Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2194
Peer-review started: October 4, 2021
First decision: November 11, 2021
Revised: November 19, 2021
Accepted: January 17, 2022
Article in press: January 17, 2022
Published online: March 6, 2022
Processing time: 148 Days and 20.2 Hours
Some studies investigated the prognostic role of several blood biomarkers, including the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR) and Glasgow prognostic score (GPS), in osteosarcoma, but their results were inconsistent with each other.
To identify the prognostic value of NLR, PLR, LMR and GPS in osteosarcoma patients through reviewing relevant studies.
The PubMed, EMBASE, Web of Science and CNKI databases were searched up to October 2, 2021. The primary and second outcomes were overall survival (OS) and disease-free survival (DFS), respectively. The hazard ratios (HRs) with 95% confidence intervals (CIs) were combined to assess the association between these indicators and prognosis of osteosarcoma patients.
A total of 13 studies involving 2087 patients were eventually included. The pooled results demonstrated that higher NLR and GPS were significantly associated with poorer OS (HR = 1.88, 95%CI: 1.38-2.55, P < 0.001; HR = 2.19, 95%CI: 1.64-2.94, P < 0.001) and DFS (HR = 1.67, 95%CI: 1.37-2.04, P < 0.001; HR = 2.50, 95%CI: 1.39-4.48, P < 0.001). However, no significant relationship of PLR and LMR and OS (P = 0.085; P = 0.338) and DFS (P = 0.396; P = 0.124) was observed.
Higher NLR and GPS were related with worse prognosis and might serve as novel prognostic indicators for osteosarcoma patients.
Core Tip: Higher neutrophil/lymphocyte ratio (NLR) and Glasgow prognostic score (GPS) were significantly associated with poorer overall survival (OS) (P < 0.001; P < 0.001) and disease-free survival (DFS) (P < 0.001; P < 0.001). However, no significant relationship of platelet/lymphocyte ratio and lymphocyte/monocyte ratio and OS (P = 0.085; P = 0.338) and DFS (P = 0.396; P = 0.124) was observed. Higher NLR and GPS were related with worse prognosis in osteosarcoma and might serve as reliable and valuable prognostic indicators for osteosarcoma patients.
- Citation: Peng LP, Li J, Li XF. Prognostic value of neutrophil/lymphocyte, platelet/lymphocyte, lymphocyte/monocyte ratios and Glasgow prognostic score in osteosarcoma: A meta-analysis. World J Clin Cases 2022; 10(7): 2194-2205
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2194.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2194
Osteosarcoma is a malignant bone tumor that seriously affects the health of children and adolescents with annual incidence of 2-3/1 million, accounting for about 20%-30% of all primary bone tumors[1]. It has the characteristics of high malignant degree, early metastasis and high mortality. Before the 1070s, the treatment of osteosarcoma was mainly based on the surgical resection with the five-year survival rate of 10%-20%[2,3]. In recent years. Through the combination of preoperative neoadjuvant chemotherapy, surgical resection, postoperative adjuvant chemotherapy and other important therapies, the five-year survival rate of osteosarcoma patients has increased to 60%-70%[4,5].
However, osteosarcoma tends to metastasize to the lungs and nearly half of osteosarcoma cases have pulmonary metastases, which is the main cause of death in patients with osteosarcoma[6]. Nevertheless, less than 20% of patients showed metastases at the time of diagnosis and the five-year survival rate of patients with pulmonary metastases is less than 30%[6,7]. Thus, some reliable and valuable prognostic indicators are still clinically needed to accurately predict the survival of osteosarcoma patients and contribute to the formulation of appropriate treatment strategies.
In recent years, many haematological indexes have been reported to show high prognostic value in cancer patients, including the neutrophil to lymphocyte ratio (NLR)[8,9], platelet to lymphocyte ratio (PLR)[10,11], lymphocyte to monocyte ratio (LMR)[10] and Glasgow prognostic score (GPS)[12-14]. Besides, the NLR has been also reported to play a role in predicting postoperative complications like the anastomotic leakage[15]. Some investigators explored the prognostic value of these indicators in osteosarcoma[16-28], but inconsistent results were reported in their studies. Whether they could be used to predict prognosis of osteosarcoma patients in clinics remains unclear.
Thus, the aim of this study was to identify the prognostic role of NLR, PLR, LMR and GPS in osteosarcoma, which might help formulate the appropriate treatment strategies for osteosarcoma patients.
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[29].
The PubMed, EMBASE, Web of Science and CNKI electronic databases were searched up to October 2, 2021. The following key works were used during the literature search: neutrophil/lymphocyte ratio, neutrophil to lymphocyte ratio, NLR, platelet/ lymphocyte ratio; platelet to lymphocyte ratio, PLR, lymphocyte/monocyte ratio, lymphocyte to monocyte ratio, LMR, Glasgow prognostic score, GPS, osteosarcoma, osteogenic sarcoma, prognostic, prognosis and survival. Besides, the references cited in the included studies were also evaluated for availability.
The following inclusion criteria were applied: (1) Patients were pathologically diagnosed with osteosarcoma; (2) Patients were divided into two groups according to the NLR, PLR, LMR or GPS and the long-term survival of patients between the two groups were compared; and (3) Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) of overall survival (OS) or disease-free survival (DFS) were reported in the articles directly.
The following exclusion criteria were applied: (1) None of the prognostic value of NLR, PLR, LMR or GPS were investigated; (2) Duplicated or overlapped data; and (3) Conference abstracts, animal trials, case reports or reviews.
The following information were collected from included studies: the name of first author, publication year, country, sample size, indicators (NLR, PLR, LMR or GPS), cutoff values of NLR, PLR or LMR, endpoints and corresponding HRs with 95%CIs.
The Newcastle-Ottawa scale (NOS) was used for the quality assessment of included studies and high-quality studies were defined as a NOS of 6 or higher[30].
In the current meta-analysis, the literature search, selection, data extraction and quality assessment were all performed by two authors independently and any disagreement was resolved by team discussion.
The HRs with 95%CIs were combined to assess the relationship between NLR, PLR, LMR and GPS and prognosis of osteosarcoma patients. The heterogeneity among included studies was evaluated by I2 statistics and Q test. When obvious heterogeneity was observed presenting as the I2 > 50% or (and) P < 0.1, the random effect model was used; otherwise, the fix effect model was used[31]. Considering the similarity, we combined the cancer-specific survival, event-free and progression-free as DFS. The sensitivity analysis was performed to detect the source of heterogeneity and evaluated the stability of pooled results. Besides, the Begg’s funnel plot and Egger’s test were conducted to detect publication bias[32]. If significant publication bias was observed presenting as a P < 0.05, then the nonparametric trim-and-fill method was used to re-estimate a corrective effect size after publication bias was adjusted[33]. All statistical analyses were conducted by STATA 15.0 software.
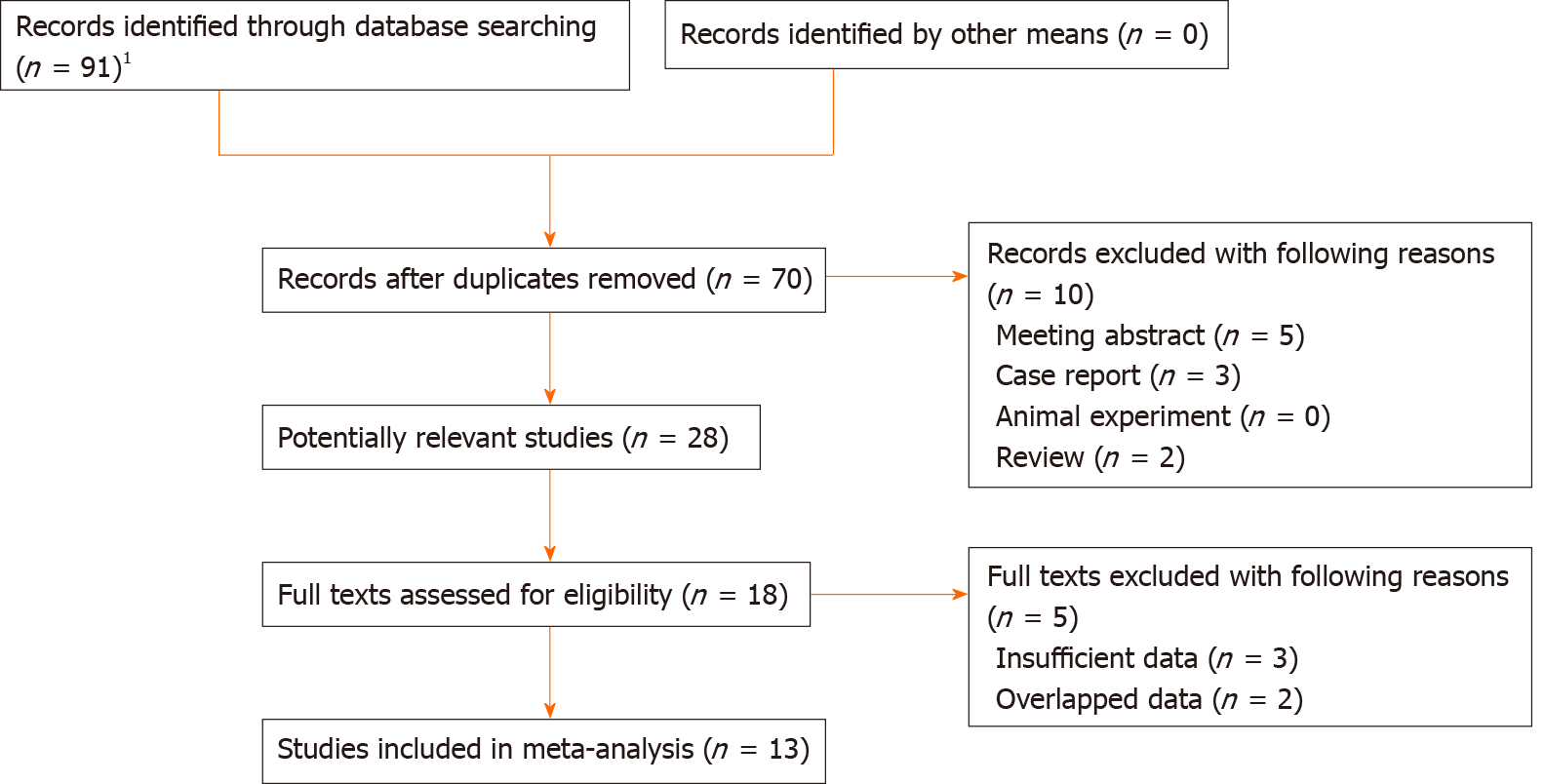
Ninety-one records were initially identified and 21 duplicated records were removed. Then 28 potentially relevant publications were assessed and 10 of them were excluded because of meeting abstract, case reports and reviews. Eighteen full texts were reviewed and a total of 13 studies were included eventually[16-28]. The detailed selection process was presented in Figure 1.
All include studies were retrospective. Most of them were from China and a total of 2087 patients were enrolled, with the sample size ranging from 55 to 327. All of them were high-quality studies with a NOS of 6 or higher. The specific information was presented in Table 1.
| Ref. | Year | Country | Sample size | Indicators | Thresholds | Endpoints | NOS |
| Liu et al[16] | 2015 | China | 327 | LMR | 3.43 | OS, EFS | 7 |
| Aggerholm-Pedersen et al[18] | 2016 | Denmark | 172 | NLR, GPS | 5.3 | OS, CSS | 7 |
| Liu et al[17] | 2016 | China | 162 | NLR, PLR, LMR, GPS | 2.57, 123.5, 4.73 | OS | 6 |
| Xia et al[19] | 2016 | China | 359 | NLR, PLR | 3.43, 122 | OS, PFS | 7 |
| Li et al[20] | 2017 | China | 216 | NLR, PLR, GPS | 2.65, 118 | OS | 6 |
| Vasquez et al[21] | 2017 | Peru | 55 | NLR, PLR | 2, 150 | OS | 6 |
| Huang et al[22] | 2018 | China | 103 | NLR, mGPS | 2.70 | OS, EFS | 7 |
| Huang et al[23] | 2019 | China | 126 | NLR, PLR | 2.1, 163.2 | OS | 7 |
| Xu et al[24] | 2019 | China | 150 | NLR, PLR, LMR | 2.7, 200, 3.0 | OS, DFS | 6 |
| Hu et al[25] | 2020 | China | 137 | LMR | 3.05 | OS | 7 |
| Yang et al[26] | 2020 | China | 133 | NLR, LMR | 2.96, 4.44 | OS, PFS | 6 |
| Yang et al[27] | 2020 | China | 77 | NLR, PLR, LMR | 2.65, 125.0, 5.16 | OS | 7 |
| Ye et al[28] | 2021 | China | 70 | NLR, PLR, LMR | 3.025, 111.5, 4.82 | OS | 7 |
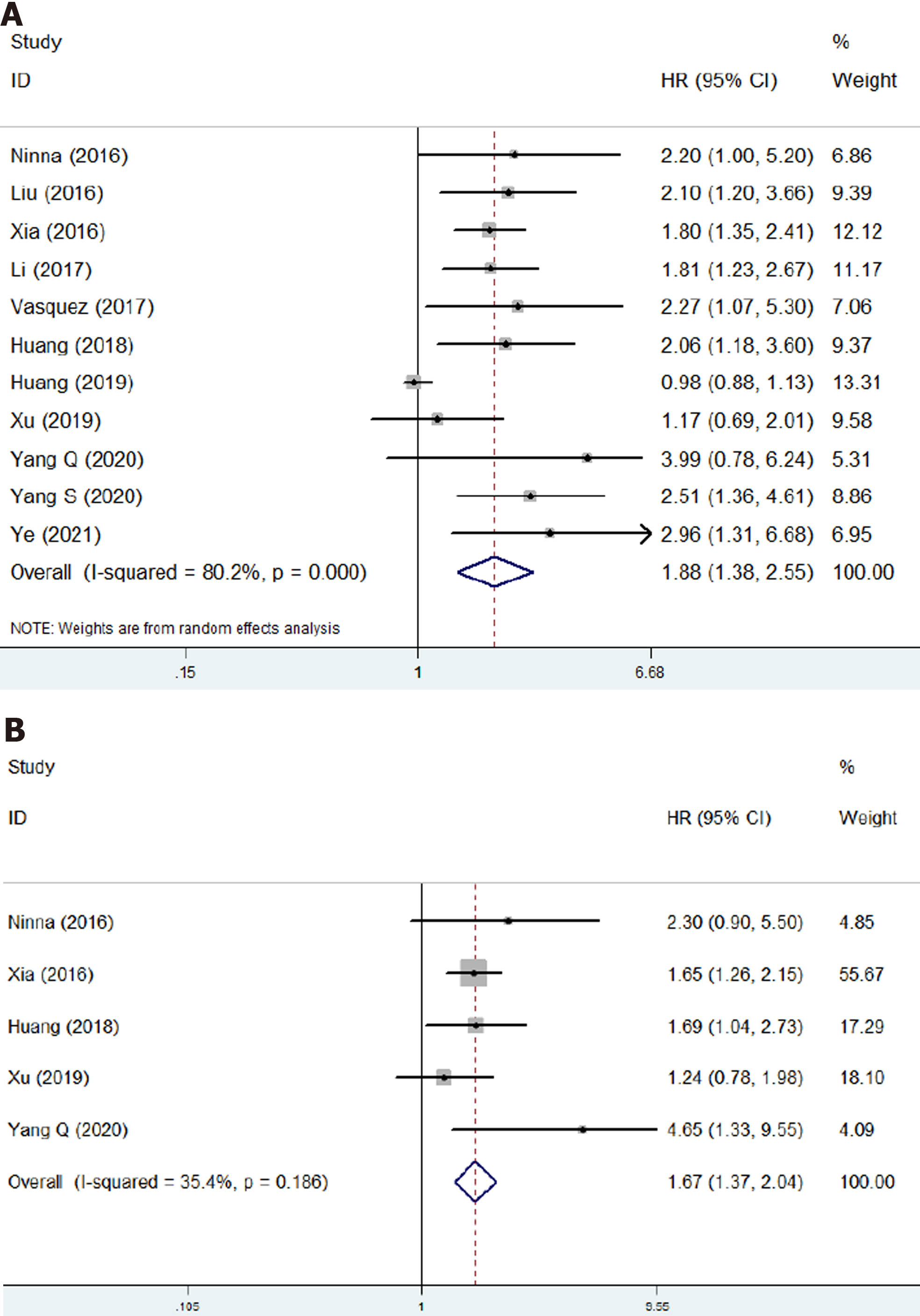
Eleven studies involving 1623 patients investigated the prognostic role of NLR in osteosarcoma[17-24,26-28]. The pooled results indicated that higher NLR was significantly associated with poor OS (HR = 1.88, 95%CI: 1.38-2.55, P < 0.001; I2 = 80.2%, P < 0.001) (Figure 2A) and DFS (HR = 1.67, 95%CI: 1.37-2.04, P < 0.001; I2 = 35.4%, P = 0.186) (Figure 2B). NLR was demonstrated to be a prognostic indicator for osteosarcoma patients (Table 2).
| No. of studies | HR | 95%CI | P value | I2(%) | P value | |
| Neutrophil/lymphocyte ratio | ||||||
| Overall survival | 11 | 1.88 | 1.38-2.55 | < 0.001 | 80.2 | < 0.001 |
| Disease-free survival | 5 | 1.67 | 1.37-2.04 | < 0.001 | 35.4 | 0.186 |
| Platelet/lymphocyte ratio | ||||||
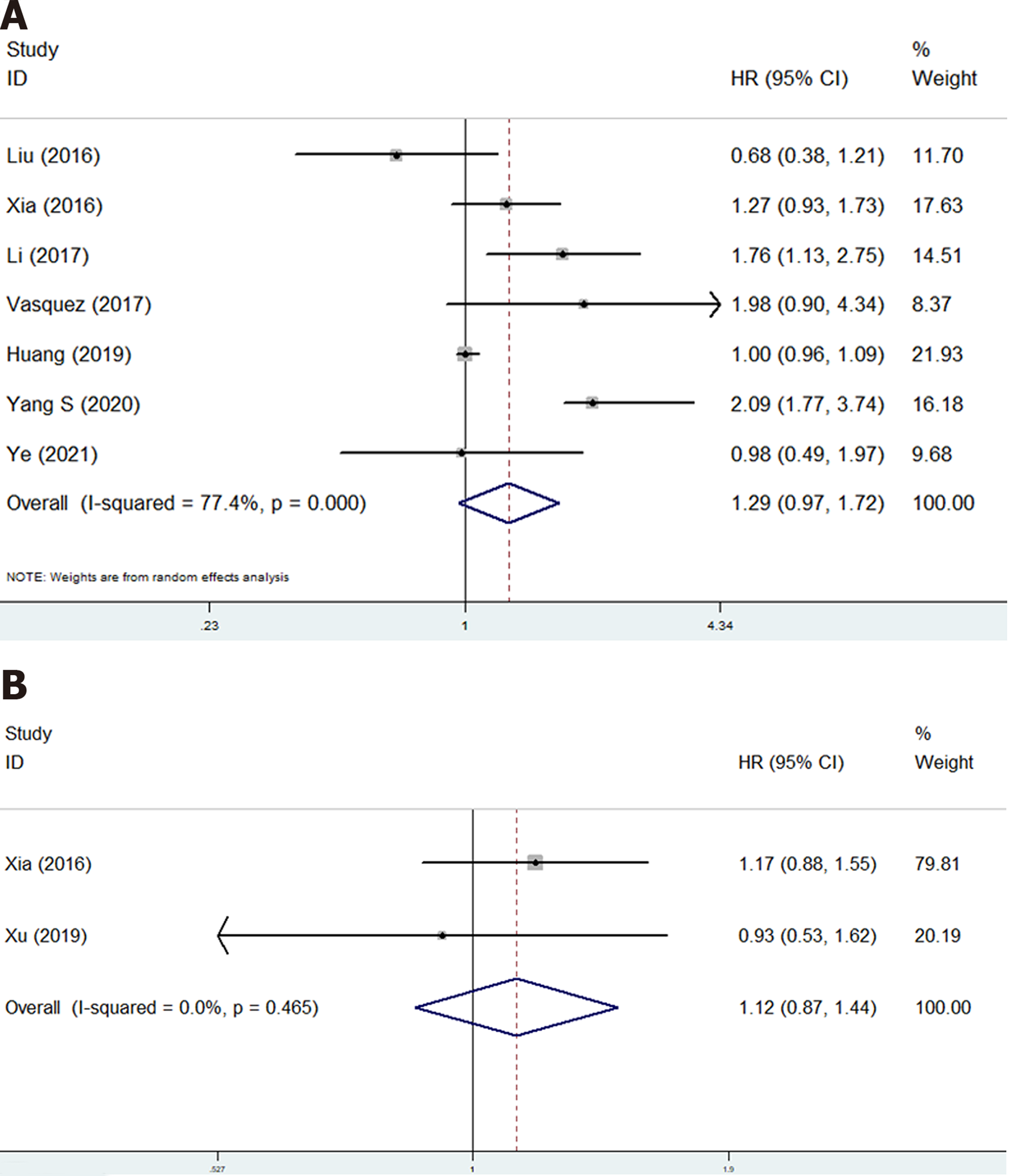
| Overall survival | 7 | 1.29 | 0.97-1.72 | 0.085 | 77.4 | < 0.000 |
| Disease-free survival | 2 | 1.12 | 0.87-1.44 | 0.396 | 0.0 | 0.465 |
| Lymphocyte/monocyte ratio | ||||||
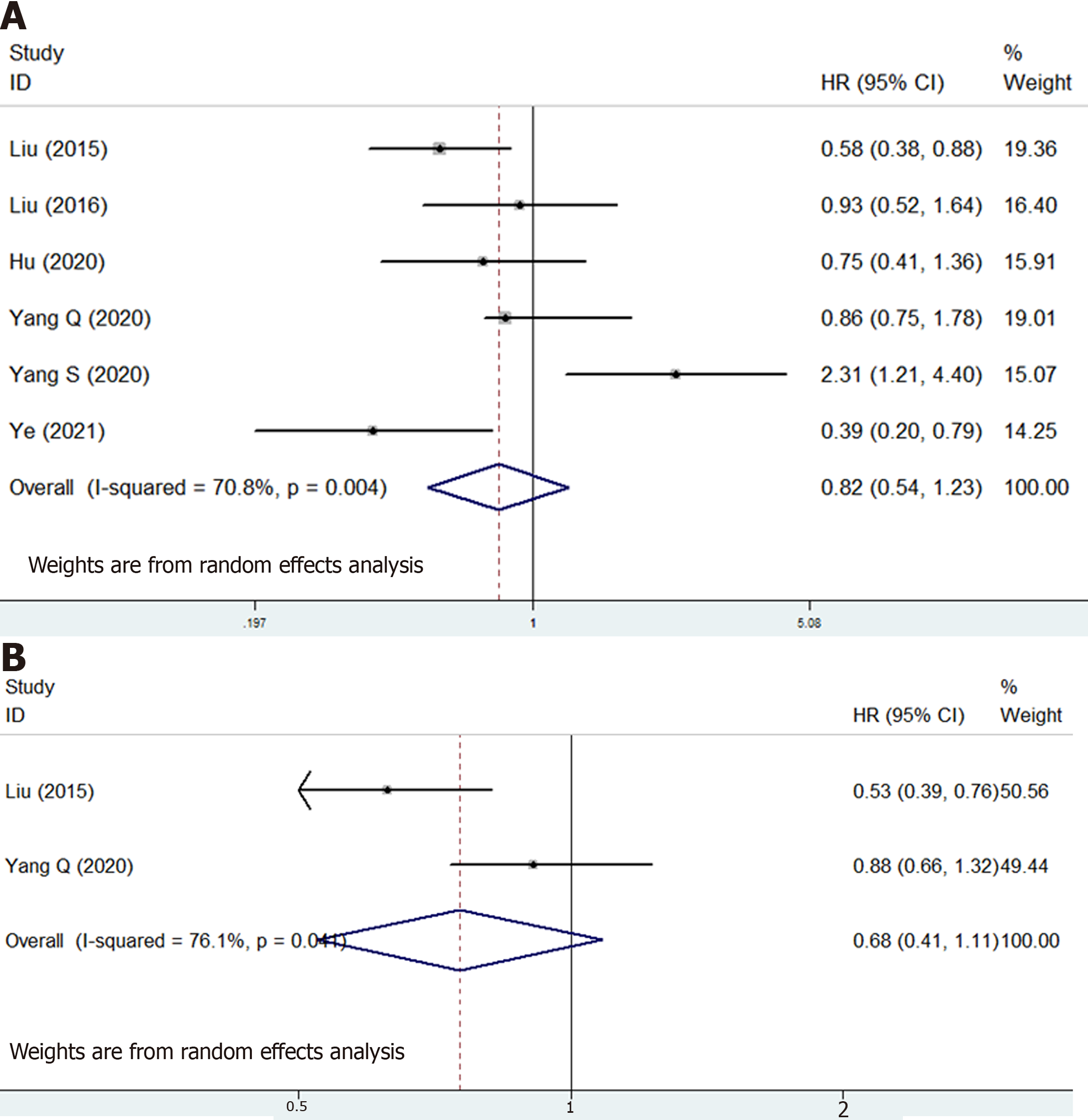
| Overall survival | 6 | 0.82 | 0.54-1.23 | 0.338 | 70.8 | 0.004 |
| Disease-free survival | 2 | 0.68 | 0.41-1.11 | 0.124 | 76.1 | 0.041 |
| Glasgow prognostic score | ||||||
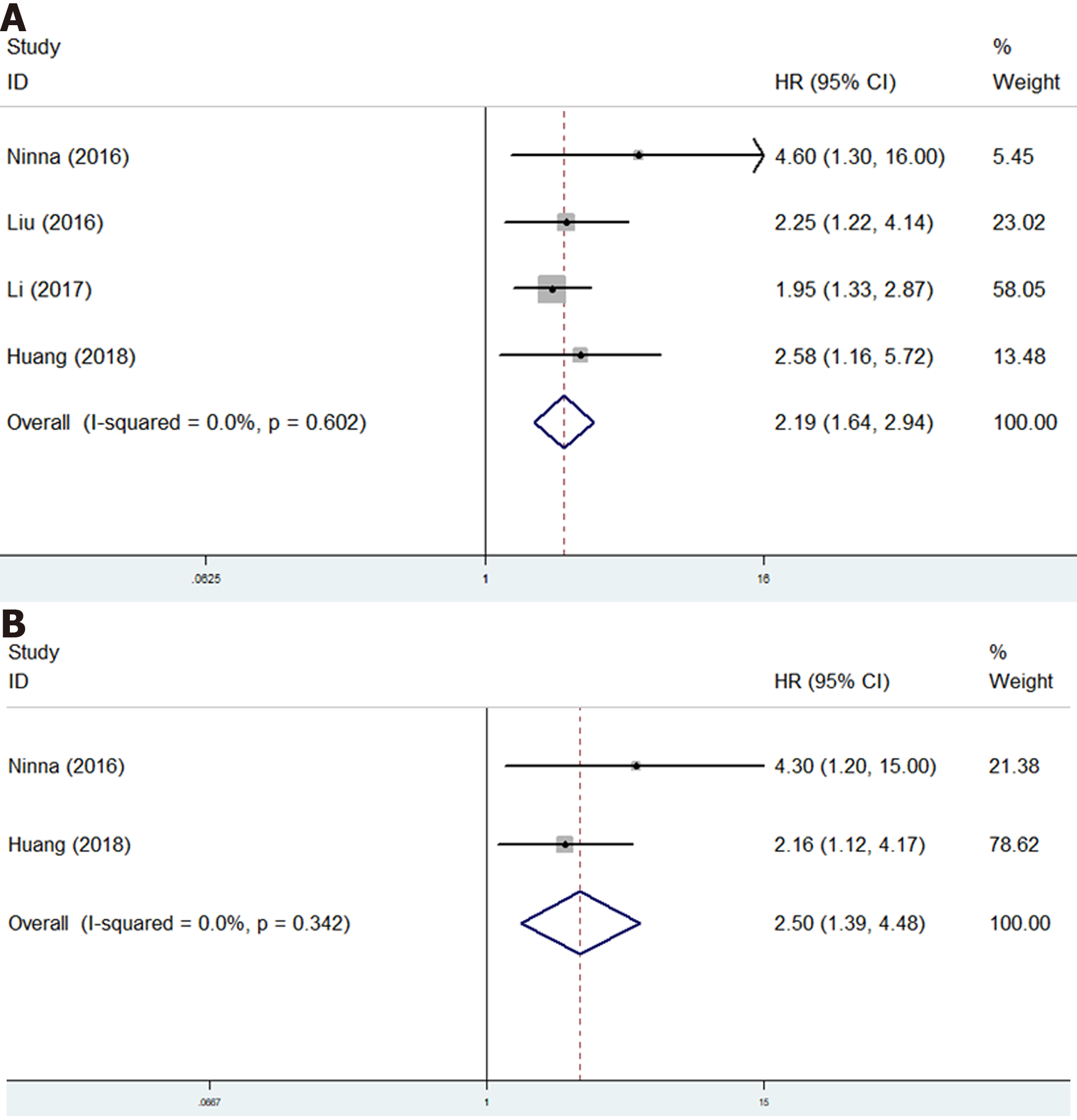
| Overall survival | 4 | 2.19 | 1.64-2.94 | < 0.001 | 0.0 | 0.602 |
| Disease-free survival | 2 | 2.50 | 1.39-4.48 | < 0.001 | 0.0 | 0.342 |
Eight studies involving 1215 patients explored the prognostic value of PLR in osteosarcoma patients[17,19-21,23,24,27,28]. However, no significant relationship of PLR with OS (HR = 1.29, 95%CI: 0.97-1.72, P = 0.085; I2 = 77.4%, P < 0.001) (Figure 3A) and DFS (HR = 1.12, 95%CI: 0.87-1.44, P < 0.001; I2 =0.0%, P = 0.465) (Figure 3B) was observed (Table 2).
Six studies involving 906 participants were included to identify the association of LMR with prognosis of osteosarcoma patients[16,17,25-28]. The pooled results manifested that LMR was not related with OS (HR = 0.82, 95%CI: 0.54-1.23, P = 0.338; I2 = 70.8%, P = 0.004) (Figure 4A) or DFS (HR = 0.68, 95%CI: 0.41-1.11, P = 0.124; I2 = 76.1%, P = 0.041) (Figure 4B) of osteosarcoma patients (Table 2).
Only four studies investigate the relationship of GPS with survival of osteosarcoma patients[17,18,20,22]. The pooled results certified that GPS was a novel predictor for OS (HR = 2.19, 95%CI: 1.64-2.94, P < 0.001; I2 = 0.0%, P = 0.602) (Figure 5A) and DFS (HR = 2.50, 95%CI: 1.39-4.48, P < 0.001; I2 = 0.0%, P = 0.342) (Figure 5B) of osteosarcoma patients (Table 2).
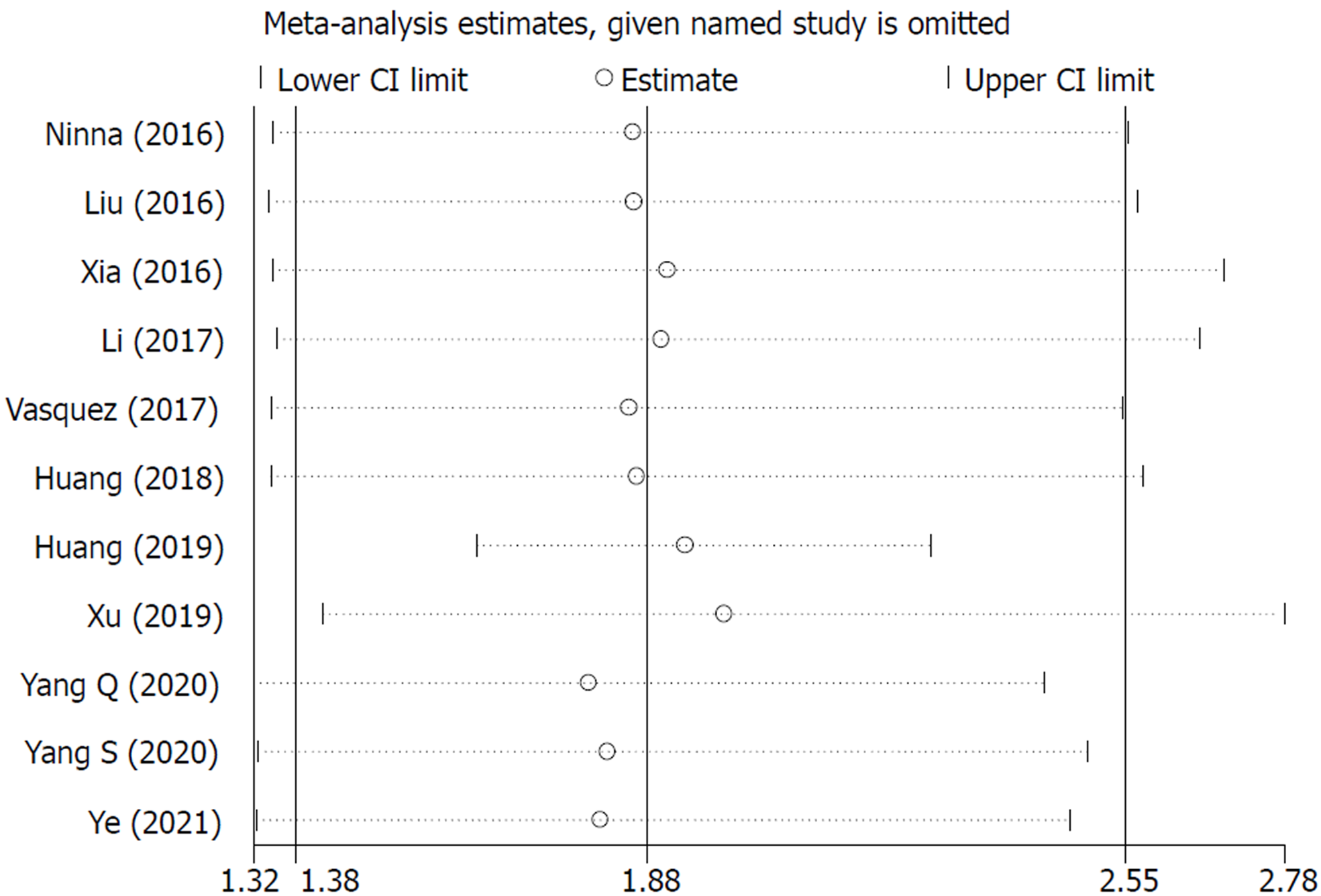
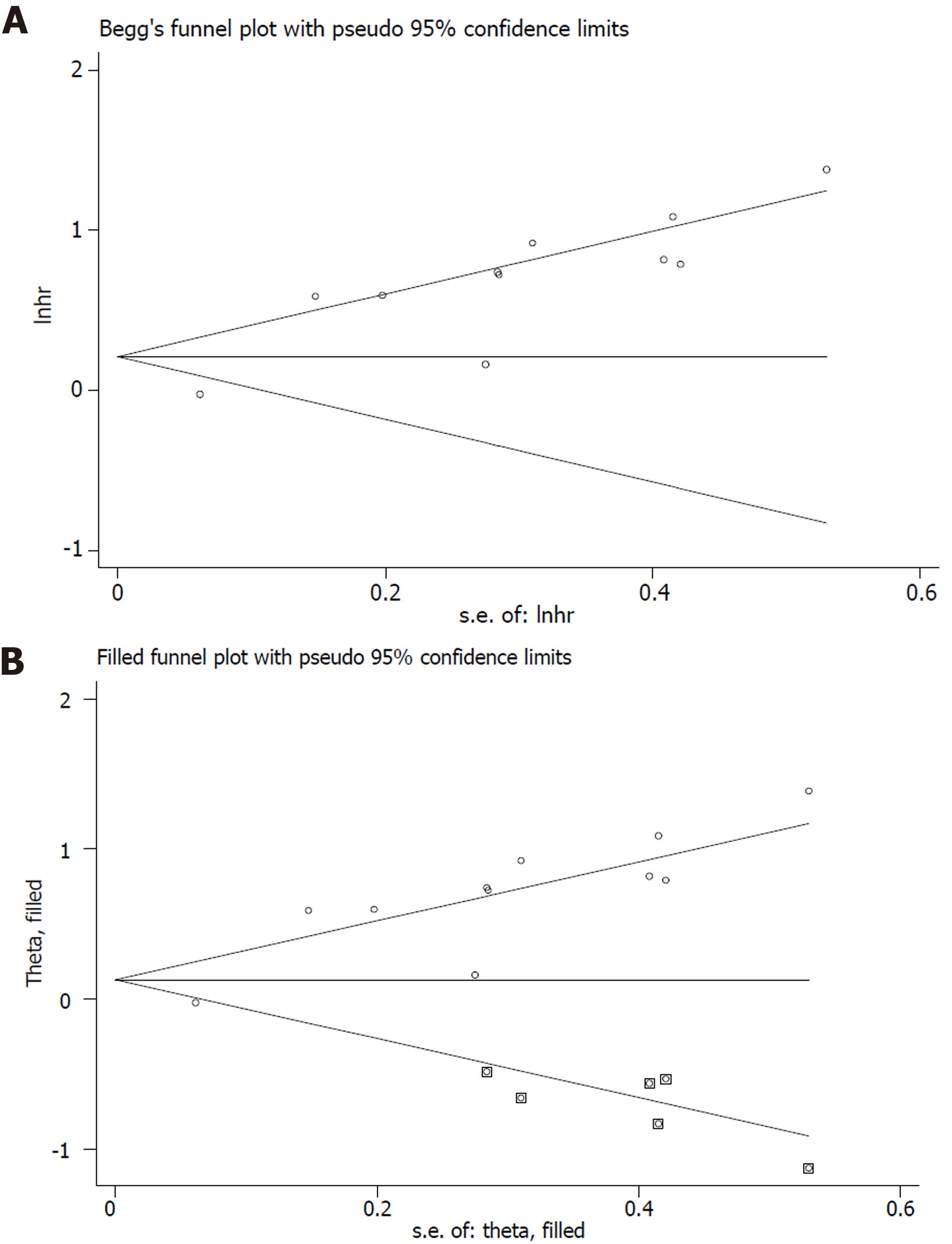
The sensitivity analysis and publication bias analysis for the association between NLR and OS of osteosarcoma patients were performed. The sensitivity analysis indicated that the pooled results were stable and reliable (Figure 6). Furthermore, the Begg’s funnel plot was asymmetric (Figure 7A) and the P value of Egger’s test was < 0.001, which indicated significant publication bias. Then the nonparametric trim-and-fill method was used and six potentially unpublished papers were found (Figure 7B). After combining these six publications, the pooled HRs for the fixed effect model and random effect model were 1.137 (95%CI: 1.036-1.247, P = 0.007) and 1.255 (95%CI: 0.952-1.654, P = 0.107), which indicated that the potentially unpublished studies might have a significant impact on the pooled results. Thus, more prospective studies with high-quality are still needed to verify our findings.
The current study demonstrated that higher NLR and GPS were independent prognostic risk factors for poorer OS and DFS of osteosarcoma patients, but no significant association of PLR and LMR with prognosis in osteosarcoma was identified. However, obvious publication bias was observed in this meta-analysis and more prospective high-quality studies are still needed to verify above findings.
It has been widely known that the systematic inflammation response is closely related with the occurrence and development of tumors. The detailed internal mechanisms have been deeply explored and introduced in previous literatures[8,33-35]. The neutrophils, lymphocytes, platelets, monocytes and C-reactive protein are all common inflammatory biomarkers and a great number of studies have revealed the prognostic role of NLR, PLR, LMR and GPS in tumors. Several met-analyses demonstrated that elevated pretreatment NLR was a prognostic risk factor in colorectal cancer (HR = 1.57, 95%CI: 1.39-1.78, P < 0.001)[36], gastric cancer (HR = 1.78, 95%CI: 1.59-1.99, P < 0.001)[37], laryngeal cancer (HR = 1.76, 95%CI: 1.53-2.03, P < 0.001)[38] and non-small cell lung cancer patients (HR = 2.86, 95%CI: 2.11-3.87, P < 0.001)[39]. Furthermore, the prognostic value of GPS has been verified in ovarian cancer (HR = 1.62, 95%CI: 1.38-1.91, P < 0.001), esophageal squamous cell cancer (HR = 1.66, 95%CI: 1.14-2.41, P = 0.008), colorectal cancer (HR = 2.20, 95%CI: 1.88-2.57, P < 0.001) and lung cancer patients (HR = 2.058, 95%CI: 1.51-2.80, P < 0.05) by several meta-analyses[14,40-42]. Similarly, the PLR and LMR have also been reported to show high prognostic value in cancer patients by some studies with high-quality evidence[33,43-47]. However, whether the NLR, PLR, LMR and GPS could serve as valuable predictors for long-term survival in osteosarcoma remained unclear. Thus, we conducted the current meta-analysis and revealed the high prognostic value of NLR and GPS in osteosarcoma patients.
In our study, the LMR was not statistically related with prognosis of osteosarcoma patients. However, among the six included studies, two of them reported significantly positive association of lower LMR with poorer OS[16,28]. Remarkably, Yang et al[27] seemed to report conflicting results. According to the Kaplan-Meier survival curve, patients with lower LMR had poorer OS than patients with higher LMR did; however, the univariate analysis indicated that higher LMR was a risk factor for worse OS[27]. After excluding this study, the pooled HR was 0.69 (95%CI: 0.55-0.87, P = 0.002) by combining the remaining studies. Thus, we deem that LMR is also a valuable prognostic indicator in osteosarcoma patients, but more relevant studies are needed to further verify this.
Actually, we suppose that there are still some fields worth further investigation. Although we identified the prognostic role of these indexes, a comparison of their prognostic values was not conducted and it is not clear whether a combination of them would show higher predictive role for prognosis in osteosarcoma patients. Whether these indicators could predict the therapeutic effects of neoadjuvant or adjuvant chemotherapy is unclear. Besides, we deem that these blood parameters might play a role in the diagnosis of osteosarcoma or its recurrence. Based on our findings, we suggested that the NLR and GPS should be considered for the assessment of disease status and formulation of therapy strategies and osteosarcoma patients with higher pretreatment NLR or GPS might receive more aggressive treatment and follow-up. Besides, it is worth exploring whether anti-inflammation treatment, reducing the NLR and GPS, is beneficial for osteosarcoma patients with high NLR or GPS.
There are several limitations in this meta-analysis. First, all included studies are retrospective with relatively small sample size, which might cause some bias. Second, most studies are from China, which limits the application of our results in other regions or countries. Third, subgroup analyses based on some important parameters such as the disease stage, age and treatment were not able to performed due to lack of relevant data.
In overall, higher NLR and GPS were related with worse prognosis and might serve as valuable prognostic indicators for osteosarcoma patients. However, more prospective high-quality studies are still needed to verify our findings.
Previous researches explored the prognostic role of the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR) and Glasgow prognostic score (GPS) in osteosarcoma, but their results were inconsistent with each other.
To verify the prognostic value of these blood indexes in osteosarcoma patients.
To verify the prognostic value of these blood indexes in osteosarcoma patients.
Several electronic databases were searched to identify relevant articles. The hazard ratio (HR) with 95% confidence interval (CI) was combined to the evaluate the association between these indicators and overall survival (OS) and disease-free survival (DFS).
Higher NLR and GPS were significantly associated with poorer OS (P < 0.001; P < 0.001) and DFS (P < 0.001; P < 0.001). However, no significant relationship of PLR and LMR and OS (P = 0.085; P = 0.338) and DFS (P = 0.396; P = 0.124) was observed.
Higher NLR and GPS were related with worse prognosis in osteosarcoma.
The NLR and GPS might serve as reliable and valuable prognostic indicators for osteosarcoma patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arigami T, Socea B S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J, Antal I, Arndt C, Brown KLB, Butterfass-Bahloul T, Calaminus G, Capra M, Dhooge C, Eriksson M, Flanagan AM, Friedel G, Gebhardt MC, Gelderblom H, Goldsby R, Grier HE, Grimer R, Hawkins DS, Hecker-Nolting S, Sundby Hall K, Isakoff MS, Jovic G, Kühne T, Kager L, von Kalle T, Kabickova E, Lang S, Lau CC, Leavey PJ, Lessnick SL, Mascarenhas L, Mayer-Steinacker R, Meyers PA, Nagarajan R, Randall RL, Reichardt P, Renard M, Rechnitzer C, Schwartz CL, Strauss S, Teot L, Timmermann B, Sydes MR, Marina N. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 430] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 2. | American Cancer Society Cancer Statistics 2021 Report. J Nucl Med. 2021;62:12N. [PubMed] |
| 3. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2966] [Article Influence: 741.5] [Reference Citation Analysis (7)] |
| 4. | Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J Clin Oncol. 2015;33:3029-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 1140] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 5. | Pruksakorn D, Teeyakasem P, Klangjorhor J, Chaiyawat P, Settakorn J, Diskul-Na-Ayudthaya P, Chokchaichamnankit D, Pothacharoen P, Srisomsap C. Overexpression of KH-type splicing regulatory protein regulates proliferation, migration, and implantation ability of osteosarcoma. Int J Oncol. 2016;49:903-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Kager L, Zoubek A, Pötschger U, Kastner U, Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M, Winkelmann W, Jundt G, Kabisch H, Reichardt P, Jürgens H, Gadner H, Bielack SS; Cooperative German-Austrian-Swiss Osteosarcoma Study Group. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 649] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 7. | Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: where do we go from here? Paediatr Drugs. 2008;10:315-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Jiang Y, Xu D, Song H, Qiu B, Tian D, Li Z, Ji Y, Wang J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis. BMJ Open. 2021;11:e048324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Zhou Q, Dong J, Sun Q, Lu N, Pan Y, Han X. Role of neutrophil-to-lymphocyte ratio as a prognostic biomarker in patients with breast cancer receiving neoadjuvant chemotherapy: a meta-analysis. BMJ Open. 2021;11:e047957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Kumarasamy C, Tiwary V, Sunil K, Suresh D, Shetty S, Muthukaliannan GK, Baxi S, Jayaraj R. Prognostic Utility of Platelet-Lymphocyte Ratio, Neutrophil-Lymphocyte Ratio and Monocyte-Lymphocyte Ratio in Head and Neck Cancers: A Detailed PRISMA Compliant Systematic Review and Meta-Analysis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Wang E, Huang H, Tang L, Tian L, Yang L, Wang S, Ma H. Prognostic significance of platelet lymphocyte ratio in patients with melanoma: A meta-analysis. Medicine (Baltimore). 2021;100:e27223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Hu X, Wang Y, Yang WX, Dou WC, Shao YX, Li X. Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:6163-6173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Li P, Li J, Lai Y, Zhou K, Wang X, Che G. The prognostic value of pretreatment Glasgow Prognostic Score in patients with esophageal cancer: a meta-analysis. Cancer Manag Res. 2019;11:8181-8190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Chen L, Wu Y, Li P, Che G. The prognostic value of modified Glasgow prognostic score in patients with esophageal squamous cell cancer: a Meta-analysis. Nutr Cancer. 2020;72:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Radulescu D, Baleanu VD, Padureanu V, Radulescu PM, Bordu S, Patrascu S, Socea B, Bacalbasa N, Surlin MV, Georgescu I, Georgescu EF. Neutrophil/Lymphocyte Ratio as Predictor of Anastomotic Leak after Gastric Cancer Surgery. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Liu T, Fang XC, Ding Z, Sun ZG, Sun LM, Wang YL. Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Bio. 2015;5:682-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Liu B, Huang Y, Sun Y, Zhang J, Yao Y, Shen Z, Xiang D, He A. Prognostic value of inflammation-based scores in patients with osteosarcoma. Sci Rep. 2016;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Aggerholm-Pedersen N, Maretty-Kongstad K, Keller J, Baerentzen S, Safwat A. The Prognostic Value of Serum Biomarkers in Localized Bone Sarcoma. Transl Oncol. 2016;9(4): 322-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Xia WK, Liu ZL, Shen D, Lin QF, Su J, Mao WD. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol. 2016;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Li YJ, Yao K, Lu MX, Zhang WB, Xiao C, Tu CQ. Prognostic value of the C-reactive protein to albumin ratio: a novel inflammation-based prognostic indicator in osteosarcoma. Onco Targets Ther. 2017;10:5255-5261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Vasquez L, León E, Beltran B, Maza I, Oscanoa M, Geronimo J. Pretreatment Neutrophil-to-Lymphocyte Ratio and Lymphocyte Recovery: Independent Prognostic Factors for Survival in Pediatric Sarcomas. J Pediatr Hematol Oncol. 2017;39:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Huang Z, Yang C, Luo Y, Deng Q, Rong X. Role of systemic inflammatory response in evaluating the prognosis of patients with osteosarcoma. J Pract Med. 2018;34:3410-3414. |
| 23. | Huang X, Hu H, Zhang W, Shao Z. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J Cell Physiol. 2019;234(10): 18408-18414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Xu K, Li B, Huang Q, Jiang D, Sun H, Zhong N, Wan W, Wei H, Xiao J. Clinical significance of traditional clinical parameters and inflammatory biomarkers for the prognosis of patients with spinal chondrosarcoma: a retrospective study of 150 patients in a single center. Eur Spine J. 2019;28(6): 1468-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Hu H, Deng X, Song Q, Lv H, Chen W, Xing X, Zhu J, Tan Z, Cheng X, Wang B, Shao Z, Zhang Y. Prognostic Value of the Preoperative Lymphocyte-to-C-Reactive Protein Ratio and Albumin-to-Globulin Ratio in Patients with Osteosarcoma. Onco Targets Ther. 2020;13: 12673-12681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Yang Q, Chen T, Yao Z, Zhang X. Prognostic value of pre-treatment Naples prognostic score (NPS) in patients with osteosarcoma. World J Surg Oncol. 2020;18(1) : 24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Yang S, Wu C, Wang L, Shan D, Chen B. Pretreatment inflammatory indexes as prognostic predictors for survival in osteosarcoma patients. Int J Clin Exp Pathol. 2020;13(3): 515-524. [PubMed] |
| 28. | Yeerhanati H, Aerhengbieke T, Aikebaier Y. Relation Between NLR, LMR, PLR and Prognosis of Osteosarcoma. Cancer Res Prev Treat. 2021;48:381-386. |
| 29. | Zhang X, Tan R, Lam WC, Yao L, Wang X, Cheng CW, Liu F, Chan JC, Aixinjueluo Q, Lau CT, Chen Y, Yang K, Wu T, Lyu A, Bian Z. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Extension for Chinese Herbal Medicines 2020 (PRISMA-CHM 2020). Am J Chin Med. 2020;48:1279-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12656] [Article Influence: 843.7] [Reference Citation Analysis (0)] |
| 31. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46527] [Article Influence: 2114.9] [Reference Citation Analysis (3)] |
| 32. | Wang Y, Li J, Chang S, Dong Y, Che G. Risk and Influencing Factors for Subsequent Primary Lung Cancer After Treatment of Breast Cancer: A Systematic Review and Two Meta-Analyses Based on Four Million Cases. J Thorac Oncol. 2021;16:1893-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 33. | Wang Y, Huang D, Xu WY, Wang YW, Che GW. Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Non-Small Cell Lung Cancer: A Meta-Analysis. Oncol Res Treat. 2019;42:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Candido S, Tomasello BMR, Lavoro A, Falzone L, Gattuso G, Libra M. Novel Insights into Epigenetic Regulation of IL6 Pathway: In Silico Perspective on Inflammation and Cancer Relationship. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Wiedlocha A, Haugsten EM, Zakrzewska M. Roles of the FGF-FGFR Signaling System in Cancer Development and Inflammation. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2021;10:5983-5997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 37. | Du S, Fang Z, Ye L, Sun H, Deng G, Wu W, Zeng F. Pretreatment neutrophil-to-lymphocyte ratio predicts the benefit of gastric cancer patients with systemic therapy. Aging (Albany NY). 2021;13:17638-17654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Yang F, Huang Q, Guan Z, Diao Q. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio in patients with laryngeal cancer: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2021;278:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang G, Chen R, Jiao S, Wang J. Pretreatment Neutrophil-to-Lymphocyte Ratio (NLR) May Predict the Outcomes of Advanced Non-small-cell Lung Cancer (NSCLC) Patients Treated With Immune Checkpoint Inhibitors (ICIs). Front Oncol. 2020;10:654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 40. | Jin J, Hu K, Zhou Y, Li W. Prognostic value of the Glasgow prognostic score in lung cancer: evidence from 10 studies. Int J Biol Markers. 2018;33:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Lu X, Guo W, Xu W, Zhang X, Shi Z, Zheng L, Zhao W. Prognostic value of the Glasgow prognostic score in colorectal cancer: a meta-analysis of 9,839 patients. Cancer Manag Res. 2019;11:229-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Xu S, Song L, Liu X. Prognostic Value of Pretreatment Glasgow Prognostic Score/Modified Glasgow Prognostic Score in Ovarian Cancer: A Systematic Review and Meta-Analysis. Nutr Cancer. 2021;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Ding N, Pang Z, Shen H, Ni Y, Du J, Liu Q. The Prognostic Value of PLR in Lung Cancer, a Meta-analysis Based on Results from a Large Consecutive Cohort. Sci Rep. 2016;6:34823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Zhang J, Zhang HY, Li J, Shao XY, Zhang CX. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:68837-68846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 45. | Jiang S, Liu J, Chen X, Zheng X, Ruan J, Ye A, Zhang S, Zhang L, Kuang Z, Liu R. Platelet-lymphocyte ratio as a potential prognostic factor in gynecologic cancers: a meta-analysis. Arch Gynecol Obstet. 2019;300(4):829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Jin J, Yang L, Liu D, Li WM. Prognostic Value of Pretreatment Lymphocyte-to-Monocyte Ratio in Lung Cancer: A Systematic Review and Meta-Analysis. Technol Cancer Res Treat. 2021;20:1533033820983085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Hu RJ, Ma JY, Hu G. Lymphocyte-to-monocyte ratio in pancreatic cancer: Prognostic significance and meta-analysis. Clin Chim Acta. 2018;481:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |