Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1263
Peer-review started: April 15, 2021
First decision: July 6, 2021
Revised: July 8, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: February 6, 2022
Processing time: 283 Days and 16.9 Hours
With the widespread application of immune checkpoint inhibitor (ICI) therapy, the number of immune-related adverse effects (irAEs) has increased over the years. Autoimmune diabetes mellitus (DM) is a rare irAEs of ICIs and can be troublesome and life threatening.
We report a 78-year-old woman with no history of diabetes who presented with hyperglycemia up to 23.4 mmol/L (random blood glucose level) after 14 courses of sintilimab. Hemoglobin A1c was 8.2%, fasting insulin was 0.29 mIU/mL, and fasting C-peptide was decreased to a level with negative autoantibodies. Combing her medical history and laboratory examination, she was diagnosed with programmed cell death (PD)-1-inhibitor-induced, new-onset autoimmune DM. After controlling her blood glucose, she was treated with daily insulin by subcutaneous injection. She was allowed to continue anti-PD-1 therapy and she still obtained some therapeutic efficacy. We also reviewed some published cases (n = 36) of PD-1/PD-ligand 1 (PD-L1) inhibitor-induced DM. We also discuss potential pathogenic mechanisms, clinical features, prognostic markers (β cell antibodies, human leukocyte antigen type, PD-L1 Level) of this rare adverse effect.
It is important for all clinicians to be aware of DM as an irAEs of ICIs.
Core tip: We report a case of programmed cell death (PD)-1-inhibitor-induced autoimmune diabetes mellitus (DM) after treatment of small cell lung cancer, and reviewed some published cases (n = 36) of PD-1/PD-ligand 1 inhibitor-induced DM. Plasma glucose monitoring is significant for preventing the occurrence of diabetic ketoacidosis.
- Citation: Yang J, Wang Y, Tong XM. Sintilimab-induced autoimmune diabetes: A case report and review of the literature. World J Clin Cases 2022; 10(4): 1263-1277
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1263.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1263
Immune checkpoint inhibitors (ICIs) have been used widely in the treatment of various advanced malignances. Programmed cell death (PD)-1 (also known as CD279) is one of the best-known ICs, and is expressed on T cells, B cells, activated monocytes, dendritic cells (DCs) and natural killer cells[1]. Its ligand PD-L1 (B7-H1, CD274) is expressed on antigen-presenting cells, macrophagocytes, nonhematopoietic cells and parenchymatous organs such as heart, lungs, placenta and liver. When PD-1 binds to PD-L1 (B7-H1, CD274)/PD-L2 (B7-DC, CD273), a signal that inhibits the proinflammatory ability of T cells and attenuates the function of cytotoxic T cells is delivered. T cell tolerance protects human tissues from immune-mediated tissue damage[2]. However, PD-1 and PD-L1 pathways are also seized by tumors, which impairs tumor immunity and facilitates tumor survival. PD-1/PD-L1 inhibitors remove the inhibitory signals of T cells, enhance cytotoxicity and increase cytokine production. Thus, ICIs can enhance the antitumor effect, but they also increase the chance of inflammatory injury (Figure 1).
According to recent research, ICIs induce immune-related adverse events (irAEs) that involve the whole body, including skin (46%–62%), gastrointestinal tract (22%–48%), autoimmune hepatitis (7%–33%), endocrine system (12%–34%), respiratory system (3%–8%) and urinary system (1%–7%)[3]. PD-1/PD-L1-inhibitor-associated autoimmune diabetes mellitus (DM) is rare, with an incidence rate of 0.1% in clinical trials[4]. ICI-induced DM (ICI-DM) is an irreversible event that can be life-threatening if not promptly recognized. Its incidence has increased with the widespread use of immunotherapy. Therefore, it is important for clinicians to fully understand the pathogenic mechanisms of these treatments and their potential irAEs.
Sintilimab is a PD-1 inhibitor that was newly approved in China for treatment of relapsed or refractory Hodgkin’s lymphoma in February 2019[5], and it is now also a feasible treatment for a variety of solid tumors, including non-small cell lung cancer and esophageal cancer. Small cell lung cancer (SCLC) is a malignant tumor with rapid metastasis and poor prognosis. Treatment of SCLC with sintilimab alone or combined with other chemotherapeutic drugs is rare and there are no reports published to describe its clinical effects. Here, we present the first case of new-onset autoimmune DM in a patient with SCLC during treatment with sintilimab, along with marked antitumor efficacy. We also provide a review of case reports of ICI-DM.
This study was conducted according to the advice of the Ethics Center of Zhejiang Provincial People’s Hospital. The patient’s written informed consent was obtained for publication of this case and any images or information that may identify the patient.
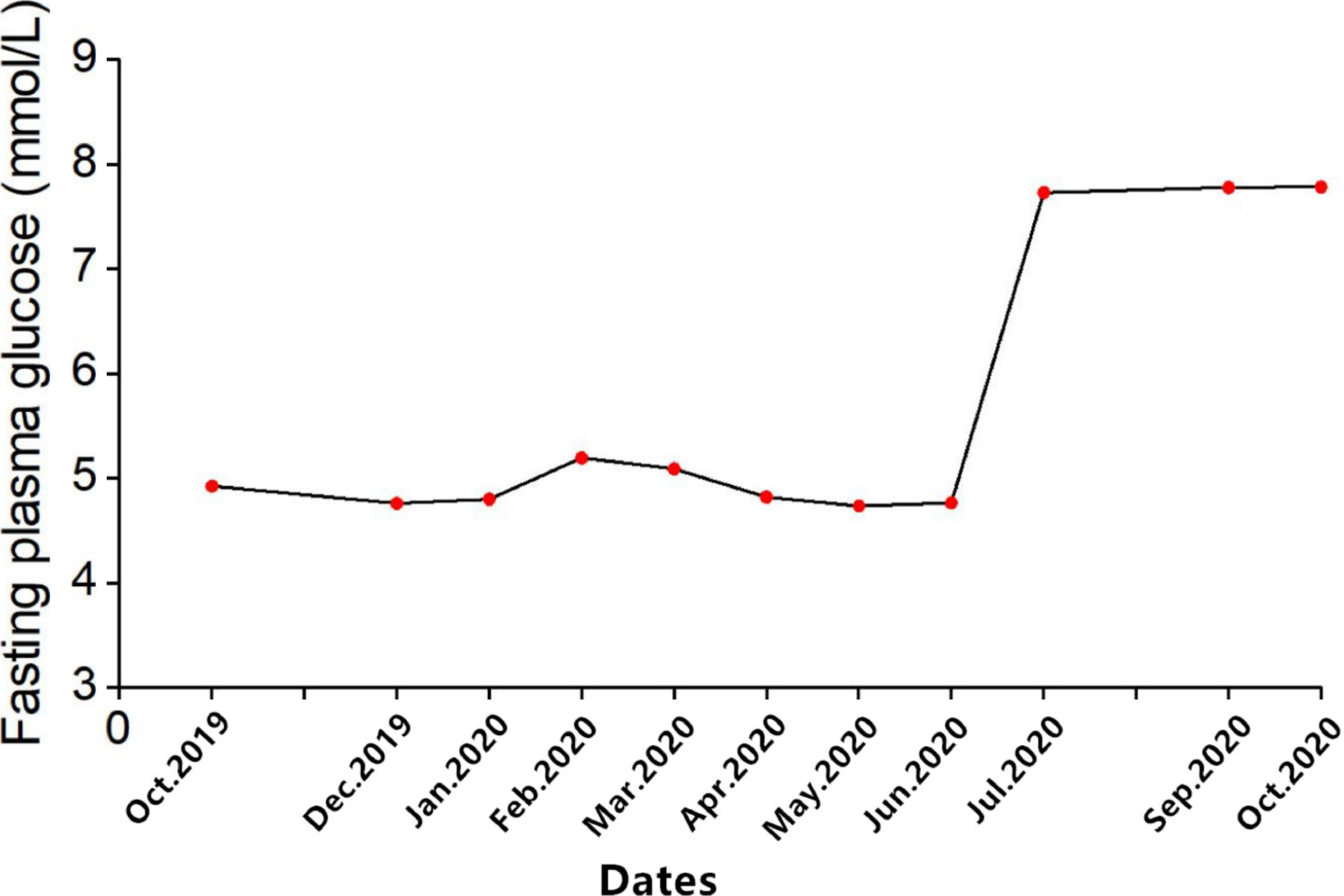
A 78-year-old Chinese woman was diagnosed with SCLC 1 year ago with no history of DM who presented with hyperglycemia up to 23.4 mmol/L (random blood glucose level) after 14 courses of sintilimab. The plasma glucose line shown in Figure 2.
The patient initially developed polyuria and polydipsia and her blood glucose level showed a mild increase after 12 cycles of sintilimab, but the treatment was continued. Two months later, the patient presented with hyperglycemia up to 23.4 mmol/L (random blood glucose level) with strong positive uric sugar (++++) and hemoglobin A1c of 8.2%.
The patient was diagnosed with SCLC on October 29, 2019 in the First Affiliated Hospital of Zhejiang University. She underwent endobronchial ultrasound-guided transbronchial needle aspiration, and the results were suggestive of poorly differentiated cell carcinoma, considered to be SCLC. Immunohistochemical staining demonstrated CKpan(+), P40(), P63(+), Ki67 (50%+), TTF-1(+), CgA(+), Syn(+), CD56(+) and CD45(). The patient immediately underwent concurrent chemotherapy and immunotherapy for SCLC (extensive). She received her first treatment, etoposide 82 mg, days 1–3; cisplatin 20 mg, days 1–3; and sintilimab 200 mg, day 1; EP plan) on October 30, 2019. The patient came to our hospital to continue treatment. After we assessed her condition, she continued the EP treatment plan, but we reformulated the doses as follows: etoposide 240 mg, days 1–3; cisplatin 250 mg, days 1–3; and sintilimab 200 mg, day 1. This therapy did control her disease well, with decreased tumor markers and no metastases found on imaging. In the following days, she came to our hospital monthly for evaluation. Her blood glucose level was normal after treatment. After five cycles with the EP plan, we changed to sintilimab 200 mg and anlotinib 8 mg q.d. because of severe gastrointestinal adverse reactions. After three cycles of the new treatment, the patient developed lower urinary tract infection, such as urinary frequency, difficulty urinating, pain with urination, and hematuria. Therefore, we had to stop anlotinib and used levofloxacin to treat the infection. Hence, we used sintilimab monotherapy, and imaging showed good antitumor effects (Figure 2). During the treatment, the patient only had mild gastrointestinal symptoms such as nausea and poor appetite.
The patient denied any other specific personal history. But she has family history of cancer, her grandmother died of lung cancer, whereas her father died of colorectal cancer.
Height: 151 cm; weight: 40.4 kg; body mass index: 17.71 kg/m2. Physical examination was no positive signs.
Other laboratory evaluation (Table 1) showed that urinary ketones were negative and blood pH was normal and the hypothalamic–pituitary–gonadal axis and hypothalamic–pituitary–adrenocortical axis were negative; but antithyroid autoantibodies were 17.80 IU/mL (normal< 4.0 IU/mL) and antithyroid peroxidase autoantibodies were 10.0 IU/mL (normal < 9.0 IU/mL). The islets antibodies tests were all negative, including anti-glutamic acid decarboxylase 65 (GADA) antibody, anti-islet cell antibody, and anti-insulin antibody tests were all negative. Moreover, zinc transporter 8 antibody levels were unavailable in our hospital. Human leukocyte antigen (HLA) class I and II, which was shown in Table 2, including HLA-A, B, C, DRB1, DQB1 and DPB1, were tested by polymerase chain reaction-sequence based typing (PCR-SBT).
| Admission bloods (normal range, units) | Results |
| BMI (kg/m2) | 18.8 |
| Finger prick glucose (mmol/L) | 23.4 |
| urinary glucose | ++++ |
| Ketones (mmol/L) | Negative |
| HbA1c [4%–6% (20–42 mmol/mol)] | 8.2% |
| C-peptide (1.0–7.1 ng/mL) | 0.22 |
| Testosterone (0.1–1.1 ng/mL) | 0.18 |
| Progesterone (0.00–0.20 ng/mL) | < 1.0 |
| Estradiol (10.00–28.00 pg/mL) | < 10 |
| FSH (26.70–133.40 IU/L) | 45.52 |
| LH (5.20–62.0 IU/L) | 18.55 |
| Prolactin (5.20–26.50 ng/mL) | 9.13 |
| 8 am ACTH (0.00-46.00 pg/mL) | 15.90 |
| 8 am cortisol (67.00-226.00 µg/L) | 113.00 |
| ARR (≤ 150 pg/mL) | 2.53 |
| TSH (15.00–65.00 mIU/L) | 0.98 |
| TT3 (0.66–1.61ug/L) | 1.03 |
| TT4 (54.40–118.50 ug/L) | 116.86 |
| FT3 (1.0–7.1 ng/L) | 3.35 |
| FT4 (1.0–7.1 ng/L) | 6.00 |
| ATG (<4.00 IU/L) | 17.80 |
| TPO (<9.00 IU/L) | 10.00 |
| GAD | Negative |
| ICA | Negative |
| IAA | Negative |
| ZnT8 | Negative |
| IL-2 (0.00–4.10 pg/mL) | 0.04 |
| IL-4 (0.10-3.20 pg/mL) | 0.01 |
| IL-6 (0.00–5.00 pg/mL) | 0.00 |
| IL-10 (0.00–5.90 pg/mL | 0.13 |
| TNF-α (0.00–6.00 pg/mL) | 0.81 |
| IFN-γ (0.00–6.00 pg/mL | 1.36 |
| IL-17A (0.00–5.90 pg/mL) | 0.70 |
| HLA type | A | B | C | DRB1 | DQB1 | DPB1 |
| Alleles | 02:01 | 35:03 | 04:01 | 04:01 | 03:01 | 02:01 |
| 24:02 | 51:05 | 14:02 | 14:03 | 03:02 | 02:01 |
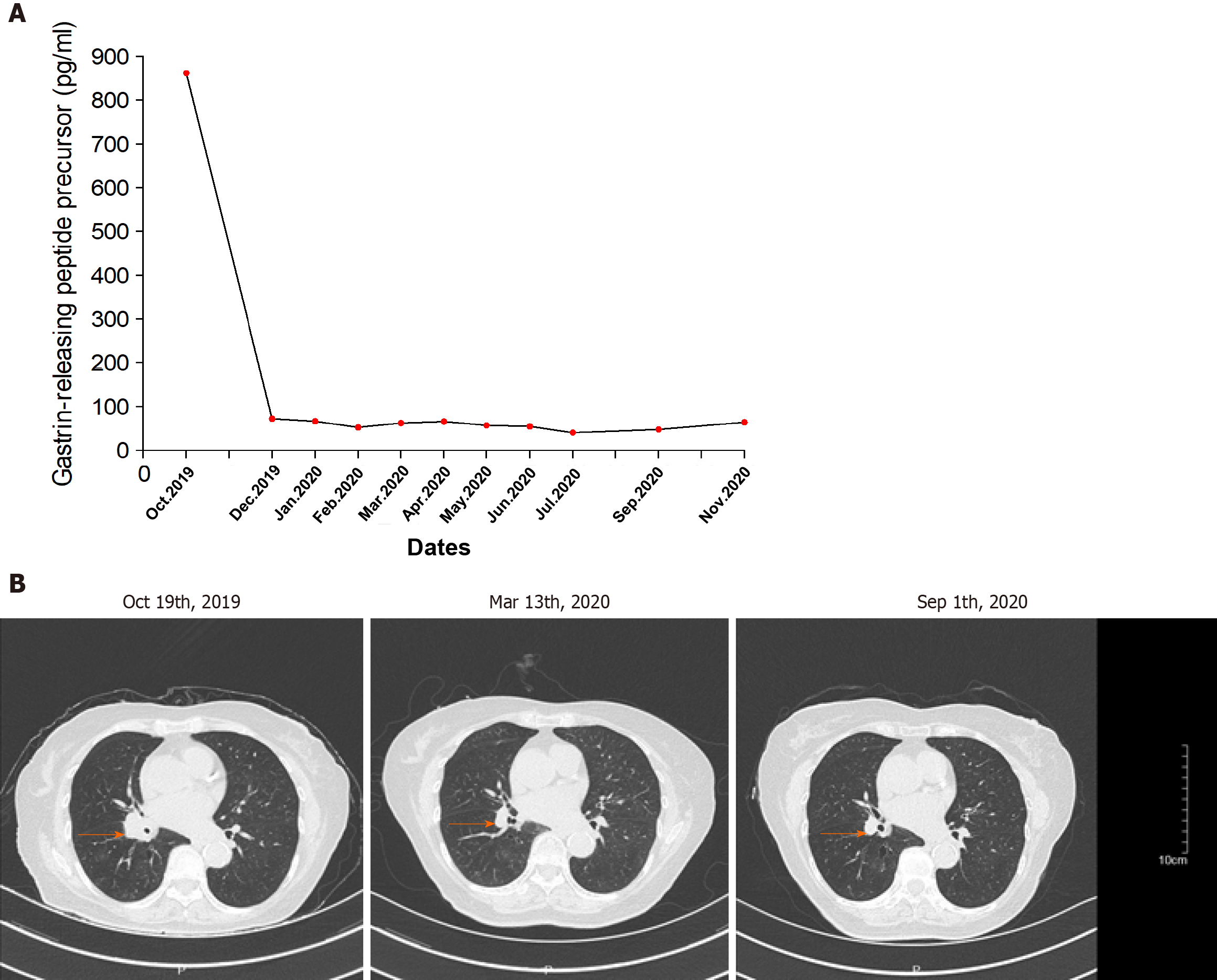
According to the computed tomography scanning of the patient’s chest (Figure 3B), the tumor was shrinking, which indicated that anti-PD-1 therapy was effective.
The patient had no history of DM or autoimmune disease before treatment, and there was no medication, infection, thromboembolic event, or other factor that could cause hyperglycemia; according to the laboratory evaluation, thus, sintilimab-induced, new-onset autoimmune DM was diagnosed.
Intravenous fluid infusion, continuous subcutaneous insulin infusion (insulin infusion pump therapy) and other supportive treatments were administered. After 10 d of insulin infusion pump therapy, the patient’s plasma glucose returned to normal levels. We performed a simple oral glucose tolerance test that revealed that the fasting and 0–2-h insulin levels were 0.29 and 2.50 IU/mL; fasting and 0–2-h C-peptide levels were 0.22 and 0.52 ng/mL, which indicated an insufficient function of pancreatic islet β cells. Subsequently, she was switched to once-daily basal insulin detemir (long-acting insulin, 10 U) plus thrice-daily premeal insulin aspart (fast acting insulin, 11 U, respectively 3U, 4U, 3U three meals) subcutaneous injection for long-term treatment.
By the time the manuscript was completed, the patient was without evidence of SCLC recurrence with no further treatment since sintilimab (Figure 3). Other endocrine adverse effects such as thyroiditis and hypophysitis did not occur. We retrieved 36 relevant case reports from 2016 to 2020 in PubMed to determine the common features of ICI-DM (Table 3)[6-37]. Table 4 summarizes the key features.
| Ref. | Sex/ Age (yr) | Primary diagnosis | Relevant history | Anti-PD-1/Anti-PD-L1 drug | Other chemo-therapies | Presentation | Other side effects | HbA1c | C peptide | Antibodies | Time with anti-PD-1 (w) | HLA |
| Araújo et al[6], 2017 | F/73 | NSCLC | N | Nivolumab | Carboplatin +pemetrexed | DKA | N | 7.20% | 0.06 ng/ml | GAD+ | 5 | High risk: DR3-DQ2/DR4-DQ8 |
| Li et al[7], 2020 | M/73 | NSCLC | N | Nivolumab | Sunitinib | DKA | N | 10.90% | 0.24 ng/mL | - | 30 | Unavailable |
| Abdullah et al[8], 2019 | M/68 | Melanoma | N | Nivolumab | None | DKA | N | Unavailable | 0.1 ng/mL | - | 4 | Unavailable |
| Kapke et al[9], 2017 | M/83 | Oral squamous cell carcinoma | Hypothyroidism | Nivolumab | None | DKA | N | Unavailable | 0.32 ng/mL | GAD+ | 12 | High risk: DRB1*08, DRB1*11, DQB1*03, DQB1*04, DQA1*04, and DQA1*05. |
| Kapke et al[9], 2017 | F/63 | Urothelial carcinoma of the bladder | Hypothyroidism | Atezolizumab | Gemcitabine + cisplatin | DKA | N | Unavailable | 0.02 ng/mL | GAD+ | 6 | High risk: DRB1*03, DRB1*04, DQB1*02, DQB1*03,DQA1*03, and DQA1*05. |
| Lowe et al[10], 2016 | M/54 | Melanoma | N | Nivolumab +ipilimumab | None | DKA | Autoimmune, thyroiditis | Unavailable | < 0.1 ng /mL | GAD+ | 19 | Unavailable |
| Rahman et al[11], 2020 | M/64 | Renal cell carcinoma | T2DM | Atezolizumab | Bevacizumab | DKA | N | Unavailable | Unavailable | GAD+ | 12 | Unavailable |
| Mengíbar et al[12], 2019 | M/55 | Urothelial carcinoma of the bladder | Family history of T1D | Durvalumab | None | DKA | Hypothyroidism | 8.40% | 0.02 ng/mL | GAD+, IA2+ | 3 | Unavailable |
| Kichloo et al[13], 2020 | F/77 | Colonic adenocarcinoma | N | Pembrolizumab | FOLFOX (leucovorin, fluorouracil, oxaliplatin | DKA | N | 8.80% | Unavailable | - | 44 | Unavailable |
| Delasos et al[14], 2020 | M/77 | Neuroendocrine tumor | N | Nivolumab | Carboplatin + etoposide | DKA | N | 8.30% | Unavailable | - | 28 | Unavailable |
| Hickmott et al[15], 2017 | M/57 | Urothelial cancer | N | Atezolizumab | Cisplatin + gemcitabine | DKA | N | 7.50% | 0.65 ng/mL | - | 15 | High risk: DRB1*11, DRB1*04; DRB3*02; DRB4*01; DQB1*03, DQB1*03 |
| Sothornwit et al[16], 2017 | F/52 | NSCLC | N | Atezolizumab | None | DKA | Transaminitis | 7.90% | 0.1 ng/ml | GAD+ | 24 | DRB1∗03, DRB1∗14, DQB1∗02, DQB1∗05 (DR3-DQ2/DR14-DQ5) |
| Changizzadeh et al[17], 2019 | M/44 | Melanoma | N | Nivolumab + ipilimumab | None | DKA | N | 6.50% | Unavailable | - | 12 | Unavailable |
| Gunawan et al[18], 2018 | M/52 | Melanoma | N | Nivolumab + ipilimumab | None | hyperglycemia Ketonuria | Hypophysitis, thyroiditis, adrenal inefficiency | 7.70% | 0.05 nmol/L (0.016 ng/ml) | - | 3 | Unavailable |
| Gunjur et al[19], 2019 | F/77 | Melanoma | N | Pembrolizumab | None | DKA | Thyroidits | 6.9% (normal range: <6.5%) | 0.07 ng/ml | GAD+,IA2+ | 3 | DRB1*04:16, DQB1*02:05 and DQA1*01:03 |
| Atkins et al[20], 2018 | M/50 | Squamous cell carcinoma of the tonsil | N | Avelumab | Utomilumab | DKA | N | 6.40% | 63 pmol/L | GAD+ | 4 | Unavailable |
| Marchand et al[21], 2019 | F/65 | Melanoma | N | Nivolumab + ipilimumab | None | DKA | Hypereosinophilia | 7.30% | <0.1 ng/mL | - | 12 | DRB1*01:01 DQA1*01DQB1*03:01 DRB1*11:01 DQA1*05 DQB1*05:01 |
| Tzoulis et al[22], 2018 | F/56 | NSCLC | N | Nivolumab | Pemetrexed + cisplatin | DKA | N | 8.20% | Undetectable | GAD+ | 7 | Unavailable |
| Porntharukchareon et al[23], 2020 | M/70 | NSCLC | N | Pembrolizumab + ipilimumab | None | DKA | IAD | 6.50% | < 0.1 ng/ml | - | 14 | Unavailable |
| Lee et al[24], 2020 | M/67 | NSCLC | T2DM | Nivolumab | Carboplatin + paclitaxel | DKA | Thyroiditis | 7.60% | <0.1 ng/mL | GAD+ | 2 | Unavailable |
| Leonardi et al[25], 2017 | M/66 | NSCLC | N | Pembrolizumab | None | hyperglycemia Ketonuria | N | 7.6% (4.2%–5.8%) | 0.3 ng/mL | GAD+ | 12 | Unavailable |
| Wong et al[26], 2020 | F/55 | Squamous cell lung carcinoma. | N | Atezolizumab | None | hyperglycemia Ketonuria | N | Unavailable | 0.6nmol/L (0.19 ng/ml) | ZnT8+ | 8 | Unavailable |
| Chokr et al[27], 2018 | F/61 | Melanoma | N | Nivolumab + ipilimumab, | None | DKA | N | 6.90% | <0.1 ng/ml. | - | 9 | Unavailable |
| Chan et al[28], 2017 | M/74 | Melanoma | N | Nivolumab + ipilimumab | None | DKA | Transaminitis | Unavailable | Unavailable | - | 14 | Unavailable |
| Zezza et al[29], 2019 | F/60 | Melanoma | T2DM | Nivolumab + ipilimumab | None | DKA | N | 7.60% | Unavailable | GAD+ICA+, IA2+ | 2 | Unavailable |
| Zezza et al[29], 2019 | F/80 | Melanoma | N | Nivolumab + ipilimumab | None | DKA | Thyroiditis | Unavailable | Unavailable | GAD+ | 3 | Unavailable |
| Shibayama et al[30], 2019 | F/79 | Merkel cell carcinoma | N | Avelumab | None | Hyperglycemia Ketonuria | N | 7.50% | <0.1 ng/mL | - | 20 | High risk: DRB1 *09:01:02 DRB1 *14:54:01 DQA1 *01:04 DQA1 *03:02 DQB1 *05:02:01 and DQB1 *03:03:02 |
| Marchand et al[21], 2019 | M/65 | Melanoma | N | Nivolumab | None | DKA | Hashimoto | 8.5% (74 mmol/mol) | <0.1 ng/mL | - | 34 | High risk: DRB1*04:01 DQA1*02 DQB1*02:02 DRB1*07:01 DQA1*03 DQB1*03:01 |
| Okamoto et al[31], 2016 | F/55 | Melanoma | N | Nivolumab | Acarbazine, + nimustine, + cisplatin + tamoxifen | Hyperglycemia Ketonuria | N | 7.00% | 1.0 ng/mL | - | 48 | High risk: DRB1*04:05-DQB1*04:01 |
| Godwin et al[32], 2017 | F/34 | NSCLC | N | Nivolumab | Carboplatin + pemetrexed | DKA | N | 7.1% (normal range 4.6–6.1%) | <0.1 ng/mL | GAD+, IA2+ ZnT8+ | 3 | A30:01, 30:02 (A30) D09:CTZ, 09:CTZ (DR9) |
| Smith-Cohn et al[33], 2017 | F/66 | Cholangiocarcinoma | N | Pembrolizumab | None | Hyperglycemia | N | 8.7% (4.2%–5.8%) | Unavailable | GAD+ | 12 | Unavailable |
| Marchand et al[21], 2019 | M/83 | Melanoma | N | Pembrolizumab | None | Hyperglycemia | Hashimoto’s disease | 9.40% | 1.0 ng/mL | - | 12 | DRB1*01:01 DQA1*01 DQB1*05:01/ DRB1*16:01 DQA1*01 DQB1*05:02 |
| Maamari et al[34], 2019-3 | F/47 | Cardiac angiosarcoma | N | Pembrolizumab | Ifosfamide, gemcitabine, docetaxel | DKA | N | 6.40% | 0.1 ng/mL | GAD+ | 3 | Unavailable |
| Tassone et al[35], 2019-9 | M/42 | Pulmonary adenocarcinoma | N | Nivolumab | None | DKA | N | Unavailable | 0.2 ng/dL (2ng/ml) | GAD+ | 12 | DRB1*03:15-DQB1*02:06 |
| Yilmas et al[36], 2020-8 | M/49 | Renal cell carcinoma | N | Nivolumab | None | DKA | N | 10.90% | 2.4 ng/mL | - | 44 | Unavailable |
| Wen et al[37], 2020 | M/56 | Hepatocellular carcinoma | N | Sintilimab | None | DKA | N | 7.80% | 1.12 ng/mL | - | 24 | DRB1*12:01 DRB1*12:02; DQB1 *05:03 DQB1 *03:01; DQA1 *01:04 DQA1 *06:01 |
| Reported cases | n (%) |
| Tumor types | |
| Melanoma | 13/36 (36.1) |
| NSCLC | 8/36 (22.2) |
| Renal cell carcinoma | 2/36 (5.6) |
| Squamous cell carcinoma | 3/36 (8.3) |
| Other cancers | 10/36 (27.8) |
| ICBs | |
| Anti PD-1 | 19/ 36 (52.7) |
| Nivolumab | 12 |
| Pembrolizumab | 6 |
| Sintilimab | 1 |
| Anti PD-L1 | 8/ 36 (22.2) |
| Avelumab | 2 |
| Atezolizumab | 5 |
| Durvalumab | 1 |
| Anti PD-1+CTLA-4 | 9/ 36 (25.0) |
| Nivolumab + ipilimumab | 8 |
| Pembrolizumab + ipilimumab | 1 |
| Demographic data | |
| Sex (F/M) | 16/20 |
| Average age (yr) | 58.8 |
| Time of diagnosis after start of (w) | 14.6 |
| Presentation | |
| DKA | 29/36 (80.6) |
| Hyperglycemia Ketonuria | 8/36 (22.2) |
| HbA1c, % (avg) | 7.8 26/36 |
| Relevant history | |
| T2DM | 3/36 (8.3) |
| Hypothyroidism | 2/36 (5) |
| Family history of T1DM | 2/36 (5) |
| None | 29/36 (80.5) |
| Antibodies | |
| GAD+ | 18/36 (50) |
| IA-2+ | 4/36 (10) |
| ZnT8+ | 2/36 (5) |
| Negative | 12/36 (33.3) |
Sintilimab is a fully humanized IgG4 monoclonal antibody that binds to PD-1, then interferes with the interaction of PD-1 and its ligands (PD-L1 and PD-L2), thus activating and restoring the function of T cells, which contributes to an obvious antitumor effect. Accordingly, some normal tissues have been damaged in this process by the increase of cytokines. ICI-DM has rarely been reported as an irAEs of anti-PD1/PD-L1 therapy, and primarily in case reports.
In our review of case reports, the main tumor types were melanoma (13/36, 36.1%) and non-SCLC (8/36, 22.2%). The different treatment regimens included monotherapy with anti-PD-1 (19/ 36, 52.7%) anti-PD-L1 (8/36, 22.2%) or a combination of anti-CTLA-4 with anti-PD-1 (9/36, 25.0%). Diabetic ketoacidosis (DKA) was the first sign of diabetes in 29 of 36 (80.5%) case reports, which is similar to 85.7% in another study[38], and the average time from initiation of anti-PD-1/PD-L1 therapy to diagnosis of ICI-DM was 14.6 wk (range 2–48 wk). Low C-peptide levels were present at diagnosis in 82% (23/28) of cases. In addition, 26 of 36 patients presented with a median glycated hemoglobin level of 7.6% (average: 7.8%; range: 6.4%–10.9%), which is the same as in other studies[39,40]. Most of the patients did not have relevant autoimmune history, which was only seen in 18.3% of our reviewed cases. These case reports have different definitions for ICI-DM. Since the syndrome has similarities with classic type 1 (T1)DM, most reports simply classified ICI-DM as T1DM, but ICI-DM has its own features. We found several significant features of ICI-DM: (1) Abrupt onset of hyperglycemia, and low to absent insulin C-peptide levels; (2) Rapid destruction of islets β cells, leading to endogenous insulin deficiency; and (3) High risk of DKA[39]. In addition to the above features, ICI-DM does not have a “honeymoon period” like juvenile T1DM, nor does it have GADA as in latent autoimmune DM in adults[41].
Similar to a previous study[39], our autoantibody analysis was positive in 50% of patients for GADA. Some studies have demonstrated that GADA-positive patients developed ICI-DM in the first 2 mo after initiation of therapy, and GADA-negative patients developed ICI-DM after 2 mo of treatment[42]. Patients with any positive diabetes autoantibodies at the time of presentation of ICI-DM have fewer cycles than those with negative autoantibodies. Our results showed that GADA-positive patients had ICI-DM onset at an average of 8 wk after immunotherapy compared with 22.8 wk in GADA-negative patients. It has been demonstrated that the interval from initiation of anti-PD-1/PD-L1 therapy and onset of ICI-DM is related to the presence/absence of GADA. Serological examination of GADA prior to anti-PD-1/PD-L1 therapy might be helpful for predicting the development of ICI-DM. In addition, several major histocompatibility complex and HLA molecules are associated with increased susceptibility to T1DM, especially HLA-DRB1, -DQB1 and –DQA1[43]. Different combinations of DRB1, DQB1 and DQA1 determine the extent of haplotypic risk. The most susceptible HLA haplotypes are DRB1*0405–DQA1*0301–DQB1*0302, followed by DRB1*0401–DQA1*0301–DQB*0302, DRB1*0301–DQA1*0501–DQB1*0201, and DRB1*0402–DQA1*0301–DQB1*0302. Subsequently, DQB1*0302 allele is the key susceptibility allele[44]. However, another study confirmed that DPB1*0301 and DPB1*0202 are also susceptible haplotypes for T1DM. Hence, the HLA typing of our patient (Table 2) showed a high risk of T1DM. Based on this evidence, there were seven patients with high-risk genes for T1DM among 13 patients tested. Accordingly, understanding the association between HLA and the development of ICI-DM by anti-PD-1/PD-L1 therapy is significant in predicting susceptible patients. When clinical features are discordant with the results of autoantibody testing, genetic risk score (GRS) could be an important addition to diagnosis of ICI-DM. This GRS summarizes risk-associated variation across the genome of T1DM[45]. One limitation of our case was the lack of information before sintilimab, such as autoimmune antibodies and genetic factors like HLA genotypes that may predispose to endocrine irAEs.
Multiple studies have indicated both the PD-1 and CTLA-4 pathways in the pathogenesis of T1DM and suggest a synergistic effect between these two negative regulatory receptors to enhance autoimmune disorders. Furthermore, the incidence of ICI-induced endocrine irAEs is significantly higher in patients treated with combination immunotherapy compared with single immunotherapy. The incidence of thyroid dysfunction is high in patients treated with single PD-1 antibodies. In contrast, the incidence of hypophysitis is highest in patients treated with ipilimumab[46]. Similarly, our review also found that combination of PD-1 inhibitor and anti-CTLA-4 therapy causes endocrine dysfunction. The most common combination was nivolumab and ipilimumab. An animal study found that single CTLA-4 blockers in nonobese diabetic (NOD) mice only induced diabetes in baby mice, while PD-1 blocked secondary diabetes in NOD mice at any age[47]. A recent case reported that ipilimumab induced T1DM. The mechanism by which single anti-CTLA-4 therapy induced ICI-DM was unclear and needs further study[48]. However, a randomized, double-blind, phase 3 study suggested that combination of immunotherapy significantly increases progression-free survival more than monotherapy does[49]. Therefore, it is important for clinicians to consider whether to continue to use combination therapy when endocrine irAEs appear.
T1DM is caused by destruction of pancreatic β cells by virus infection, genetic factors and autoimmune disorders[32]. Accordingly, the main mechanism of ICI-DM may be islet cell damage. There is an active interaction between β cells and immune cells during insulitis. This kind of interaction usually has a largely negative effect on β cells. An animal study has shown that PD-1 deficiency accelerates the occurrence and frequency of T1DM in NOD mice, and infiltration of pancreatic islets by T cells with strong T helper 1 polarization[50]. In addition, animal and human experiments have shown that PD-L1 in insulin-positive cells of T1DM, but absent in nondiabetic individuals and type 2 DM, is mainly due to islet β cell expression[6]. The present data indicate that interferon (IFN)-α and IFN-γ are the main regulators of PD-L1 expression in human pancreatic β cells, especially IFN-γ. IFN-γ suppresses autoreactive T cells by upregulating PD-L1. In other words, PD-L1 protects islet β cells to delay progression of DM and even prevent its onset[50-52]. Yet, IFN-α and IFN-γ induce proinflammatory responses. For instance, HLA class I upregulation, cytokine production and endoplasmic reticulum stress are harmful to the human body, including the pancreas. Inhibition of signal transducer and activator of transcription 2 can prevent IFNα-induced HLA class I expression, and at the same time allow PD-L1 upregulation[53], but this lacks clinical validation. Therefore, the level of PD-L1 expression may serve as an additional criterion for irAEs after ICI treatment. PD-L1 expression can also be used as a prognostic marker of immunotherapy[54]. In our patient, in spite of tumor necrosis factor-α, IL-1β and IFN-γ, all of the cytokines were normal during treatment with sintilimab, which suggest the particular pathogenic mechanism of ICI-DM. However, the precise mechanism mediating ICI-DM is still unclear. Further studies are required to elucidate the pathogenesis and background factors for this form of DM.
This is the second case of sintilimab-induced autoimmune DM. The first one was a recently published case report of autoimmune DM diagnosed in a patient with hepatocellular carcinoma[37]. What makes our case different from others is that there was no DKA in the process of DM. This may be because patients were regularly monitored for plasma glucose level. This illustrates the importance of regular monitoring of glucose during immunotherapy for inhibiting progression of DM. Furthermore, based on the information collected in our review, we recommend measuring PD-L1 expression, HLA typing, islet cell antibody testing, C peptide measurement, or even determining T1DM-associated GRS for clinicians before or during immunotherapy. We can compare symptom severity and therapeutic efficacy in DM patients with or without a history of DM after treatment with PD-1/PD-L1 inhibitors in the future, so as to evaluate whether patients with potential risk of DM are suitable for treatment with PD-1/PD-L1 inhibitors.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gebbia V S-Editor: Wang JL L-Editor: Kerr C P-Editor: Wang JL
| 1. | Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 2. | Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 4139] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 3. | Heinzerling L, de Toni EN, Schett G, Hundorfean G, Zimmer L. Checkpoint Inhibitors. Dtsch Arztebl Int. 2019;116:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 4. | Akturk HK, Michels AW. Adverse events associated with immune checkpoint inhibitors: a new era in autoimmune diabetes. Curr Opin Endocrinol Diabetes Obes. 2020;27:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Hoy SM. Sintilimab: First Global Approval. Drugs. 2019;79:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (2)] |
| 6. | Araújo M, Ligeiro D, Costa L, Marques F, Trindade H, Correia JM, Fonseca C. A case of fulminant Type 1 diabetes following anti-PD1 immunotherapy in a genetically susceptible patient. Immunotherapy. 2017;9:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Li W, Wang H, Chen B, Zhao S, Zhang X, Jia K, Deng J, He Y, Zhou C. Anti PD-1 monoclonal antibody induced autoimmune diabetes mellitus: a case report and brief review. Transl Lung Cancer Res. 2020;9:379-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Abdullah HMA, Elnair R, Khan UI, Omar M, Morey-Vargas OL. Rapid onset type-1 diabetes and diabetic ketoacidosis secondary to nivolumab immunotherapy: a review of existing literature. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kapke J, Shaheen Z, Kilari D, Knudson P, Wong S. Immune Checkpoint Inhibitor-Associated Type 1 Diabetes Mellitus: Case Series, Review of the Literature, and Optimal Management. Case Rep Oncol. 2017;10:897-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, Hanks BA. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer. 2016;4:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Rahman W, Conley A, Silver KD. Atezolizumab-induced type 1 diabetes mellitus in a patient with metastatic renal cell carcinoma. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Mengíbar JL, Capel I, Bonfill T, Mazarico I, Espuña LC, Caixàs A, Rigla M. Simultaneous onset of type 1 diabetes mellitus and silent thyroiditis under durvalumab treatment. Endocrinol Diabetes Metab Case Rep. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Kichloo A, Albosta MS, McMahon S, Movsesian K, Wani F, Jamal SM, Aljadah M, Singh J. Pembrolizumab-Induced Diabetes Mellitus Presenting as Diabetic Ketoacidosis in a Patient With Metastatic Colonic Adenocarcinoma. J Investig Med High Impact Case Rep. 2020;8:2324709620951339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Delasos L, Bazewicz C, Sliwinska A, Lia NL, Vredenburgh J. New onset diabetes with ketoacidosis following nivolumab immunotherapy: A case report and review of literature. J Oncol Pharm Pract. 2021;27:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Hickmott L, De La Peña H, Turner H, Ahmed F, Protheroe A, Grossman A, Gupta A. Anti-PD-L1 atezolizumab-Induced Autoimmune Diabetes: a Case Report and Review of the Literature. Target Oncol. 2017;12:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Sothornwit J, Phunmanee A, Pongchaiyakul C. Atezolizumab-Induced Autoimmune Diabetes in a Patient With Metastatic Lung Cancer. Front Endocrinol (Lausanne). 2019;10:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Changizzadeh PN, Mukkamalla SKR, Armenio VA. Combined checkpoint inhibitor therapy causing diabetic ketoacidosis in metastatic melanoma. J Immunother Cancer. 2017;5:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Gunawan F, George E, Roberts A. Combination immune checkpoint inhibitor therapy nivolumab and ipilimumab associated with multiple endocrinopathies. Endocrinol Diabetes Metab Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Gunjur A, Klein O, Kee D, Cebon J. Anti-programmed cell death protein 1 (anti-PD1) immunotherapy induced autoimmune polyendocrine syndrome type II (APS-2): a case report and review of the literature. J Immunother Cancer. 2019;7:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 20. | Atkins PW, Thompson DM. Combination avelumab and utomilumab immunotherapy can induce diabetic ketoacidosis. Diabetes Metab. 2018;44:514-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Marchand L, Thivolet A, Dalle S, Chikh K, Reffet S, Vouillarmet J, Fabien N, Cugnet-Anceau C, Thivolet C. Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: description of pancreatic endocrine and exocrine phenotype. Acta Diabetol. 2019;56:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Tzoulis P, Corbett RW, Ponnampalam S, Baker E, Heaton D, Doulgeraki T, Stebbing J. Nivolumab-induced fulminant diabetic ketoacidosis followed by thyroiditis. Endocrinol Diabetes Metab Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Porntharukchareon T, Tontivuthikul B, Sintawichai N, Srichomkwun P. Pembrolizumab- and ipilimumab-induced diabetic ketoacidosis and isolated adrenocorticotropic hormone deficiency: a case report. J Med Case Rep. 2020;14:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Lee S, Morgan A, Shah S, Ebeling PR. Rapid-onset diabetic ketoacidosis secondary to nivolumab therapy. Endocrinol Diabetes Metab Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Leonardi GC, Oxnard GR, Haas A, Lang JP, Williams JS, Awad MM. Diabetic Ketoacidosis as an Immune-related Adverse Event from Pembrolizumab in Non-Small Cell Lung Cancer. J Immunother. 2017;40:249-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Wong M, Nandi N, Sinha A. A unique case of atezolizumab-induced autoimmune diabetes. AACE Clin Case Rep. 2020;6:e30-e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Chokr N, Farooq H, Guadalupe E. Fulminant Diabetes in a Patient with Advanced Melanoma on Nivolumab. Case Rep Oncol Med. 2018;2018:8981375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Chan PY, Hall P, Hay G, Cohen VML, Szlosarek PW. A major responder to ipilimumab and nivolumab in metastatic uveal melanoma with concomitant autoimmunity. Pigment Cell Melanoma Res. 2017;30:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Zezza M, Kosinski C, Mekoguem C, Marino L, Chtioui H, Pitteloud N, Lamine F. Combined immune checkpoint inhibitor therapy with nivolumab and ipilimumab causing acute-onset type 1 diabetes mellitus following a single administration: two case reports. BMC Endocr Disord. 2019;19:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Shibayama Y, Kameda H, Ota S, Tsuchida K, Cho KY, Nakamura A, Miyoshi H, Atsumi T. Case of fulminant type 1 diabetes induced by the anti-programmed death-ligand 1 antibody, avelumab. J Diabetes Investig. 2019;10:1385-1387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Okamoto M, Okamoto M, Gotoh K, Masaki T, Ozeki Y, Ando H, Anai M, Sato A, Yoshida Y, Ueda S, Kakuma T, Shibata H. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7:915-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 32. | Godwin JL, Jaggi S, Sirisena I, Sharda P, Rao AD, Mehra R, Veloski C. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer. 2017;5:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Smith-Cohn MA, Gill D, Voorhies BN, Agarwal N, Garrido-Laguna I. Case report: pembrolizumab-induced Type 1 diabetes in a patient with metastatic cholangiocarcinoma. Immunotherapy. 2017;9:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Maamari J, Yeung SJ, Chaftari PS. Diabetic ketoacidosis induced by a single dose of pembrolizumab. Am J Emerg Med. 2019;37:376.e1-376.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Tassone F, Colantonio I, Gamarra E, Gianotti L, Baffoni C, Magro G, Borretta G. Nivolumab-induced fulminant type 1 diabetes (T1D): the first Italian case report with long follow-up and flash glucose monitoring. Acta Diabetol. 2019;56:489-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Yilmaz M. Nivolumab-induced type 1 diabetes mellitus as an immune-related adverse event. J Oncol Pharm Pract. 2020;26:236-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Wen L, Zou X, Chen Y, Bai X, Liang T. Sintilimab-Induced Autoimmune Diabetes in a Patient With the Anti-tumor Effect of Partial Regression. Front Immunol. 2020;11:2076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed Cell Death-1 Inhibitor-Induced Type 1 Diabetes Mellitus. J Clin Endocrinol Metab. 2018;103:3144-3154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (2)] |
| 39. | de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, Van der Auwera BJ, Gorus FK, Roep BO, Aspeslagh S, Neyns B, Velkeniers B, Kharagjitsingh AV. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol. 2019;181:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 40. | Quandt Z, Young A, Anderson M. Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin Exp Immunol. 2020;200:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 41. | Bingley PJ. Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab. 2010;95:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 42. | Usui Y, Udagawa H, Matsumoto S, Imai K, Ohashi K, Ishibashi M, Kirita K, Umemura S, Yoh K, Niho S, Osame K, Goto K. Association of Serum Anti-GAD Antibody and HLA Haplotypes with Type 1 Diabetes Mellitus Triggered by Nivolumab in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:e41-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Koeleman BP, Lie BA, Undlien DE, Dudbridge F, Thorsby E, de Vries RR, Cucca F, Roep BO, Giphart MJ, Todd JA. Genotype effects and epistasis in type 1 diabetes and HLA-DQ trans dimer associations with disease. Genes Immun. 2004;5:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P; Type 1 Diabetes Genetics Consortium. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 589] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 45. | Khdair SI, Jarrar W, Jarrar YB, Bataineh S, Al-Khaldi O. Association of HLA-DRB1 and -DQ Alleles and Haplotypes with Type 1 Diabetes in Jordanians. Endocr Metab Immune Disord Drug Targets. 2020;20:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm Metab Res. 2019;51:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 47. | Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H Jr, Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 577] [Cited by in RCA: 644] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 48. | Tsiogka A, Jansky GL, Bauer JW, Koelblinger P. Fulminant type 1 diabetes after adjuvant ipilimumab therapy in cutaneous melanoma. Melanoma Res. 2017;27:524-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2362] [Cited by in RCA: 2779] [Article Influence: 347.4] [Reference Citation Analysis (0)] |
| 50. | Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102:11823-11828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 358] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 51. | Osum KC, Burrack AL, Martinov T, Sahli NL, Mitchell JS, Tucker CG, Pauken KE, Papas K, Appakalai B, Spanier JA, Fife BT. Interferon-gamma drives programmed death-ligand 1 expression on islet β cells to limit T cell function during autoimmune diabetes. Sci Rep. 2018;8:8295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 52. | Pizarro C, García-Díaz DF, Codner E, Salas-Pérez F, Carrasco E, Pérez-Bravo F. PD-L1 gene polymorphisms and low serum level of PD-L1 protein are associated to type 1 diabetes in Chile. Diabetes Metab Res Rev. 2014;30:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Colli ML, Hill JLE, Marroquí L, Chaffey J, Dos Santos RS, Leete P, Coomans de Brachène A, Paula FMM, Op de Beeck A, Castela A, Marselli L, Krogvold L, Dahl-Jorgensen K, Marchetti P, Morgan NG, Richardson SJ, Eizirik DL. PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-α and-γ via IRF1 induction. EBioMedicine. 2018;36:367-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 54. | Sanchez-Paulete AR, Labiano S, Rodriguez-Ruiz ME, Azpilikueta A, Etxeberria I, Bolaños E, Lang V, Rodriguez M, Aznar MA, Jure-Kunkel M, Melero I. Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. Eur J Immunol. 2016;46:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |