cells in active sarcoidosis are thought to be integral to the development of sarcoid granuloma[21].
Increased production of IFN-α has been linked to Th1 polarization and Th2 inactivation with an enhanced level of granuloma-promoting cytokines such as interleukin (IL)-2, IL-8, IL-12, IL-18, and IFN-γ[22].
It is likely that the TNF-α soluble receptor is an unopposed type I IFN product that promotes a shift toward a Th1/Th2 profile, and the neutralization of soluble TNF-α can promote the activation of specific autoreactive T cells[23].
Irrespective of slight differences among the three above injections, they share some common significant characteristics such as favorable Th1 and inactivated Th2 profiles in terms of immune characteristics.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the ensuing COVID-19 have afflicted 608.6 million people in a worldwide pandemic, and as of 12 September 2022, deaths approaching 6.51 million have been reported. Obviously, safe and effective vaccines are needed urgently.
The mRNA vaccine is a lipid nanoparticle-formulated, nucleoside-modified RNA vaccine that encodes a prefusion-stabilized, membrane-anchored SARS-CoV-2 full-length spike protein[24-27].
Regarding T cell immune reaction to the mRNA vaccine, concurrent production of neutralizing antibodies, activation of virus-specific CD4+ and CD8+ T cells, and robust release of immune-modulatory cytokines such as IFN-γ represent a coordinated immune response to counter viral intrusion[28].
The T cell-related immune characteristics such as a favorable Th1 and inactivated Th2 profiles by the mRNA vaccine[28] have shown common immune characteristics similar to that of ICIs[21], IFN[22], TNF-α[23] which induce DISR.
In the context of common immune characteristics, mRNA can induce DISR under some presently-unknown conditions[24].
Recently a 44-year-old male patient has demonstrated mRNA vaccine-associated sarcoidosis the so-called DISR as confirmed by histopathological examination, and lymphadenopathy as disclosed by FDG-PET/CT. The patient had received the first dose of the mRNA vaccine a few days before CTCA/CMR and the second dose the day before FDG-PET/CT. Further examination of FDG PET/CT images revealed a triangular uptake of intramuscular FDG at the injection site in the left arm. Since the second dose of the vaccine was given in the interval between the CTCA/CMR and the PET/CT in the ipsilateral arm, and the enlargement of the left axillary lymph node was more prominent in the PET/CT scan than in the CTCA, the intramuscular FDG was interpreted as indicative of an inflammatory reaction. Nonetheless, to further determine the underlying nature of ilo-mediastinal lymphadenopathies, endobronchial ultrasound-guided transbronchial needle aspiration was carried out on stations 4R and 11R, and histopathological analysis revealed a sarcoidal-type granulomatous inflammation[29]. This report, however, showed discrepancy between the location of the enlargement (the left axillary lymph node) and the location of histopathological examination (ilo-mediastinal lymph node) after the second dose of the vaccine. Finally, the authors diagnosed this case as the so-called DISR[29].
In addition, very recently two histologically confirmed sarcoidosis cases due to BNT162b2 vaccination have been reported.
One is 61-year-old man after first mRNA vaccination. He developed sarcoidosis as manifested as uveitis, bilateral hilar lymphadenopathy, angiotensinconverting enzyme elevation, and epithelioid and giant cell granuloma formation in the lung soon after the first BNT162b2 injection[30].
Another is 43-year-old man who presented intermittent cough after the third dose of COVID-19 vaccination. 18 F-FDG PET/CT showed high uptake of one solitary nodule in the right middle lobe, mediastinal lymph nodes, bilateral hila, and multiple nodules under the right pleura, mimicking the malignancy.
Nevertheless, the biopsy confirmed distinct noncaseating granulomas[31].
In the present case, during the clinical course, no other particular drugs, injections, checkpoint inhibitors, HAART, IFN and TNF-α antagonists, BRAF inhibitors methotrexate and BCG, except for the mRNA vaccine, were administered.
The elevated level of sIL-2R observed in the present case was compatible with the previous papers[32-35].
Taken together with above reports[29-31], the present case suggests that the sarcoidosis-like reaction might be induced by the mRNA vaccination.
Above mRNA induced DISR cases were multiple nodules of DISR. Even though the present case was a single nodule of DISR, the present case was also considered as mRNA induced DISR compatible with Chopra's criteria that does not exclude a single nodule of DISR from DISR.
To the best of our knowledge, the present case may be the first on mRNA-induced HSLR, especially that to date no other such case has been reported in the literature.
The novel Omicron (B.1.1.529) variant, first identified in South Africa on November 24, 2021, has put the whole world on red alert[36]. Based on the unprecedented number of mutations (> 32 mutations in the Spike protein), and enhanced transmissibility (three times more infectious and severe than the original Wuhan strain). The World Health Organization announced on 26 November 2021 that the novel Omicron variant was of concern. As of February 2022, it has grown into the dominant variant all over the world.
Fortunately, rechallenge of drug-induced sarcoidosis like reaction was not confirmed two months after the third booster mRNA vaccination. That, however, does not necessarily deny the possibility of DISR with mRNA vaccination in the present case.
Further accumulation of relative cases is needed to clarify the clinical characteristics of mRNA-induced sarcoidosis-like reaction, its prevalence, predisposition, and past history.
The authors thank Mss. Noriko Yorifuji and Yukako Nagai for scientific advice, and Mss. Minako Miyauchi and Mika Matsui for technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kian W, Israel; Li G, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Chopra A, Nautiyal A, Kalkanis A, Judson MA. Drug-Induced Sarcoidosis-Like Reactions. Chest. 2018;154:664-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 2. | Montaudié H, Pradelli J, Passeron T, Lacour JP, Leroy S. Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol. 2017;176:1060-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Firwana B, Ravilla R, Raval M, Hutchins L, Mahmoud F. Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract. 2017;23:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Danlos FX, Pagès C, Baroudjian B, Vercellino L, Battistella M, Mimoun M, Jebali M, Bagot M, Tazi A, Lebbé C. Nivolumab-Induced Sarcoid-Like Granulomatous Reaction in a Patient With Advanced Melanoma. Chest. 2016;149:e133-e136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Church LWP, Chopra A, Judson MA. Paradoxical Reactions and the Immune Reconstitution Inflammatory Syndrome. Microbiol Spectr. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Martí N, Martin JM, Mayordomo E, Calduch L, Jordá E. Cutaneous and pulmonary sarcoidosis in a patient with human immunodeficiency virus: a late feature of immune restoration syndrome. Clin Exp Dermatol. 2011;36:306-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Miranda EJ, Leite OH, Duarte MI. Immune reconstitution inflammatory syndrome associated with pulmonary sarcoidosis in an HIV-infected patient: an immunohistochemical study. Braz J Infect Dis. 2011;15:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Cardoso C, Freire R, Alves A, Oliveira A. Interferon-induced sarcoidosis. BMJ Case Rep. 2011;2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Joshita S, Shirahata K, Yazaki Y, Okaniwa S, Nakamura Y, Kimura T, Noami S, Horigome R, Yagi H, Ito N, Yamazaki A, Akahane Y, Umemura T, Yoshizawa K, Tanaka E, Ota M. Cutaneous sarcoidosis in a chronic hepatitis C patient receiving pegylated interferon and ribavirin therapy. Hepatol Res. 2013;43:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Rodríguez-Lojo R, Almagro M, Barja JM, Piñeyro F, Pérez-Varela L, Del Pozo J, Yebra-Pimentel MT, Fonseca E. Subcutaneous Sarcoidosis during Pegylated Interferon Alfa and Ribavirin Treatment for Chronic Hepatitis C. Dermatol Res Pract. 2010;2010:230417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Gîlcă GE, Diaconescu S, Bălan GG, Timofte O, Ştefănescu G. Sarcoidosis associated with infliximab therapy in ulcerative colitis: A case report. Medicine (Baltimore). 2017;96:e6156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Olivier A, Gilson B, Lafontaine S, Pautot JX, Bindi P. [Pulmonary and renal involvement in a TNFα antagonist drug-induced sarcoidosis]. Rev Med Interne. 2012;33:e25-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Garrido MC, Gutierrez C, Riveiro-Falkenbach E, Ortiz P, Rodriguez-Peralto JL. BRAF Inhibitor-Induced Antitumoral Granulomatous Dermatitis Eruption in Advanced Melanoma. Am J Dermatopathol. 2015;37:795-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Jansen YJ, Janssens P, Hoorens A, Schreuer MS, Seremet T, Wilgenhof S, Neyns B. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Leal L, Agut-Busquet E, Romani J, Sabat M, Yebenes M, Saez A, Luelmo J. Cutaneous granulomatous panniculitis and sarcoidal granulomatous papular eruption in a patient with metastatic melanoma treated with a BRAF inhibitor. J Dermatol. 2016;43:715-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Kim SR, Hayashi Y, Kudo M, Matsuoka T, Imoto S, Sasaki K, Shintani S, Song KB, Park SY, Kim JH, Ando K, Koterazawa T, Kim KI, Ninomiya T. Inflammatory pseudotumor of the liver in a patient with chronic hepatitis C: difficulty in differentiating it from hepatocellular carcinoma. Pathol Int. 1999;49:726-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Zen Y, Fujii T, Nakanuma Y. Hepatic pseudolymphoma: a clinicopathological study of five cases and review of the literature. Mod Pathol. 2010;23:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Nakamura Y, Klimstra DS, Komuta M, Zen Y. Digestive System Tumours. WHO Classification of Tumours. 5th Edition. Intrahepatic cholangiocarcinoma; International Agency for Research on Cancer: Lyon, France, 2019: 254-259. |
| 20. | Sasaki M, Matsubara T, Kakuda Y, Sato Y, Nakanuma Y. Immunostaining for polycomb group protein EZH2 and senescent marker p16INK4a may be useful to differentiate cholangiolocellular carcinoma from ductular reaction and bile duct adenoma. Am J Surg Pathol. 2014;38:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, Jackson JR, Shahabi V. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 627] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 22. | Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P, Holden DA, Forrester JM, Lazarus A, Wysocka M, Trinchieri G, Karp C. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol. 1996;156:4952-4960. [PubMed] |
| 23. | Salvatierra J, Magro-Checa C, Rosales-Alexander JL, Raya-Alvarez E. Acute sarcoidosis as parotid fever in rheumatoid arthritis under anti-tumor necrosis factor-alpha therapy. Rheumatology (Oxford). 2011;50:1346-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10712] [Article Influence: 2142.4] [Reference Citation Analysis (1)] |
| 25. | Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1414] [Article Influence: 282.8] [Reference Citation Analysis (0)] |
| 26. | Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Walsh EE, Frenck R, Falsey AR, Dormitzer PR, Gruber WC, Şahin U, Jansen KU. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 1055] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 27. | Walsh EE, Frenck R, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Thompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Sahin U, Gruber WC. RNA-Based COVID-19 Vaccine BNT162b2 Selected for a Pivotal Efficacy Study. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 28. | Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, Brachtendorf S, Lörks V, Sikorski J, Hilker R, Becker D, Eller AK, Grützner J, Boesler C, Rosenbaum C, Kühnle MC, Luxemburger U, Kemmer-Brück A, Langer D, Bexon M, Bolte S, Karikó K, Palanche T, Fischer B, Schultz A, Shi PY, Fontes-Garfias C, Perez JL, Swanson KA, Loschko J, Scully IL, Cutler M, Kalina W, Kyratsous CA, Cooper D, Dormitzer PR, Jansen KU, Türeci Ö. Concurrent human antibody and TH1 type T-cell responses elicited by a COVID-19 RNA vaccine. medRxiv. 2020;. [DOI] [Full Text] |
| 29. | Bauckneht M, Aloè T, Tagliabue E, Cittadini G, Guadagno A, Morbelli S, Barisione E. Beyond Covid-19 vaccination-associated pitfalls on [18F]Fluorodeoxyglucose (FDG) PET: a case of a concomitant sarcoidosis. Eur J Nucl Med Mol Imaging. 2021;48:2661-2662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Numakura T, Murakami K, Tamada T, Yamaguchi C, Inoue C, Ohkouchi S, Tode N, Sano H, Aizawa H, Sato K, Mitsune A, Kurosawa H, Nakazawa T, Sugiura H. A Novel Development of Sarcoidosis Following COVID-19 Vaccination and a Literature Review. Intern Med. 2022;61:3101-3106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Song X, Shao F, Lan X. The Onset of Sarcoidosis After COVID-19 Vaccination Revealed by the 18 F-FDG PET. Clin Nucl Med. 2022;47:869-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Gungor S, Ozseker F, Yalcinsoy M, Akkaya E, Can G, Eroglu H, Genc NS. Conventional markers in determination of activity of sarcoidosis. Int Immunopharmacol. 2015;25:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Vorselaars AD, Verwoerd A, van Moorsel CH, Keijsers RG, Rijkers GT, Grutters JC. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J. 2014;43:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Ogata-Suetsugu S, Hamada N, Takayama K, Tsubouchi K, Arimura-Omori M, Nakanishi Y. The clinical value of serum soluble interleukin-2 receptor in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Grutters JC, Fellrath JM, Mulder L, Janssen R, van den Bosch JM, van Velzen-Blad H. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis: a clinical evaluation. Chest. 2003;124:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Graham F. Daily briefing: Omicron coronavirus variant puts scientists on alert. Nature. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |